Abstract
Abnormal lipid metabolism is associated with gestational diabetes mellitus (GDM) and is observed in neonates with abnormal fetal growth. However, the underlying specific changes in the lipoprotein profile remain poorly understood. Thus, in the present study we used a novel nuclear magnetic resonance (NMR)-based approach to profile the umbilical cord serum lipoproteins. Two-dimensional diffusion-ordered 1H-NMR spectroscopy showed that size, lipid content, number and concentration of particles within their subclasses were similar between offspring born to control (n = 74) and GDM (n = 62) mothers. Subsequent data stratification according to newborn birth-weight categories, i.e., small (n = 39), appropriate (n = 50) or large (n = 49) for gestational age (SGA, AGA and LGA, respectively), showed an interaction between GDM and birth-weight categories for intermediate-density lipoproteins (IDL)-cholesterol content and IDL- and low-density lipoproteins (LDL)-triglyceride content, and the number of medium very low-density lipoproteins (VLDL) and LDL particles specifically in AGA neonates. Moreover, in a 2-year follow-up study, we observed that small LDL particles were independently associated with offspring obesity at 2 years (n = 103). Collectively, our data demonstrate that GDM disturbs triglyceride and cholesterol lipoprotein content across birth-weight categories, with AGA neonates born to GDM mothers displaying a profile more similar to that of adults with dyslipidemia. Furthermore, an altered fetal lipoprotein pattern was associated with the development of obesity at 2 years.
1. Introduction
Fetal growth and development constitute a particularly vulnerable period in life that is greatly affected by the maternal environment. Prenatal exposure to nutritional stressors has been associated with fetal programming, which can impact both metabolism and physiology and, consequently, predispose to later development of cardiovascular and metabolic diseases, including obesity [1]. In this context, it has been proposed that cardiovascular disease can begin early in life [2] and that atherosclerosis may originate during the fetal period [3].
Birth-weight is strongly determined by neonatal fat mass and gestational age, and fetal growth disorders can result from impaired maternal and fetal lipid metabolism. In fact, the levels and composition of cord blood lipids, apolipoproteins and lipoproteins are affected by both maternal and fetal factors [4,5,6]. Disturbed lipid profiles at birth have been described in small and large for gestational age (SGA and LGA, respectively) neonates [6,7,8,9]. When compared with appropriate for gestational age (AGA) peers, SGA neonates show higher levels of triglycerides, triglyceride-enriched very low-density lipoproteins (VLDL), low-density lipoproteins (LDL) and high-density lipoproteins (HDL), and lower levels of total cholesterol [6,9,10]. By contrast, LGA neonates display higher LDL, HDL and total cholesterol levels than AGA neonates [8].
Diabetic pregnancies are associated with a higher incidence of fetal growth disorders, and there is evidence that disturbances in maternal metabolism strongly contribute to these situations [11]. Changes in cord blood lipoprotein concentrations have been reported in mothers with type 1 diabetes mellitus, including an increased cholesterol content of LDL and a decrease in HDL [12,13]. The situation appears more complex in gestational diabetes mellitus (GDM), with some studies showing no differences with normal glucose-tolerant mothers and others showing lower HDL and higher VLDL and LDL cholesterol concentrations [14]. Additionally, qualitative changes in HDL remodeling resulting in an altered functionality have been reported in GDM neonates [15], but that does not seem to affect newborn cholesterol metabolism in both obese and well-controlled GDM mothers [16]. Thus, new strategies for an in-depth analysis of the lipoprotein profile in GDM are still required.
Interestingly, 1H-nuclear magnetic resonance (1H-NMR)-based analysis of lipoproteins has established that the number of LDL and HDL particles is a more powerful index of cardiovascular risk than classical cholesterol determinations, given the large variability in the amount of cholesterol per particle and in particle size [17]. 1H-NMR-based tests have also demonstrated the incomplete conversion of VLDL into LDL in diabetes, which results in a higher prevalence of VLDL and small and dense LDL particles [17].
Given the heterogeneity of growth patterns and the inconclusive findings in the cord blood lipoprotein profile of infants from GDM mothers, a comprehensive characterization of the main lipoproteins, including the assessment of the size and number of particles, is necessary to identify possible alterations in fetal lipoprotein metabolism and their potential consequences for fetal health. Thus, in the present study, we used the Liposcale test, a novel advanced lipoprotein assessment method based on 2D diffusion-ordered 1H-NMR [18], to examine possible correlations between fetal growth disorders and differences in umbilical cord blood lipoprotein profile. Additionally, we explored the potential association with offspring outcomes, including obesity, at 2 years of age.
2. Materials and Methods
2.1. Study Subjects
All the mother–offspring pairs included in this study belonged to a carefully selected pre-birth cohort, and the mothers gave birth at the Department of Obstetrics of the Hospital Universitari de Tarragona Joan XXIII between June 2010 and May 2017. GDM and control women were recruited at their first prenatal visit to the Obstetrics Department and were followed until delivery. In this study, the participants were stratified according to the birth-weight category, and we included a similar proportion of infants born to mothers with GDM and control women who were AGA, SGA or LGA. The inclusion criteria at the end of pregnancy were: (1) singleton pregnancy, (2) accurate gestational age confirmed by an ultrasound examination before 20 weeks of gestation, (3) absence of fetal anomalies, (4) cord blood serum availability and (5) maternal lipid information from the third trimester exam. Neonates with major congenital anomalies, intrauterine infections or born from women with chronic and inflammatory diseases were excluded. One hundred and thirty-six neonate-and-mother pairs fulfilled these criteria (74 control pairs and 62 pairs with GDM) and were included in this study, which was performed in accordance with the tenets of the Declaration of Helsinki and whose protocol was reviewed and approved by the Hospital Universitari de Tarragona Research Ethics Board (ref: 243/2016). All participants provided informed consent before inclusion.
All mothers were screened for GDM between 24–28 weeks of pregnancy following the Spanish Diabetes and Pregnancy Group recommendations [19]. Subjects with a 1 h 50 g glucose challenge test ≥140 mg/dL underwent a 3 h 100 g oral glucose tolerance test. Subjects with two or more values above the threshold proposed by the National Diabetes Data Group [20] were considered to have GDM, whereas those with all values below the threshold were classified as controls.
Care for GDM was managed according to the Spanish guidelines for diagnosis and therapy of GDM [19]. Women with GDM were given an individualized diet with at least 40% carbohydrates, and they were instructed to self-monitor blood glucose 6 times a day (fasting and 1 h postprandial). Insulin therapy was recommended when fasting glucose and/or 1 h post-prandial values were repeatedly over 95 mg/dL (5.3 mmol/L) or over 140 mg/dL (7.8 mmol/L), respectively. According to these criteria, 31 women were treated only with diet, and 31 women also required insulin.
Maternal and umbilical cord blood samples were stored in a biobank collection along with the associated clinical data.
2.2. Clinical and Demographic Data
Demographic and obstetric information on participants was collected via an interviewer-administered questionnaire, which paid particular attention to GDM risk factors. Maternal anthropometry included height, pre-pregnancy weight and weight at the end of pregnancy. Pre-pregnancy weight was self-reported and compared with the weight recorded in the first prenatal visit (before the 10th week of pregnancy) to ensure concordance. Pre-pregnancy body mass index (BMI) and final BMI were calculated as pre-pregnancy weight (kg)/height (m)2 and final weight/height (m)2, respectively. Similarly, gestational weight gain (GWG) was calculated as final weight—pre-pregnancy weight.
Infant data included sex, gestational age, method of delivery and anthropometry. Neonatal length and weight were measured after delivery using a measuring board to the nearest 0.1 cm and a calibrated scale to the nearest 10 g. Ponderal index (PI) was calculated as birth weight (g)/length (cm)3. Suprailiac skinfold thickness was measured within the first 48 h of life and was used to calculate the fat mass percentage. [21] Neonates were classified according to gestational age- and sex-specific growth charts of the World Health Organization (WHO) [22]. Infants with birth weights adjusted for sex and gestational age below the 10th percentile were considered SGA, while those with birth weights above the 90th percentile were included in the LGA group. Infants with birth weights between the 10th and 90th percentile adjusted for gestational age were included in the AGA group. The distribution of neonates according to birth-weight category was: 25 AGA, 25 SGA and 24 LGA in the control group and 25 AGA, 14 SGA and 23 LGA in the GDM group.
2.3. Infant Growth and Child BMI
Height and weight information from birth up to 2 years of age was collected for 103 children. We defined obesity as a BMI ≥ 85th percentile according to age- and sex-specific BMI tables of the WHO growth standards [22].
2.4. Umbilical Cord Blood Collection
Umbilical cord blood was obtained immediately after delivery. Serum was immediately separated by centrifugation, divided into aliquots and stored at −80 °C until further analysis.
2.5. Laboratory Analysis
Maternal fasting serum samples were obtained between gestational week 33 and 36 to determine glucose, triglycerides, total and HDL-cholesterol in an ADVIA 2400 (Siemens AG, Munich, Germany) autoanalyzer by standard enzymatic methods [23]. LDL cholesterol was calculated using the Friedewald formula. Plasma insulin was determined by immunoassay in an ADVIA Centaur System (Siemens AG, Munich, Germany). This assay shows a cross-reactivity of 0.1% to intact human proinsulin and the primary circulating split form des-31,32-proinsulin. Insulin resistance was estimated using homeostatic model assessment of insulin resistance (HOMA)-IR, as previously described [24].
2.6. 1H-NMR Spectroscopy-Based Cord Blood Lipoprotein Profiling
Cord blood serum samples were analyzed using the 2D diffusion-ordered 1H-NMR-based Liposcale test (Biosfer Teslab, Reus, Spain) [18]. This technique has shown to be reliable with samples stored at −80 °C for more than a decade [25]. The test provides information about size, lipid concentration (cholesterol and triglycerides), number of particles and concentration of particles within their subclasses (large, medium and small) for the main classes of lipoproteins, i.e., VLDL, LDL, intermediate-density lipoprotein (IDL) and HDL. 1H-NMR spectra were recorded on a Bruker Avance III 600 spectrometer (Bruker BioSpin, Rheinstetten, Germany).
2.7. Statistical Analysis
SPSS software v20.0 (IBM, Armonk, NY, USA) was used for statistical analysis. Data were presented as percentages for categorical variables, mean (±SD) for normally distributed continuous variables, and median (interquartile range) for non-normally distributed variables. Normal distribution of the data was tested with the Kolmogorov–Smirnov test. Non-normally distributed quantitative variables were used after log10 transformation, when required. For comparisons of proportions, differences between groups were analyzed using the chi-square test, while comparisons between normally and non-normally distributed quantitative variables were performed using unpaired t-test or Mann–Whitney U test. One-way analysis of variance (ANOVA) was used to test differences among three or more groups. Potential interactions between GDM and birth-weight categories were assessed by two-way ANOVA followed by Bonferroni post hoc test to adjust for multiple comparisons. Spearman’s rank correlation coefficients were used for the analysis of the relationships between 1H-NMR-assessed lipoprotein profile and maternal and offspring metabolic and clinical variables. To control the false discovery rate (FDR), the Benjamini–Hochberg (B–H) procedure was used, and only those values significant with the B–H correction were considered [26]. Logistic regression was used to investigate the independence of the association between 1H-NMR-assessed large LDL and small LDL particles, offspring obesity (percentile ≥ 85th = 1), and the normal weight (percentile < 85th = 0), after adjustment for potential confounders (GDM, gestational age at delivery, birth weight, sex, pre-gestational BMI and gestational weight gain). p < 0.05 was considered statistically significant.
3. Results
3.1. Clinical Characteristics and Cord Blood 1H-NMR-Based Lipoprotein Profile of the Studied Population
Clinical and metabolic characteristics of the two groups, i.e., control and GDM, are shown in Table 1. Clinical and laboratory parameters and the 1H-NMR lipoprotein profile were similar between GDM and control groups with the exception of GWG, which was significantly lower in the GDM group (Table 1). In the control group, 42 pregnant women were normal weight, 18 overweight and 14 obese. In the GDM group, 27 pregnant women were normal weight, 20 overweight and 15 obese. Likewise, GWG (7.2 ± 4.4 vs. 10.1 ± 5.1 kg; p = 0.023) and fasting glucose (78 ± 8 vs. 89 ± 12 mg/dL; p < 0.001) were lower in the GDM group of women treated only with diet compared to those who needed insulin therapy (data not shown). In the 1HNMR-assessed lipoprotein profile, no difference was observed between the two groups, except for a lower concentration of medium HDL-P in GDM treated with insulin compared with those treated with diet (10.3 ± 1.2 vs. 9.6 ± 1.4; p = 0.026) (data not shown).

Table 1.
Maternal and neonatal characteristics and cord blood 1H-NMR-assessed lipoprotein profile according to gestational diabetes.
One hundred and thirty-six neonates born to GDM (n = 62) and control (n = 74) mothers were categorized into three groups based on birth-weight categories according to age- and sex-weight specific charts (Table 2). As expected, there were significant differences in birth weight, percentage of fat mass and PI across groups, increasing from the SGA to the LGA group. There were also differences between birth-weight groups for GWG, final BMI and cord blood insulin. To note, in the GDM group the type of intervention (only diet or diet plus insulin) was distributed similarly in the three birth-weight groups (p = 0.321) (data not shown).

Table 2.
Maternal and neonatal characteristics and cord blood 1H-NMR-assessed lipoprotein profile according to birth-weight groups.
In the 1HNMR-assessed cord blood lipoprotein profile, the cholesterol content in VLDL, LDL and HDL lipoproteins was different between the three birth-weight groups, with the LGA and AGA groups showing the highest cholesterol content in VLDL and HDL lipoproteins, respectively, and the SGA group showing the lowest cholesterol content in LDL and HDL lipoproteins. Regarding triglyceride content, SGA showed a higher VLDL triglyceride content than AGA and LGA, whereas AGA showed higher HDL triglyceride content than SGA and LGA. No differences were observed in LDL lipoproteins (Table 2).
Lipoprotein particle size and number were also different across the three groups. The number of VLDL-particles (VLDL-P) was highest in the SGA group and lowest in the LGA group, which was consistent with the differences observed among particle sizes (large, medium and small VLDL-P). The number of LDL-P was lower in the SGA group than in the AGA and LGA groups, and the same distribution was observed for large and small LDL-P. The number of HDL-P was highest in the AGA group and showed an inverse U distribution when compared with the LGA and SGA groups. This phenomenon was observed specifically for small particles (Table 2).
3.2. GDM Alters the Cord Blood Lipoprotein Profile across Birth-Weight Categories
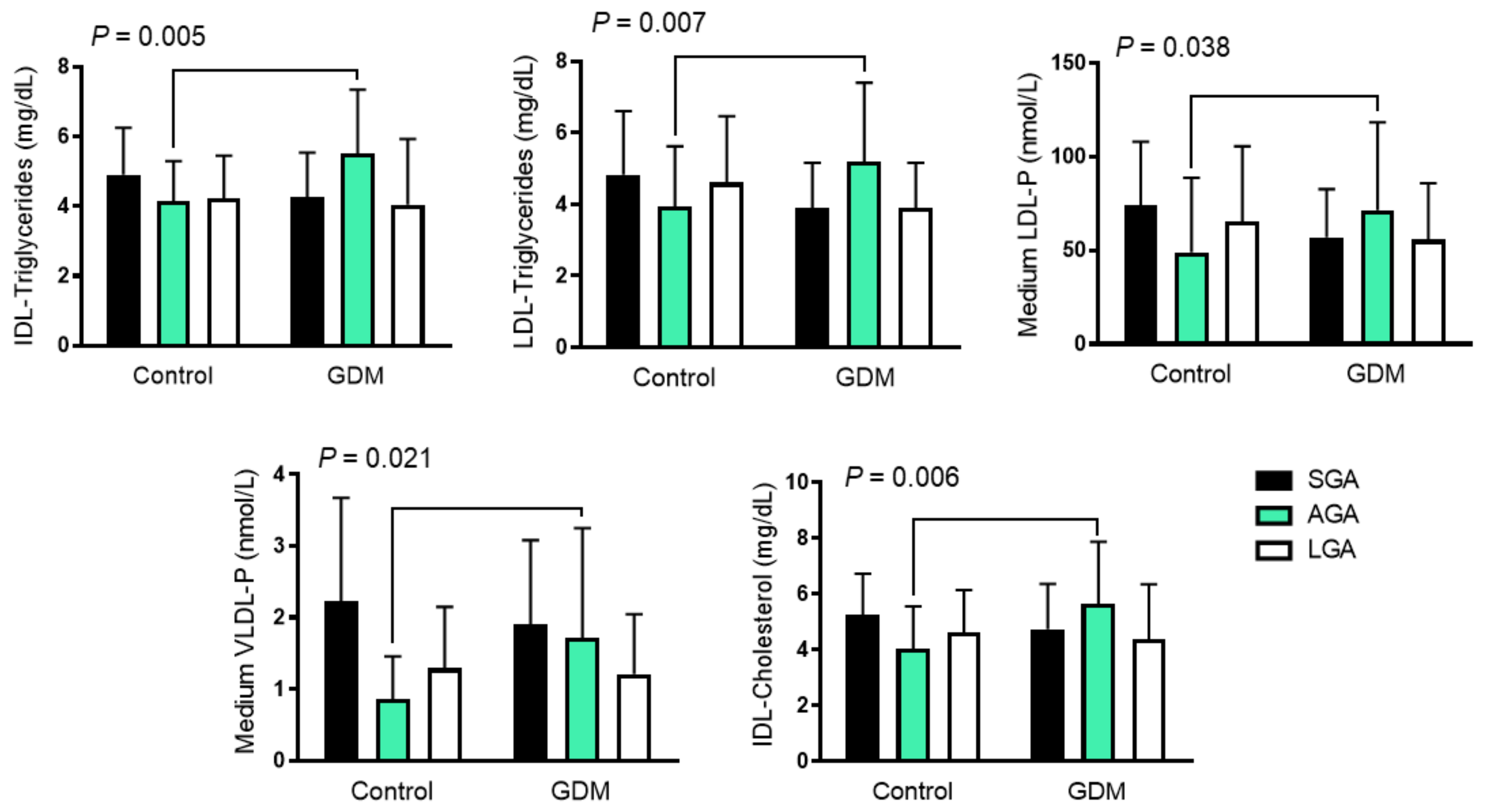
While the cord blood lipoprotein profile was similar in offspring born to GDM and control mothers, we found some interactions when we assessed the effect of both birth-weight categories and GDM. AGA neonates born to GDM mothers had higher IDL-cholesterol and -triglyceride content, and higher LDL-triglyceride content than the SGA and LGA groups, in contrast to those born to control mothers who had lower concentrations. The same pattern was also observed with medium VLDL-P and LDL-P, which followed an inversed U distribution (Figure 1 and Table 3).
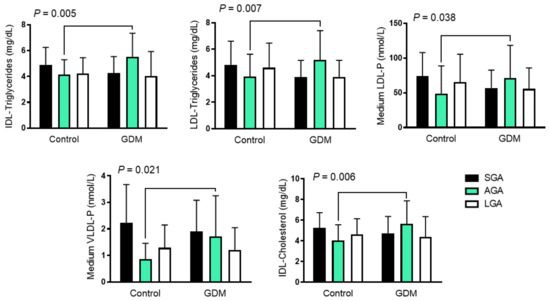
Figure 1.
Differences in cord blood 1H-NMR-assessed lipoprotein pattern among growth groups in GDM and control mothers. Data are shown as mean ± SD and were analyzed using two-way ANOVA. GDM: gestational diabetes mellitus; LDL low-density lipoprotein; VLDL: very-low-density lipoprotein; IDL: intermediate-density lipoproteins; IDL-C: cholesterol content in IDL; IDL-TG: Triglyceride content in IDL; LDL-TG: triglyceride content in LDL; LDL-P: LDL number of particles; VLDL-P: VLDL number of particles; AGA: appropriate for gestational age; LGA: large for gestational age, SGA; small for gestational age.

Table 3.
Cord blood lipid profile determined by 1H-NMR-based methods across the birth-weight categories in GDM and control women.
3.3. Relationship of 1H-NMR-Assessed Lipoprotein Profile with Clinical and Laboratory Parameters
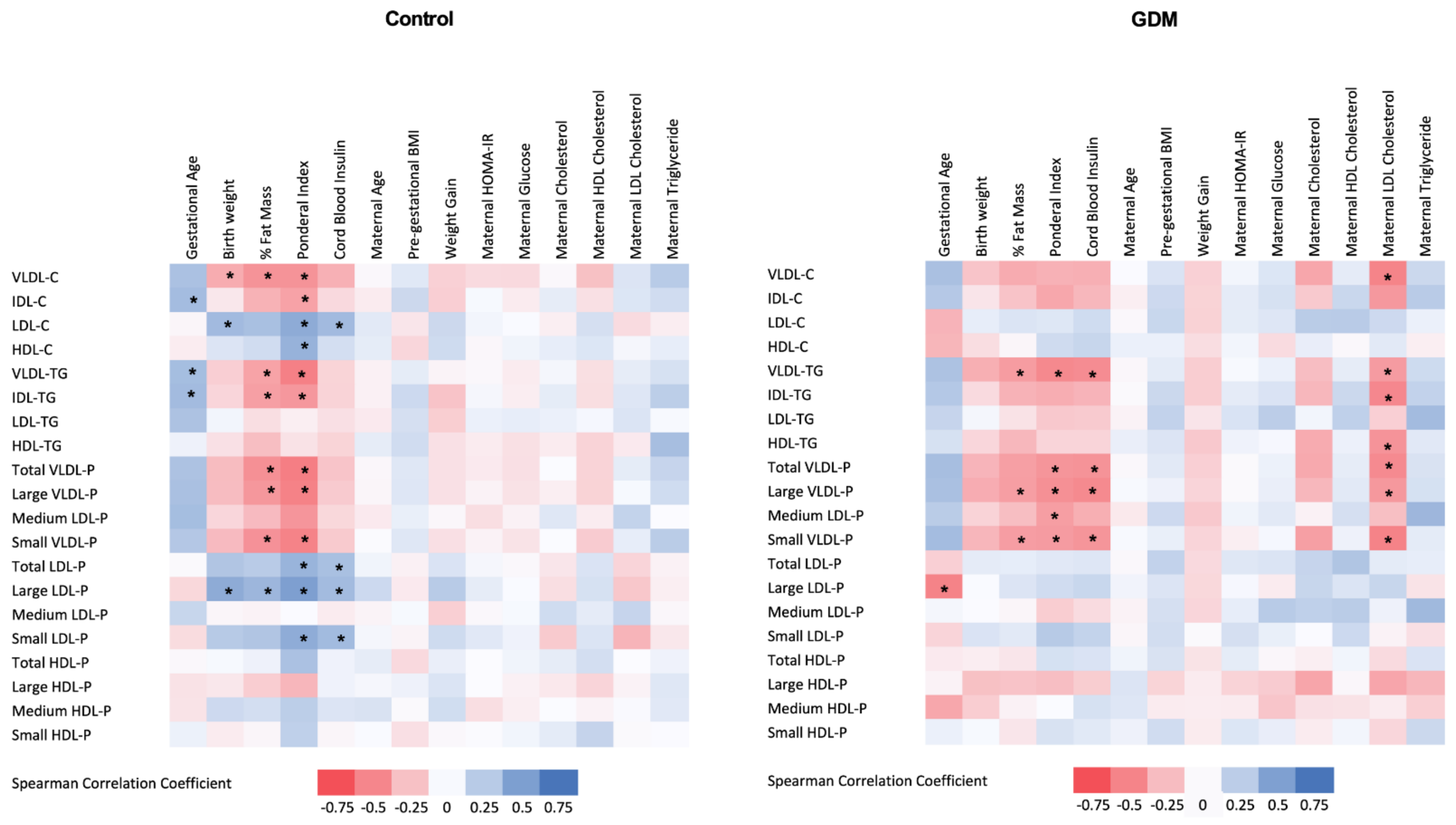
Subsequently, we examined the relationship between the 1H-NMR-based lipoprotein profile and the maternal clinical and laboratory parameters as well as the neonatal outcomes. This analysis was conducted separately in both GDM and control groups, and results are shown in Figure 2. In the GDM group, maternal LDL-cholesterol determined by standard methods was negatively associated with cord blood VLDL-P (total number, large and small particles), cholesterol and triglyceride content in VLDL, and IDL- and HDL-triglyceride content.
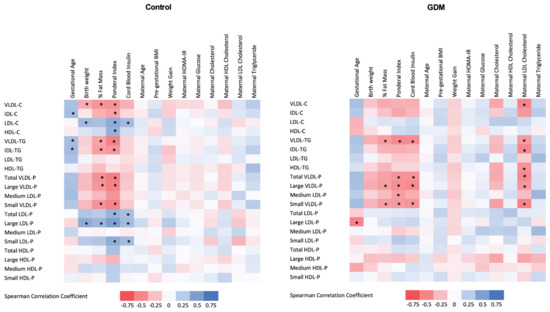
Figure 2.
Heat map of the associations between cord blood 1H-NMR-assessed lipoprotein profile and maternal and neonatal clinical variables in control (left panel) and GDM (right panel) mothers. GDM: gestational diabetes mellitus; % Fat Mass: percentage of fat mass; Cb: cord blood; HDL: high-density lipoprotein; LDL low-density lipoprotein; VLDL: very-low-density lipoprotein; IDL: intermediate-density lipoprotein; VLDL-C: cholesterol content in VLDL; VLDL-TG: triglyceride content in VLDL; VLDL-P: VLDL number of particles; IDL-C: cholesterol content in IDL; IDL-TG: triglyceride content in IDL; LDL-C: cholesterol content in LDL; LDL-TG: triglyceride content in LDL; LDL-P: LDL number of particles; HDL-C: cholesterol content in HDL; HDL-TG: triglyceride content in HDL; HDL-P: HDL number of particles; Pre-pregnancy BMI: pre-gestational body mass index; HOMA-IR: homeostatic model assessment for insulin resistance. Spearman correlation coefficients. * Indicates significant associations after applying B–H procedure for FDR correction.
LDL-cholesterol concentrations were negatively associated with birth weight in the GDM group, while no association was observed in the control group. Cord blood insulin was strongly and positively associated with small and large LDL-P and LDL-cholesterol content in the control group, while a negative relationship was observed with VLDL-P and VLDL-triglyceride content in the GDM offspring.
In both groups, neonatal adiposity was negatively correlated with cord blood VLDL-P, VLDL- and IDL-triglyceride content, and positively associated with cord blood LDL-cholesterol content and LDL-P, and these associations were stronger in the control group.
3.4. Cord Blood 1H-NMR Lipoprotein Profile Is Associated with Obesity at Two Years
To assess whether the 1H-NMR-determined cord blood lipoprotein profile could be used as a biomarker for offspring outcomes, we explored its potential association with obesity at 2 years of life in a subset of participants. From the 103 children available for the follow-up study, 78 had a normal weight and 25 were obese. Obese children were born to women with higher pregestational BMI (25.6 ± 5.0 vs. 28.2 ± 6.7 kg/m2; p = 0.036), had higher birth weight (3281 ± 609 vs. 3632 ± 480 g; p = 0.010) and were more exposed to GDM during the intrauterine life compared with normal-weight children (17 of the 25 obese children and 34 of the 78 normal-weight children were born to a mother with GDM; p = 0.034). Additionally, they showed a higher number of cord blood small (352 ± 29 vs. 326 ± 51 nmol/L; p = 0.019) and large LDL-P (98 ± 11 vs. 91 ± 13 nmol/L; p = 0.022). No other differences were observed between the two groups. To further assess the independence of these associations, we performed logistic regression analysis. We found that small LDL-P were associated with infant obesity at 2 years after adjusting for potential confounders (p = 0.023), whereas large LDL-P showed a trend (p = 0.058) (Table 4). No differences in sex, GDM or birth-weight category distribution, birth weight, maternal pre-pregnancy BMI, GWG or cord blood lipid profile were observed between the children that could not be followed up and those that remained in the study.

Table 4.
Adjusted odds ratio for the association between large and small LDL particles with the development of obesity at 2 years of age.
4. Discussion
Both GDM and abnormal growth patterns have been associated with long-term adverse outcomes in offspring. Similarly, changes in the lipoprotein composition have been proposed as potential markers of cardiovascular diseases later in life. Taking advantage of a thorough lipoprotein profiling based on 1H-NMR, we showed for the first time that GDM modifies the umbilical cord blood lipoprotein profile in AGA neonates. In particular, GDM alters IDL lipoproteins, triglyceride content in LDL, and medium-size VLDL-P and LDL-P in those children. By contrast, GDM offspring belonging to the LGA and SGA groups have lipoprotein profiles more similar to those of controls. Additionally, we found that cord blood small LDL-P, known to be associated with atherosclerosis development, had predictive value for later obesity in the offspring.
Both under and overnutrition in utero affects the lipoprotein profile of neonates [4,5,6,7]. SGA neonates are reported to exhibit higher cord blood triglyceride concentrations [6,9,27], higher VLDL and IDL concentrations, and lower HDL concentrations when compared with equivalent AGA neonates [10]. Some of these findings have also been reported in fetal macrosomia [6,8] and GDM pregnancies [28]. However, standard lipid profiling failed to identify differences between offspring born to healthy pregnant and GDM mothers [29]. Nonetheless, as shown in other metabolic disorders such as diabetic dyslipidemia [17], a more in-depth characterization of the lipoprotein profile could provide more accurate data on the regulation of lipoprotein metabolism in fetal life and its potential implications for metabolism later in life. Thus, using 1H-NMR-based cord blood lipoprotein profiling, we detected differences according to fetal growth categories in GDM women, revealing a disturbed cholesterol and triglyceride metabolism predominantly in AGA neonates. This pattern may denote an excessive transfer of triglycerides to LDL, and the further increased cholesterol-poor LDL particles in the liver [30]. Furthermore, nutritional factors and dysfunctional HDL lipoproteins [31], as described in cord blood of infants of GDM mothers [15], may induce an abnormal hepatic lipase activation, also increasing IDL half-life. This scenario is similar to the dyslipidemia associated with diabetes and insulin-resistant states, where an increased generation of IDL, small and dense LDL particles, and triglyceride-enriched HDL particles is observed [30], and which has been related to an increased atherogenic risk. These findings appear to suggest that postnatal insulin resistance, which has been described in offspring of GDM women, may be programmed in utero and would be present even in AGA neonates, further suggesting that good glycemic control during pregnancy is not enough to prevent long-term complications, as has been previously reported [32,33].
Since treatment with insulin at the end of pregnancy may activate placental nutrient transport to the fetus and promote placental fatty acid transfer [34], we assessed potential differences in the advanced lipoprotein profile and the distribution of infants between the two groups in case it could be a confounding factor. No differences in the 1HNMR-assessed lipoprotein profiles between GDM women treated with diet or insulin were noticed, except for a lower concentration of medium HDL-P in the insulin-treated group, as we have stated in the results section. Additionally, a similar distribution according to birth-weight categories was found between the diet- and insulin-treated groups. For this reason, they were analyzed together.
Despite the differences observed in some lipoproteins and lipid particles in AGA infants of GDM women and controls, these differences were not seen in the rest of the lipoprotein particles or in the groups characterized by more severe alterations in growth, SGA and LGA, suggesting that in these cases, the pattern of growth and fat accumulation could dilute the effect produced by GDM. However, this has to be interpreted cautiously since evidence of cord blood lipoproteins as biomarkers for cardiovascular disease later in life is still scarce. In this regard, results in adult life are often extrapolated to fetal life, despite the fact that they might have a different interpretation. In fact, differences in lipoprotein composition between adults and fetuses have been described, including excess apoE on fetal HDL particles, which are large in size, lack paraoxonase I, and might have lower anti-oxidant capacity [35,36], as well as small LDL poorer in lipid content [37]. These findings highlight the need for a better understanding of how lipid metabolism in utero relates to lipid metabolism in adults and, in turn, how these metabolic changes in the fetus impact adult cardiovascular health.
Previous studies exploring the potential relationship between prenatal lipid metabolism and adverse metabolic outcomes in offspring have generated inconsistent results [38,39,40,41,42,43]. Following other reports [44,45], we confirmed that GDM, pre-pregnancy BMI, and GWG during pregnancy are all associated with offspring obesity in early life. Furthermore, we found that small LDL-P in cord blood were associated with early obesity, even after controlling for confounding factors. These findings support the notion that disturbances in lipoprotein metabolism at birth may have lasting effects independently of birth weight or maternal metabolic status.
There is evidence that an altered fetal lipoprotein profile is associated with aorta intima thickness in SGA and LGA neonates [8,27], indicating a potentially increased atherosclerotic risk already at birth. We are aware that our results cannot establish a direct link between the 1H-NMR-assessed lipoprotein profile, observed in GDM-AGA newborns, and a potentially increased atherogenic risk. Nevertheless, the present study offers new clues to understand the high metabolic and cardiovascular risk in the offspring of pregnant GDM women [46]. Long-term studies are guaranteed to confirm whether cord blood 1H-NMR-based lipoprotein profiling can be implemented as a useful biomarker of later metabolic diseases beyond 2 years of age.
One of the main limitations in observational studies is the inability to attribute causation between the observed associations. However, we considered several critical confounding variables to mitigate bias in the analysis. Thus, the main prenatal factors were addressed, and the groups were comparable for maternal BMI and birth-weight categories. Of note, to reach a sufficient sample size in the three birth-weight categories, the SGA and LGA groups were overrepresented, and further population-based studies are needed to determine the role of lipoprotein composition and subfractions in the pathogenesis of metabolic diseases in offspring. Furthermore, given the relevance of the placenta in the passage of nutrients and in the regulation of fetal metabolism and growth, it would be important to establish whether there is a parallelism between the lipoprotein pattern and placental metabolic pathways.
The strengths of this study include a longitudinal birth cohort with almost complete maternal data that establish a temporal relationship between the outcome and the exposure to GDM. The novelty of the lipoprotein assessment, which allowed us to identify different fetal metabolic behaviors, is also a big asset in the experimental methods.
5. Conclusions
GDM disturbs triglyceride and cholesterol lipoprotein concentrations across birth categories, with GDM-AGA neonates showing a profile more similar to that of adults with dyslipidemia and atherosclerosis than those born to normal glucose-tolerant mothers. Moreover, an altered fetal lipoprotein pattern is associated with obesity development at 2 years. Overall, these findings suggest that the fetal lipoprotein profile might be an early biomarker for the development of later diseases.
Author Contributions
F.A.-C.: conceptualization, methodology, formal analysis, investigation, writing—original draft, visualization. E.M.-M.: methodology, investigation. M.B.: methodology, investigation, resources, supervision. A.G.: methodology, investigation, resources. A.M.-M.: writing—original draft, visualization. O.F.: methodology, investigation, resources. N.A.: methodology, investigation, and resources. S.F.-V.: conceptualization, resources, writing—review and editing, supervision, project administration, funding acquisition. J.V.: conceptualization, methodology, validation, formal analysis, investigation, resources, data curation, writing—original draft, writing—review and editing, visualization, supervision, project administration, funding acquisition. A.M.: conceptualization, methodology, validation, resources, writing—review and editing, supervision, project administration, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by grants from the Spanish Ministry of Economy and Competitiveness (PI 15/01562 and PI 18/00516 to A.M., RTI2018-093919-B-I00 to S.F.-V., PI14/00228 and PI17/01503 to J.V.) co-financed by the European Regional Development Fund (ERDF) “A way to make Europe/Investing in your future”. The Spanish Biomedical Research Center in Diabetes and Associated Metabolic Disorders (CIBERDEM) (CB07708/0012) is an initiative of the Instituto de Salud Carlos III. F.A.-C. is funded by a predoctoral fellowship from AGAUR, Spain (2017FI_B_00632). S.F.-V. acknowledges the Miguel Servet tenure-track program (CP10/00438 and CPII16/00008) from the Fondo de Investigación Sanitaria, co-financed by the ERDF.
Institutional Review Board Statement
This study was performed in accordance with the tenets of the Declaration of Helsinki, and its protocol was reviewed and approved by the Institutional Ethics Committee of the Hospital Universitari de Tarragona (ref: 243/2016).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are available from the authors upon reasonable request.
Acknowledgments
We want to particularly acknowledge all of the patients and volunteers involved in this study for their collaboration. We also acknowledge the BioBank IISPV (PT17/0015/0029) integrated into the Spanish National Biobanks Network. Finally, we wish to highlight the essential contribution of the midwives and the Gynecology and Obstetrics Department of the Hospital Universitari Joan XXIII de Tarragona.
Conflicts of Interest
N.A. is a stockowner in Biosfer Teslab and has a patent for the lipoprotein profiling described in the present manuscript. The other authors declare that they have no competing interest.
References
- Barker, D.J. The Fetal and Infant Origins of Disease. Eur. J. Clin. Investig. 1995, 25, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Srinivasan, S.R.; Wattigney, W.A.; Bao, W.; Berenson, G.S. Usefulness of Childhood Low-Density Lipoprotein Cholesterol Level in Predicting Adult Dyslipidemia and Other Cardiovascular Risks. Arch. Intern. Med. 1996, 156, 1315–1320. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.S. Fetal and Childhood Onset of Adult Cardiovascular Diseases. Pediatr. Clin. N. Am. 2004, 51, 1697–1719. [Google Scholar] [CrossRef] [PubMed]
- Loughrey, C.M.; Rimm, E.; Heiss, G.; Rifai, N. Race and Gender Differences in Cord Blood Lipoproteins. Atherosclerosis 2000, 148, 57–65. [Google Scholar] [CrossRef]
- Lane, D.M.; McConathy, W.J. Factors Affecting the Lipid and Apolipoprotein Levels of Cord Sera. Pediatr. Res. 1983, 17, 83–91. [Google Scholar] [CrossRef]
- Aletayeb, S.M.H.; Dehdashtian, M.; Aminzadeh, M.; Moghaddam, A.R.E.; Mortazavi, M.; Malamiri, R.A.; Habibzadeh, M.; Javaherizadeh, H. Correlation between Umbilical Cord Blood Lipid Profile and Neonatal Birth Weight. Pediatr. Pol. 2013, 88, 521–525. [Google Scholar] [CrossRef][Green Version]
- Kumar, A.; Gupta, A.; Malhotra, V.K.; Agarwal, P.S.; Thirupuram, S.; Gaind, B. Cord Blood Lipid Levels in Low Birth Weight Newborns. Indian Pediatr. 1989, 26, 571–574. [Google Scholar]
- Koklu, E.; Akcakus, M.; Kurtoglu, S.; Koklu, S.; Yikilmaz, A.; Coskun, A.; Gunes, T. Aortic Intima-Media Thickness and Lipid Profile in Macrosomic Newborns. Eur. J. Pediatr. 2007, 166, 333–338. [Google Scholar] [CrossRef]
- Miranda, J.; Simões, R.V.; Paules, C.; Cañueto, D.; Pardo-Cea, M.A.; García-Martín, M.L.; Crovetto, F.; Fuertes-Martin, R.; Domenech, M.; Gómez-Roig, M.D.; et al. Metabolic Profiling and Targeted Lipidomics Reveals a Disturbed Lipid Profile in Mothers and Fetuses with Intrauterine Growth Restriction. Sci. Rep. 2018, 8, 13614. [Google Scholar] [CrossRef]
- Kim, S.M.; Lee, S.M.; Kim, S.J.; Kim, B.J.; Shin, S.; Kim, J.R.; Cho, K.H. Cord and Maternal Sera from Small Neonates Share Dysfunctional Lipoproteins with Proatherogenic Properties: Evidence for Barker’s Hypothesis. J. Clin. Lipidol. 2017, 11, 1318–1328.e3. [Google Scholar] [CrossRef]
- Herrera, E.; Desoye, G. Maternal and Fetal Lipid Metabolism under Normal and Gestational Diabetic Conditions. Horm. Mol. Biol. Clin. Investig. 2016, 26, 109–127. [Google Scholar] [CrossRef]
- Fordyce, M.K.; Duncan, R.; Chao, R.; Christakis, M.; Hsia, S.L.; Robertson, E.; Kafatos, A.; Christakis, G. Cord Blood Serum in Newborns of Diabetic Mothers. J. Chronic Dis. 1983, 36, 263–268. [Google Scholar] [CrossRef]
- Kilby, M.D.; Neary, R.H.; Mackness, M.I.; Durrington, P.N. Fetal and Maternal Lipoprotein Metabolism in Human Pregnancy Complicated by Type I Diabetes Mellitus 1. J. Clin. Endocrinol. Metab. 1998, 83, 1736–1741. [Google Scholar] [CrossRef]
- Chan, T.C.; Schwartz, J.J.; Garcia, R.E.; Chin, H.P.; Barndt, R. Total Serum Cholesterol and Plasma Lipoprotein Cholesterol Concentrations in Cord Sera of Newborns from Hispanic Mothers with Gestational Diabetes. Artery 1988, 15, 203–216. [Google Scholar]
- Sreckovic, I.; Birner-Gruenberger, R.; Besenboeck, C.; Miljkovic, M.; Stojakovic, T.; Scharnagl, H.; Marsche, G.; Lang, U.; Kotur-Stevuljevic, J.; Jelic-Ivanovic, Z.; et al. Gestational Diabetes Mellitus Modulates Neonatal High-Density Lipoprotein Composition and Its Functional Heterogeneity. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2014, 1841, 1619–1627. [Google Scholar] [CrossRef]
- Miettinen, H.E.; Rönö, K.; Koivusalo, S.B.; Eriksson, J.G.; Gylling, H. Effect of Gestational Diabetes Mellitus on Newborn Cholesterol Metabolism. Atherosclerosis 2018, 275, 346–351. [Google Scholar] [CrossRef]
- Mallol, R.; Rodriguez, M.A.; Brezmes, J.; Masana, L.; Correig, X. Human Serum/Plasma Lipoprotein Analysis by NMR: Application to the Study of Diabetic Dyslipidemia. Prog. Nucl. Magn. Reson. Spectrosc. 2013, 70, 1–24. [Google Scholar] [CrossRef]
- Mallol, R.; Amigó, N.; Rodríguez, M.A.; Heras, M.; Vinaixa, M.; Plana, N.; Rock, E.; Ribalta, J.; Yanes, O.; Masana, L.; et al. Liposcale: A Novel Advanced Lipoprotein Test Based on 2D Diffusion-Ordered 1H NMR Spectroscopy. J. Lipid Res. 2015, 56, 737–746. [Google Scholar] [CrossRef]
- Grupo Español de Diabetes y Embarazo (GEDE); Grupo Español de Diabetes y Embarazo Asistencia a la Gestante con Diabetes. Guía de práctica clínica actualizada en 2014 Avances en Diabetología. Av. Diabetol. 2015, 31, 45–59. [Google Scholar] [CrossRef]
- Alberti, K.G.; Zimmet, P.Z. Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications. Part 1: Diagnosis and Classification of Diabetes Mellitus Provisional Report of a WHO Consultation. Diabet. Med. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- Catalano, P.M.; Thomas, A.J.; Avallone, D.A. Amini SB Anthropometric Estimation of Neonatal Body Composition. Am. J. Obs. Gynecol. 1995, 173, 1176–1181. [Google Scholar] [CrossRef]
- WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weightfor- Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Martinez-Perez, B.; Ejarque, M.; Gutierrez, C.; Nuñez-Roa, C.; Roche, K.; Vila-Bedmar, R.; Ballesteros, M.; Redondo-Angulo, I.; Planavila, A.; Villarroya, F.; et al. Angiopoietin-like Protein 8 (ANGPTL8) in Pregnancy: A Brown Adipose Tissue–Derived Endocrine Factor with a Potential Role in Fetal Growth. Transl. Res. 2016, 178, 12. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis Model Assessment: Insulin Resistance and Beta Cell Function from Fasting Plasma Glucose and Insulin Concentrations in Man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Pichler, G.; Amigo, N.; Tellez-Plaza, M.; Pardo-Cea, M.; Dominguez-Lucas, A.; VG, M.; Monleon, D.; Martin-Escudero, J.; Ascaso, J.; Chaves, F.; et al. LDL Particle Size and Composition and Incident Cardiovascular Disease in a South-European Population: The Hortega-Liposcale Follow-up Study. Int. J. Cardiol. 2018, 264, 172–178. [Google Scholar] [CrossRef]
- Haynes, W. Benjamini–Hochberg Method. In Encyclopedia of Systems Biology; Springer: New York, NY, USA, 2013; p. 78. [Google Scholar]
- Koklu, E.; Kurtoglu, S.; Akcakus, M.; Koklu, S.; Buyukkayhan, D.; Gumus, H.; Yikilmaz, A. Increased Aortic Intima-Media Thickness Is Related to Lipid Profile in Newborns with Intrauterine Growth Restriction. Horm. Res. Paediatr. 2006, 65, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Schaefer-Graf, U.M.; Graf, K.; Kulbacka, I.; Kjos, S.L.; Dudenhausen, J.; Vetter, K.; Herrera, E. Maternal Lipids as Strong Determinants of Fetal Environment and Growth in Pregnancies with Gestational Diabetes Mellitus. Diabetes Care 2008, 31, 1858–1863. [Google Scholar] [CrossRef] [PubMed]
- Schaefer-Graf, U.M.; Meitzner, K.; Ortega-Senovilla, H.; Graf, K.; Vetter, K.; Abou-Dakn, M.; Herrera, E. Differences in the Implications of Maternal Lipids on Fetal Metabolism and Growth between Gestational Diabetes Mellitus and Control Pregnancies. Diabet. Med. 2011, 28, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Hassing, H.C.; Surendran, R.P.; Mooij, H.L.; Stroes, E.S.; Nieuwdorp, M.; Dallinga-Thie, G.M. Pathophysiology of Hypertriglyceridemia. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2012, 1821, 826–832. [Google Scholar] [CrossRef]
- Chatterjee, C.; Sparks, D.L. Hepatic Lipase, High Density Lipoproteins, and Hypertriglyceridemia. Am. J. Pathol. 2011, 178, 1429–1433. [Google Scholar] [CrossRef]
- Gillman, M.W.; Oakey, H.; Baghurst, P.A.; Volkmer, R.E.; Robinson, J.S.; Crowther, C.A.; Gillman, M.W.; Oakey, H.; Baghurst, P.A.; Volkmer, R.E.; et al. Effect of Treatment of Gestational Diabetes Mellitus on Obesity in the next Generation. Diabetes Care 2010, 33, 964–968. [Google Scholar] [CrossRef]
- Landon, M.B.; Rice, M.M.; Varner, M.W.; Casey, B.M.; Reddy, U.M.; Wapner, R.J.; Rouse, D.J.; Biggio, J.R.; Thorp, J.M.; Chien, E.K.; et al. Mild Gestational Diabetes Mellitus and Long-Term Child Health. Diabetes Care 2015, 38, 445–452. [Google Scholar] [CrossRef]
- Ruiz-Palacios, M.L.; Ruiz-Alcaraz, A.J.; Sanchez-Campillo, M.; Larqué, E. Role of Insulin in Placental Transport of Nutrients in Gestational Diabetes Mellitus. Annu. Nutr. Metab. 2017, 70, 16–25. [Google Scholar] [CrossRef]
- Fujita, H.; Okada, T.; Inami, I.; Makimoto, M.; Hosono, S.; Minato, M.; Takahashi, S.; Mugishima, H.; Yamamoto, T. Heterogeneity of High-Density Lipoprotein in Cord Blood and Its Postnatal Change. Clin. Chim. Acta 2008, 389, 93–97. [Google Scholar] [CrossRef]
- Sreckovic, I.; Birner-Gruenberger, R.; Obrist, B.; Stojakovic, T.; Scharnagl, H.; Holzer, M.; Scholler, M.; Philipose, S.; Marsche, G.; Lang, U.; et al. Distinct Composition of Human Fetal HDL Attenuates Its Anti-Oxidative Capacity. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2013, 1831, 737–746. [Google Scholar] [CrossRef]
- Gugliucci, A.; Numaguchi, M.; Caccavello, R.; Kimura, S. Small-Dense Low-Density Lipoproteins Are the Predominant ApoB-100-Containing Lipoproteins in Cord Blood. Clin. Biochem. 2014, 47, 475–477. [Google Scholar] [CrossRef]
- Shokry, E.; Marchioro, L.; Uhl, O.; Bermúdez, M.G.; García-Santos, J.A.; Segura, M.T.; Campoy, C.; Koletzko, B. Transgenerational Cycle of Obesity and Diabetes: Investigating Possible Metabolic Precursors in Cord Blood from the PREOBE Study. Acta Diabetol. 2019, 56, 1073–1082. [Google Scholar] [CrossRef]
- Simpson, J.; Smith, A.D.; Fraser, A.; Sattar, N.; Callaway, M.; Lindsay, R.S.; Lawlor, D.A.; Nelson, S.M. Cord Blood Adipokines and Lipids and Adolescent Nonalcoholic Fatty Liver Disease. J. Clin. Endocrinol. Metab. 2016, 101, 4661–4668. [Google Scholar] [CrossRef]
- Hellmuth, C.; Uhl, O.; Standl, M.; Demmelmair, H.; Heinrich, J.; Koletzko, B.; Thiering, E. Cord Blood Metabolome Is Highly Associated with Birth Weight, but Less Predictive for Later Weight Development. Obes. Facts 2017, 10, 85–100. [Google Scholar] [CrossRef]
- Stratakis, N.; Gielen, M.; Margetaki, K.; de Groot, R.H.M.; Apostolaki, M.; Chalkiadaki, G.; Vafeiadi, M.; Leventakou, V.; Karachaliou, M.; Godschalk, R.W.; et al. Polyunsaturated Fatty Acid Status at Birth, Childhood Growth, and Cardiometabolic Risk: A Pooled Analysis of the MEFAB and RHEA Cohorts. Eur. J. Clin. Nutr. 2018, 18, 175. [Google Scholar] [CrossRef]
- Standl, M.; Thiering, E.; Demmelmair, H.; Koletzko, B.; Heinrich, J. Age-Dependent Effects of Cord Blood Long-Chain PUFA Composition on BMI during the First 10 Years of Life. Br. J. Nutr. 2014, 111, 2024–2031. [Google Scholar] [CrossRef]
- Donahue, S.M.A.; Rifas-Shiman, S.L.; Gold, D.R.; Jouni, Z.E.; Gillman, M.W.; Oken, E. Prenatal Fatty Acid Status and Child Adiposity at Age 3 y: Results from a US Pregnancy Cohort. Am. J. Clin. Nutr. 2011, 93, 780–788. [Google Scholar] [CrossRef]
- Hillier, T.A.; Pedula, K.L.; Schmidt, M.M.; Mullen, J.A.; Charles, M.-A.; Pettitt, D.J. Childhood Obesity and Metabolic Imprinting. Diabetes Care 2007, 30, 2287–2292. [Google Scholar] [CrossRef]
- Voerman, E.; Santos, S.; Patro Golab, B.; Amiano, P.; Ballester, F.; Barros, H.; Bergström, A.; Charles, M.-A.; Chatzi, L.; Chevrier, C.; et al. Maternal Body Mass Index, Gestational Weight Gain, and the Risk of Overweight and Obesity across Childhood: An Individual Participant Data Meta-Analysis. PLoS Med. 2019, 16, e1002744. [Google Scholar] [CrossRef]
- Pathirana, M.M.; Lassi, Z.S.; Roberts, C.T.; Andraweera, P.H. Cardiovascular Risk Factors in Offspring Exposed to Gestational Diabetes Mellitus in Utero: Systematic Review and Meta-Analysis. J. Dev. Orig. Health Dis. 2020, 18, 850. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).