Comparison of the Efficacy and Safety of Biologics (Secukinumab, Ustekinumab, and Guselkumab) for the Treatment of Moderate-to-Severe Psoriasis: Real-World Data from a Single Korean Center
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Procedure
2.3. Outcomes
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
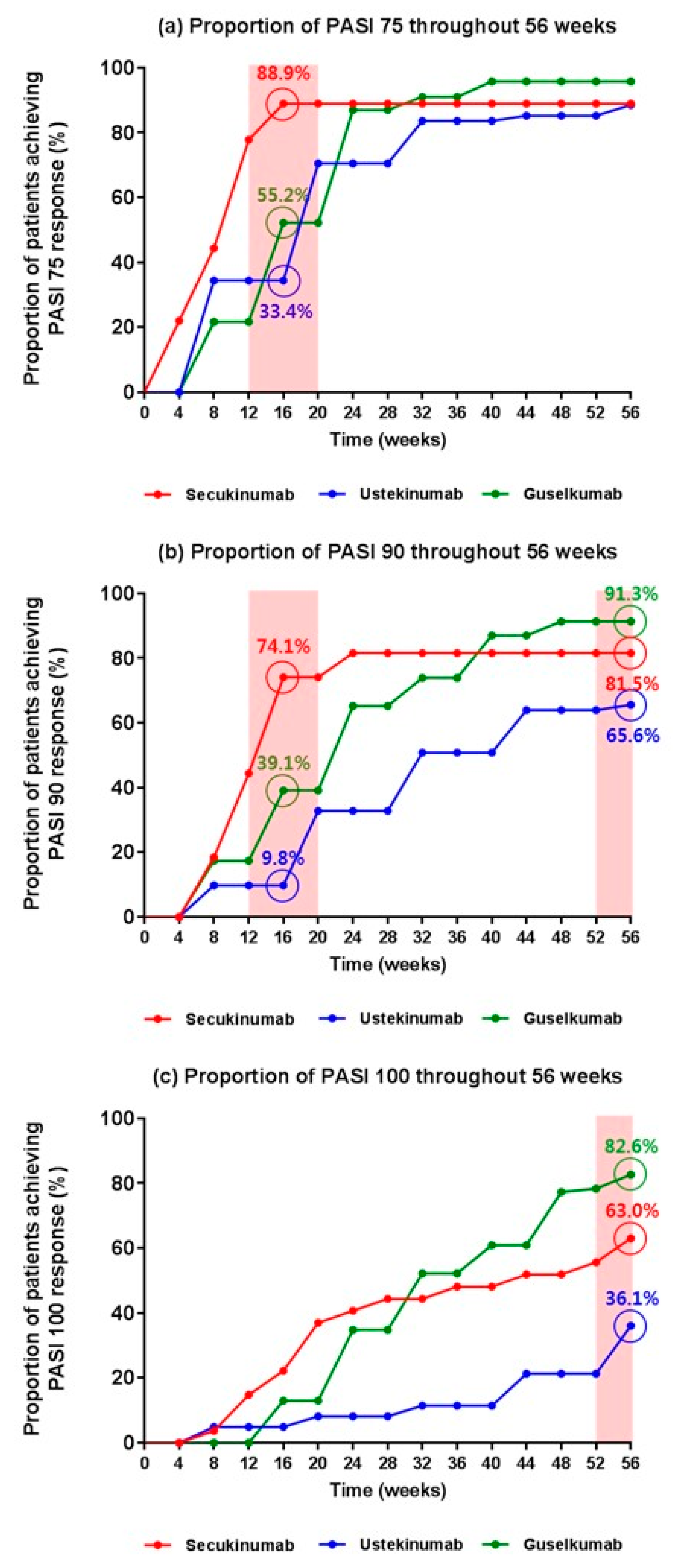
3.2. Direct Comparison of the Efficacy of the Biologics in Real-World Practice
3.3. Direct Comparisons of Biologic Safety in Real-World Practice
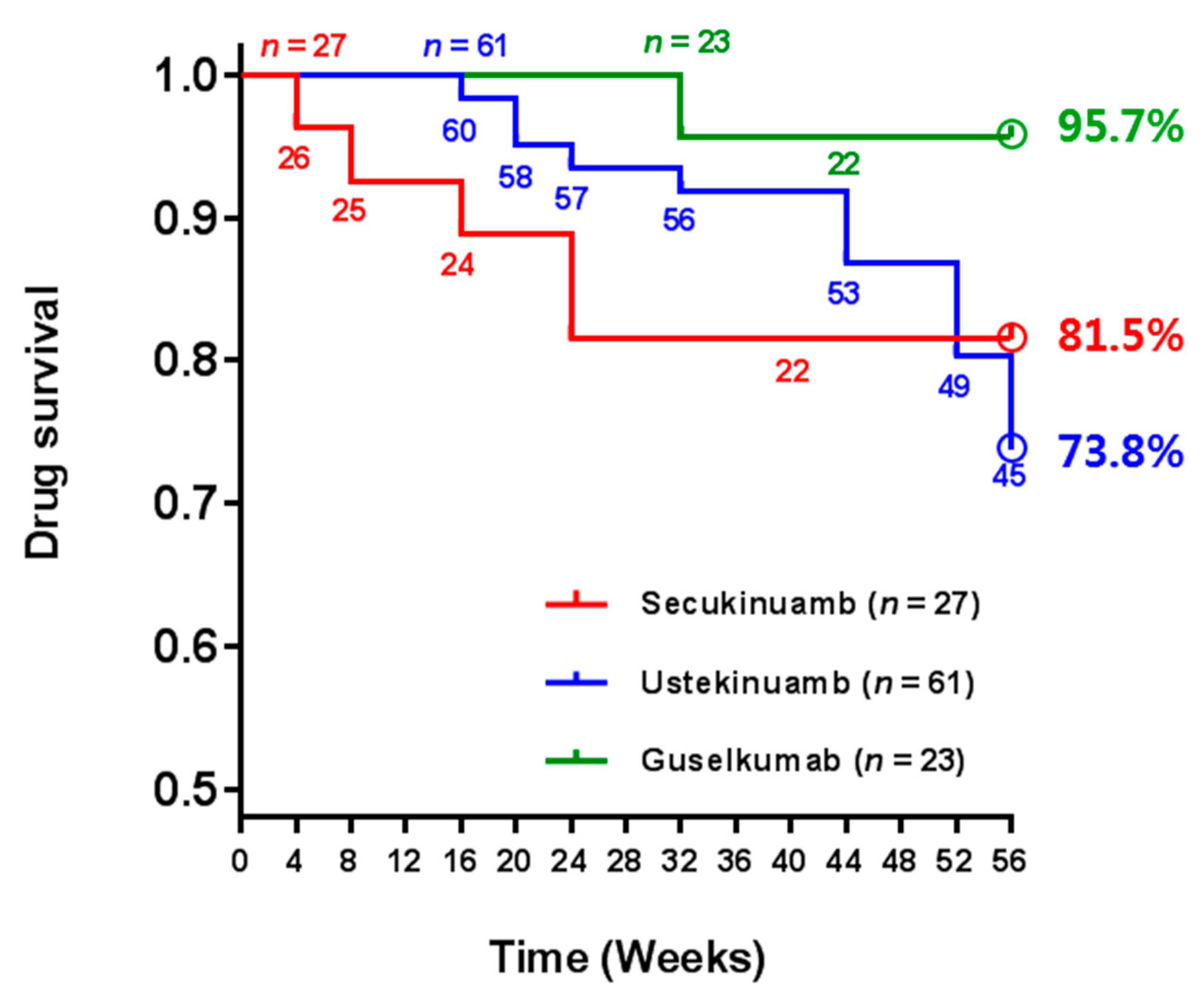
3.4. Direct Comparisons of Biologic Drug Survival up to 56 Weeks in Real-World Practice
3.5. Factors Affecting Drug Survival in Real-World Practice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amoruso, G.F.; Nisticò, S.P.; Iannone, L.; Russo, E.; Rago, G.; Patruno, C.; Bennardo, L. Ixekizumab May Improve Renal Function in Psoriasis. Healthcare 2021, 9, 543. [Google Scholar] [CrossRef] [PubMed]
- Dastoli, S.; Nisticò, S.P.; Morrone, P.; Patruno, C.; Leo, A.; Citraro, R.; Gallelli, L.; Russo, E.; De Sarro, G.; Bennardo, L. Colchicine in Managing Skin Conditions: A Systematic Review. Pharmaceutics 2022, 14, 294. [Google Scholar] [CrossRef]
- Parisi, R.; Symmons, D.P.; Griffiths, C.E.M.; Ashcroft, D.M. Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team. Global epidemiology of psoriasis: A systematic review of incidence and prevalence. J. Investig. Dermatol. 2013, 133, 377–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parisi, R.; Iskandar, I.Y.K.; Kontopantelis, E.; Augustin, M.; Griffiths, C.E.M.; Ashcroft, D.M. National, regional, and worldwide epidemiology of psoriasis: Systematic analysis and modelling study. BMJ 2020, 369, m1590. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kang, S.; Park, J.S.; Jo, S.J. Prevalence of psoriasis in Korea: A population-based epidemiological study using the Korean National Health Insurance Database. Ann. Dermatol. 2017, 29, 761–767. [Google Scholar] [CrossRef] [Green Version]
- Rapp, S.R.; Feldman, S.R.; Exum, M.L.; Fleischer, A.B.; Reboussin, D.M. Psoriasis causes as much disability as other major medical diseases. J. Am. Acad. Dermatol. 1999, 41, 401–407. [Google Scholar] [CrossRef]
- Jungo, P.; Maul, J.T.; Djamei, V.; von Felten, S.; Kolios, A.G.A.; Czernielewsk, J.; Yawalkar, N.; Odermatt, O.; Laffitte, E.; Anliker, M.; et al. Superiority in quality of life improvement of biologics over conventional systemic drugs in a Swiss real-life psoriasis registry. Dermatology 2016, 232, 655–663. [Google Scholar] [CrossRef] [Green Version]
- Blauvelt, A.; Reich, K.; Tsai, T.-F.; Tyring, S.; Vanaclocha, F.; Kingo, K.; Ziv, M.; Pinter, A.; Vender, R.; Hugot, S.; et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate-to-severe plaque psoriasis up to 1 year: Results from the CLEAR study. J. Am. Acad. Dermatol. 2017, 76, 60–69.e69. [Google Scholar] [CrossRef] [PubMed]
- Paul, C.; Griffiths, C.E.M.; van de Kerkhof, P.C.M.; Puig, L.; Dutronc, Y.; Henneges, C.; Dossenbach, M.; Hollister, K.; Reich, K. Ixekizumab provides superior efficacy compared with ustekinumab over 52 weeks of treatment: Results from IXORA-S, a phase 3 study. J. Am. Acad. Dermatol. 2019, 80, 70–79.e73. [Google Scholar] [CrossRef] [Green Version]
- Gordon, K.B.; Strober, B.; Lebwohl, M.; Augustin, M.; Blauvelt, A.; Poulin, Y.; Papp, K.A.; Sofen, H.; Puig, L.; Foley, P.; et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): Results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet 2018, 392, 650–661. [Google Scholar] [CrossRef]
- Girolomoni, G.; Strohal, R.; Puig, L.; Bachelez, H.; Barker, J.; Boehncke, W.H.; Prinz, J.C. The role of IL-23 and the IL-23/TH17 immune axis in the pathogenesis and treatment of psoriasis. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1616–1626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Cesare, A.; Di Meglio, P.; Nestle, F.O. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J. Investig. Dermatol. 2009, 129, 1339–1350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lebwohl, M.; Strober, B.; Menter, A.; Gordon, K.; Weglowska, J.; Puig, L.; Papp, K.; Spelman, L.; Toth, D.; Kerdel, F.; et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N. Engl. J. Med. 2015, 373, 1318–1328. [Google Scholar] [CrossRef]
- Blauvelt, A.; Papp, K.A.; Griffiths, C.E.M.; Randazzo, B.; Wasfi, Y.; Shen, Y.-K.; Li, S.; Kimball, A.B. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: Results from the phase III, double-blinded, placebo- and active comparator–controlled VOYAGE 1 trial. J. Am. Acad. Dermatol. 2017, 76, 405–417. [Google Scholar] [CrossRef] [Green Version]
- Reich, K.; Armstrong, A.W.; Foley, P.; Song, M.; Wasfi, Y.; Randazzo, B.; Li, S.; Shen, Y.K.; Gordon, K.B. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: Results from the phase III, double-blind, placebo- and active comparator–controlled VOYAGE 2 trial. J. Am. Acad. Dermatol. 2017, 76, 418–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Megna, M.; Fabbrocini, G.; Ruggiero, A.; Cinelli, E. Efficacy and safety of risankizumab in psoriasis patients who failed anti-IL-17, anti-12/23 and/or anti IL-23: Preliminary data of a real-life 16-week retrospective study. Dermatol. Ther. 2020, 33, e14144. [Google Scholar] [CrossRef] [PubMed]
- Reich, K.; Gooderham, M.; Thaçi, D.; Crowley, J.J.; Ryan, C.; Krueger, J.G.; Tsai, T.-F.; Flack, M.; Gu, Y.; Williams, D.A.; et al. Risankizumab compared with adalimumab in patients with moderate-to-severe plaque psoriasis (IMMvent): A randomised, double-blind, active-comparator-controlled phase 3 trial. Lancet 2019, 394, 576–586. [Google Scholar] [CrossRef]
- Warren, R.B.; Blauvelt, A.; Poulin, Y.; Beeck, S.; Kelly, M.; Wu, T.; Geng, Z.; Paul, C. Efficacy and safety of risankizumab vs. secukinumab in patients with moderate-to-severe plaque psoriasis (IMMerge): Results from a phase III, randomized, open-label, efficacy–assessor-blinded clinical trial. Br. J. Dermatol. 2021, 184, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Youn, S.W.; Yu, D.Y.; Kim, T.Y.; Kim, B.S.; Lee, S.C.; Lee, J.H.; Choe, Y.B.; Lee, J.H.; Choi, J.H.; Roh, J.Y.; et al. Efficacy and safety of guselkumab compared with placebo and adalimumab in Korean patients with moderate-to-severe psoriasis: Post-hoc analysis from the phase III, double-blind, placebo- and active-comparator-controlled VOYAGE 1/2 trials. J. Dermatolog. Treat. 2022, 33, 535–541. [Google Scholar] [CrossRef]
- Reich, K.; Song, M.; Li, S.; Jiang, J.; Youn, S.W.; Tsai, T.F.; Choe, Y.B.; Huang, Y.H.; Gordon, K.B. Consistent responses with guselkumab treatment in Asian and non-Asian patients with psoriasis: An analysis from VOYAGE 1 and VOYAGE 2. J. Dermatol. 2019, 46, 1141–1152. [Google Scholar] [CrossRef]
- Kwon, S.H.; Lee, E.S. Secukinumab Response in Korean Patients with Moderate to Severe Plaque-Type Psoriasis Irrespective of Previous Biologic Use: 1-Year Experience at a Single Center. Ann. Dermatol. 2020, 32, 255–257. [Google Scholar] [CrossRef] [PubMed]
- Youn, S.W.; Yu, D.Y.; Kim, B.S.; Kim, Y.; Kim, K.J.; Choi, J.H.; Son, S.W.; Lee, E.S.; Ro, Y.S.; Park, Y.L.; et al. Stelara PMS investigators. Clinical outcomes in adult patients with plaque psoriasis treated with ustekinumab under real-world practice in Korea: A prospective, observational, multi-center, postmarketing surveillance study. J. Dermatol. 2021, 48, 778–785. [Google Scholar] [CrossRef]
- Hsiao, C.-Y.; Chiu, H.-Y.; Wang, T.-S.; Tsai, T.-F. Serial QuantiFERON-TB Gold testing in patients with psoriasis treated with ustekinumab. PLoS ONE 2017, 12, e0184178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saunte, D.M.; Mrowietz, U.; Puig, L.; Zachariae, C. Candida infections in patients with psoriasis and psoriatic arthritis treated with interleukin-17 inhibitors and their practical management. Br. J. Dermatol. 2017, 177, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Egeberg, A.; Ottosen, M.B.; Gniadecki, R.; Broesby-Olsen, S.; Dam, T.N.; Bryld, L.E.; Rasmussen, M.K.; Skov, L. Safety, efficacy and drug survival of biologics and biosimilars for moderate-to-severe plaque psoriasis. Br. J. Dermatol. 2018, 178, 509–519. [Google Scholar] [CrossRef] [Green Version]
- Yiu, Z.Z.N.; Mason, K.J.; Hampton, P.J.; Reynolds, N.J.; Smith, C.H.; Lunt, M.; Griffiths, C.E.M.; Warren, R.B.; BADBIR Study Group. Drug survival of adalimumab, ustekinumab and secukinumab in patients with psoriasis: A prospective cohort study from the British Association of Dermatologists Biologics and Immunomodulators Register (BADBIR). Br. J. Dermatol. 2020, 183, 294–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sbidian, E.; Mezzarobba, M.; Weill, A.; Coste, J.; Rudant, J. Persistence of treatment with biologics for patients with psoriasis: A real-world analysis of 16 545 biologic-naïve patients from the French National Health Insurance database (SNIIRAM). Br. J. Dermatol. 2019, 180, 86–93. [Google Scholar] [CrossRef] [Green Version]
- Graier, T.; Salmhofer, W.; Jonak, C.; Weger, W.; Kölli, C.; Gruber, B.; Sator, P.G.; Prillinger, K.; Mlynek, A.; Schütz-Bergmayr, M.; et al. Biologic drug survival rates in the era of anti-interleukin-17 antibodies: A time-period-adjusted registry analysis. Br. J. Dermatol. 2021, 184, 1094–1105. [Google Scholar] [CrossRef] [PubMed]
- Torres, T.; Puig, L.; Vender, R.; Lynde, C.; Piaserico, S.; Carrascosa, J.M.; Gisondi, P.; Daudén, E.; Conrad, C.; Mendes-Bastos, P.; et al. Drug survival of IL-12/23, IL-17 and IL-23 inhibitors for psoriasis treatment: A retrospective multi-country, multicentric cohort study. Am. J. Clin. Dermatol. 2021, 22, 567–579. [Google Scholar] [CrossRef]
- Mourad, A.; Straube, S.; Armijo-Olivo, S.; Gniadecki, R. Factors predicting persistence of biologic drugs in psoriasis: A systematic review and meta-analysis. Br. J. Dermatol. 2019, 181, 450–458. [Google Scholar] [CrossRef]
- Doshi, J.A.; Takeshita, J.; Pinto, L.; Li, P.; Yu, X.; Rao, P.; Viswsanathan, H.N.; Gelfand, J.M. Biologic therapy adherence, discontinuation, switching, and restarting among patients with psoriasis in the US Medicare population. J. Am. Acad. Dermatol. 2016, 74, 1057–1065.e1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warren, R.B.; Smith, C.H.; Yiu, Z.Z.N.; Ashcroft, D.M.; Barker, J.N.W.N.; Burden, A.D.; Lunt, M.; McElhone, K.; Ormerod, A.D.; Owen, C.M.; et al. Differential drug survival of biologic therapies for the treatment of psoriasis: A prospective observational cohort study from the British Association of Dermatologists Biologic Interventions Register (BADBIR). J. Investig. Dermatol. 2015, 135, 2632–2640. [Google Scholar] [CrossRef]
- Shalom, G.; Cohen, A.D.; Ziv, M.; Eran, C.B.; Feldhamer, I.; Freud, T.; Berman, E.; Oren, S.; Hodak, E.; Pavlovsky, L. Biologic drug survival in Israeli psoriasis patients. J. Am. Acad. Dermatol. 2017, 76, 662–669.e661. [Google Scholar] [CrossRef] [PubMed]
- Graier, T.; Weger, W.; Sator, P.-G.; Salmhofer, W.; Gruber, B.; Jonak, C.; Kölli, C.; Schütz-Bergmayr, M.; Vujic, I.; Ratzinger, G.; et al. Effectiveness and clinical predictors of drug survival in psoriasis patients receiving apremilast: A registry analysis. JAAD Int. 2021, 2, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Jacobi, A.; Rustenbach, S.J.; Augustin, M. Comorbidity as a predictor for drug survival of biologic therapy in patients with psoriasis. Int. J. Dermatol. 2016, 55, 296–302. [Google Scholar] [CrossRef]
| Secukinumab (n = 27) | Ustekinumab (n = 61) | Guselkumab (n = 23) | p-Value | |
|---|---|---|---|---|
| Age (years) Mean (SD) | 47.9 (15.6) | 47.8 (15.3) | 43.6 (15.1) | 0.82 |
| Sex (female) | 8 (29.6%) | 23 (37.7%) | 8 (34.8%) | 0.76 |
| Body mass index, kg/m2 Mean (SD) Obesity (BMI ≥ 30) | 26.0 (4.9) 4 (14.8%) | 24.6 (4.1) 9 (14.8%) | 26.0 (5.1) 3 (13.0%) | 0.82 0.99 |
| Body surface area (%) Mean (SD) | 25.0 (8.2) | 26.8 (11.4) | 27.4 (11.0) | 0.27 |
| Initial PASI Mean (SD) | 17.9 (6.2) | 17.5 (7.5) | 20.0 (9.2) | 0.14 |
| Previous treatments Only systemic immunosuppressive Treatments † Sequential combination of immunosuppressive treatments and phototherapy †† Alcohol | 12 (44.4%) 15 (55.6%) 14 (51.9%) | 30 (49.2%) 31 (50.8%) 33 (54.1%) | 11 (47.8%) 12 (52.2%) 15 (65.2%) | 0.92 0.92 0.59 |
| Smoking | 9 (33.3%) | 29 (47.5%) | 11 (47.8%) | 0.43 |
| Hypertension | 8 (29.6%) | 12 (19.7%) | 7 (30.4%) | 0.45 |
| Diabetes | 5 (18.5%) | 3 (4.9%) | 1 (4.3%) | 0.07 |
| Previous hepatitis history | 1 (3.7%) | 2 (3.3%) | 3 (13.0%) | 0.19 |
| Previous tuberculosis history | 0 (0.0%) | 6 (9.8%) | 2 (8.7%) | 0.25 |
| Dyslipidemia Cardiovascular disease Psoriatic arthritis | 7 (25.9%) 1 (3.7%) 2 (7.4%) | 7 (11.5%) 2 (3.3%) 4 (6.6%) | 7 (30.4%) 1 (4.3%) 3 (13.0%) | 0.08 0.97 0.62 |
| Secukinumab (n = 27) | Ustekinumab (n = 61) | Guselkumab (n = 23) | p-Value | |
|---|---|---|---|---|
| Patients with ≥1 AE Tuberculosis Viral infection Fungal infection Injection site reaction Worsening of psoriasis Conjunctivitis Systemic allergic reaction Arthralgia | 5 (18.5%) 0 (0.0%) 1 (3.7%) 0 (0.0%) 2 (7.4%) 0 (0.0%) 1 (3.7%) 1 (3.7%) 0 (0.0%) | 11 (18.0%) 5 (8.2%) 0 (0.0%) 4 (6.6%) 1 (1.6%) 1 (1.6%) 0 (0.0%) 0 (0.0%) 0 (0.0%) | 4 (17.4%) 0 (0.0%) 0 (0.0%) 1 (4.3%) 1 (4.3%) 0 (0.0%) 1 (4.3%) 0 (0.0%) 1 (4.3%) | 0.99 0.07 0.22 0.39 0.42 0.66 0.30 0.22 0.15 |
| SAE affecting drug survivals | 3 (11.1%) | 1 (1.6%) | 0 (0.0%) | <0.01 ** |
| Secukinumab (n = 27) | Ustekinumab (n = 61) | Guselkumab (n = 23) | |
|---|---|---|---|
| Drug survival for up to 56 weeks Median drug survival weeks (95% CI) Patients lost during follow-up period Biologic discontinuation Severe adverse event Loss of efficacy Complete remission | 22/27 (81.5%) 48.4 (41.9–55.0) 5/27 (18.5%) 2 (7.4%) 2 (7.4%) 0 (0.0%) 0 (0.0%) | 45/61 (73.8%) 52.4 (50.0–54.8) 16/61 (26.2%) 3 (4.9%) 1 (1.6%) 1 (1.6%) 1 (1.6%) | 22/23 (95.7%) 55.0 (52.8–57.1) 1/23 (4.3%) 0 (0.0%) 0 (0.0%) 0 (0.0%) 0 (0.0%) |
| Biologic change Severe adverse event Loss of efficacy | 3 (11.1%) 1 (3.7%) 2 (7.4%) | 13 (21.3%) 0 (0.0%) 13 (21.3%) | 1 (4.3%) 0 (0.0%) 1 (4.3%) |
| HR (95% CI) | p-Value | |
|---|---|---|
| Female sex Obesity (BMI ≥ 30) Initial PASI | 1.38 (1.12–1.68) 1.59 (0.77–3.26) 0.91 (0.82–1.02) | 0.04 * 0.36 0.09 |
| Alcohol consumption | 1.99 (0.45–8.85) | 0.36 |
| Current smoker | 0.67 (0.35–1.28) | 0.22 |
| Hypertension | 1.36 (1.13–1.57) | 0.02 * |
| Diabetes | 1.84 (0.64–5.32) | 0.26 |
| Hepatitis | 1.18 (0.36–3.88) | 0.79 |
| Tuberculosis | 1.45 (0.57–4.00) | 0.47 |
| Dyslipidemia | 0.75 (0.31–1.83) | 0.53 |
| Psoriatic arthritis | 0.88 (0.32–2.42) | 0.81 |
| After guselkumab introduction | 3.42 (2.81–4.17) | <0.01 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, S.-W.; Lim, S.H.; Jeon, J.J.; Heo, Y.-W.; Choi, M.S.; Hong, S.-P. Comparison of the Efficacy and Safety of Biologics (Secukinumab, Ustekinumab, and Guselkumab) for the Treatment of Moderate-to-Severe Psoriasis: Real-World Data from a Single Korean Center. Biomedicines 2022, 10, 1058. https://doi.org/10.3390/biomedicines10051058
Jung S-W, Lim SH, Jeon JJ, Heo Y-W, Choi MS, Hong S-P. Comparison of the Efficacy and Safety of Biologics (Secukinumab, Ustekinumab, and Guselkumab) for the Treatment of Moderate-to-Severe Psoriasis: Real-World Data from a Single Korean Center. Biomedicines. 2022; 10(5):1058. https://doi.org/10.3390/biomedicines10051058
Chicago/Turabian StyleJung, Seung-Won, Sung Ha Lim, Jae Joon Jeon, Yeon-Woo Heo, Mi Soo Choi, and Seung-Phil Hong. 2022. "Comparison of the Efficacy and Safety of Biologics (Secukinumab, Ustekinumab, and Guselkumab) for the Treatment of Moderate-to-Severe Psoriasis: Real-World Data from a Single Korean Center" Biomedicines 10, no. 5: 1058. https://doi.org/10.3390/biomedicines10051058







