The Role of the Metabolism of Zinc and Manganese Ions in Human Cancerogenesis
Abstract
:1. Introduction
2. Regulation of Zn/Mn Homeostasis
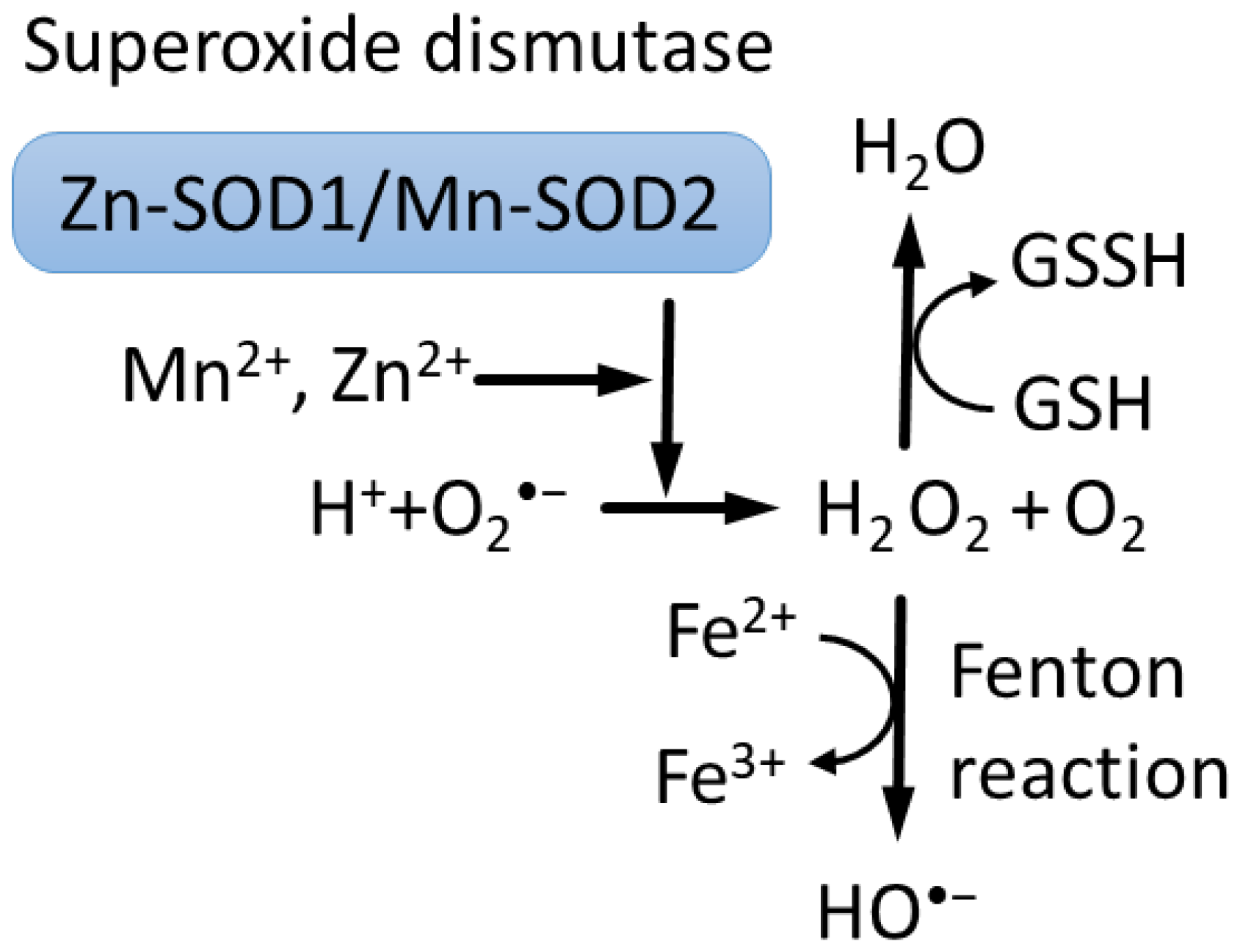
2.1. Zn, Mn and ROS Detoxification Reactions
2.2. Cellular Distribution of Mn and Toxicity
2.3. Regulation of Zn Homeostasis and Toxicity
2.4. Zn and Mn Transport
2.5. Zn and Mn Transporters and Cancerogenesis
2.6. Transcriptional Regulation by Zn and Mn
2.7. Mn- and Zn-Mediated Signal Transduction Pathways
2.8. Differential Effects of Mn and Zn in Normal and Cancer Cells
3. Effect of Zn and Mn on cGAS-STING Pathway, Immune Response and Cancerogenesis
4. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cotruvo, J.A.; Stubbe, J. Metallation and mismetallation of iron and manganese proteins in vitro and in vivo: The class I ribonucleotide reductases as a case study. Metallomics 2012, 4, 1020–1036. [Google Scholar] [CrossRef] [Green Version]
- Smith, M.R.; Fernandes, J.; Go, Y.-M.; Jones, D.P. Redox dynamics of manganese as a mitochondrial life-death switch. Biochem. Biophys. Res. Commun. 2017, 482, 388–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunter, T.E.; Gavin, C.E.; Gunter, K.K. The case for manganese interaction with mitochondria. Neurotoxicology 2009, 30, 727–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, W.; Ren, S.; Graziano, J.H. Manganese inhibits mitochondrial aconitase: A mechanism of manganese neurotoxicity. Brain Res. 1998, 799, 334–342. [Google Scholar] [CrossRef]
- Chen, J.-Y.; Tsao, G.C.; Zhao, Q.; Zheng, W. Differential cytotoxicity of Mn(II) and Mn(III): Special reference to mitochondrial [Fe-S] containing enzymes. Toxicol. Appl. Pharmacol. 2001, 175, 160–168. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, J.; Hao, L.; Bijli, K.M.; Chandler, J.D.; Orr, M.; Hu, X.; Jones, D.P.; Go, Y.-M. From the Cover: Manganese Stimulates Mitochondrial H2O2 Production in SH-SY5Y Human Neuroblastoma Cells over Physiologic as well as Toxicologic Range. Toxicol. Sci. 2017, 155, 213–223. [Google Scholar] [CrossRef] [Green Version]
- Sensi, S.L.; Ton-That, D.; Weiss, J.H. Mitochondrial sequestration and Ca2+-dependent release of cytosolic Zn2+ loads in cortical neurons. Neurobiol. Dis. 2002, 10, 100–108. [Google Scholar] [CrossRef] [Green Version]
- Ma, T.; Zhao, L.; Zhang, J.; Tang, R.; Wang, X.; Liu, N.; Zhang, Q.; Wang, F.; Li, M.; Shan, Q.; et al. A pair of transporters controls mitochondrial Zn2+ levels to maintain mitochondrial homeostasis. Protein Cell 2022, 13, 180–202. [Google Scholar] [CrossRef]
- Abiria, S.A.; Krapivinsky, G.; Sah, R.; Santa-Cruz, A.G.; Chaudhuri, D.; Zhang, J.; Adstamongkonkul, P.; DeCaen, P.G.; Clapham, D.E. TRPM7 senses oxidative stress to release Zn2+ from unique intracellular vesicles. Proc. Natl. Acad. Sci. USA 2017, 114, E6079–E6088. [Google Scholar] [CrossRef] [Green Version]
- Lu, Q.; Haragopal, H.; Slepchenko, K.G.; Stork, C.; Li, Y.V. Intracellular zinc distribution in mitochondria, ER and the Golgi apparatus. Int. J. Physiol. Pathophysiol. Pharmacol. 2016, 8, 35–43. [Google Scholar]
- Chabosseau, P.; Woodier, J.; Cheung, R.; Rutter, G.A. Sensors for measuring subcellular zinc pools. Metallomics 2018, 10, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yu, X.; Ding, H.; Zhao, Y.; Hu, C.; Feng, J. Comparing the Influence of High Doses of Different Zinc Salts on Oxidative Stress and Energy Depletion in IPEC-J2 Cells. Biol. Trace Elem. Res. 2020, 196, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Gu, M.; Hu, M.; Pinchi, P.; Chen, W.; Ryan, M.; Nold, T.; Bannaga, A.; Xu, H. Lysosomal Zn2+ release triggers rapid, mitochondria-mediated, non-apoptotic cell death in metastatic melanoma. Cell Rep. 2021, 37, 109848. [Google Scholar] [CrossRef] [PubMed]
- Gazaryan, I.G.; Krasinskaya, I.P.; Kristal, B.S.; Brown, A.M. Zinc irreversibly damages major enzymes of energy production and antioxidant defense prior to mitochondrial permeability transition. J. Biol. Chem. 2007, 282, 24373–24380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudolf, E.; Rudolf, K. Increases in Intracellular Zinc Enhance Proliferative Signaling as well as Mitochondrial and Endolysosomal Activity in Human Melanocytes. Cell. Physiol. Biochem. 2017, 43, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, A.S.; Bao, B. Molecular Mechanisms of Zinc as a Pro-Antioxidant Mediator: Clinical Therapeutic Implications. Antioxidants 2019, 8, 164. [Google Scholar] [CrossRef] [Green Version]
- Wei, Q.; Wang, J.; Wang, M.-H.; Yu, F.; Dong, Z. Inhibition of apoptosis by Zn2+ in renal tubular cells following ATP depletion. Am. J. Physiol. Ren. Physiol. 2004, 287, F492–F500. [Google Scholar] [CrossRef]
- Zhao, Y.; Tan, Y.; Dai, J.; Li, B.; Guo, L.; Cui, J.; Wang, G.; Shi, X.; Zhang, X.; Mellen, N.; et al. Exacerbation of diabetes-induced testicular apoptosis by zinc deficiency is most likely associated with oxidative stress, p38 MAPK activation, and p53 activation in mice. Toxicol. Lett. 2011, 200, 100–106. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, Z.; Wang, Q.; Zhang, J.; Wang, L.; Zhang, Q.; Li, H.; Wu, S. Manganese chloride induces histone acetylation changes in neuronal cells: Its role in manganese-induced damage. Neurotoxicology 2018, 65, 255–263. [Google Scholar] [CrossRef]
- Bonke, E.; Zwicker, K.; Dröse, S. Manganese ions induce H2O2 generation at the ubiquinone binding site of mitochondrial complex II. Arch. Biochem. Biophys. 2015, 580, 75–83. [Google Scholar] [CrossRef]
- Slepchenko, K.G.; Lu, Q.; Li, Y.V. Cross talk between increased intracellular zinc Zn2+ and accumulation of reactive oxygen species in chemical ischemia. Am. J. Physiol. Cell Physiol. 2017, 313, C448–C459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bin, B.-H.; Lee, S.-H.; Bhin, J.; Irié, T.; Kim, S.; Seo, J.; Mishima, K.; Lee, T.R.; Hwang, D.; Fukada, T.; et al. The epithelial zinc transporter ZIP10 epigenetically regulates human epidermal homeostasis by modulating histone acetyltransferase activity. Br. J. Dermatol. 2019, 180, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Qiao, X.; Xie, T.; Fu, W.; Li, H.; Zhao, Y.; Guo, M.; Feng, Y.; Chen, L.; Zhao, Y.; et al. SLC-30A9 is required for Zn2+ homeostasis, Zn2+ mobilization, and mitochondrial health. Proc. Natl. Acad. Sci. USA 2021, 118, e2023909118. [Google Scholar] [CrossRef] [PubMed]
- Abdo, A.I.; Tran, H.B.; Hodge, S.; Beltrame, J.F.; Zalewski, P.D. Zinc homeostasis alters zinc transporter protein expression in vascular endothelial and smooth muscle cells. Biol. Trace Elem. Res. 2021, 199, 2158–2171. [Google Scholar] [CrossRef]
- Hardyman, J.E.J.; Tyson, J.; Jackson, K.A.; Aldridge, C.; Cockell, S.J.; Wakeling, L.A.; Valentine, R.A.; Ford, D. Zinc sensing by metal-responsive transcription factor 1 (MTF1) controls metallothionein and ZnT1 expression to buffer the sensitivity of the transcriptome response to zinc. Metallomics 2016, 8, 337–343. [Google Scholar] [CrossRef] [Green Version]
- Kukic, I.; Kelleher, S.L.; Kiselyov, K. Zn2+ efflux through lysosomal exocytosis prevents Zn2+-induced toxicity. J. Cell Sci. 2014, 127, 3094–3103. [Google Scholar] [CrossRef] [Green Version]
- Stelling, M.P.; Soares, M.A.; Cardoso, S.C.; Motta, J.M.; de Abreu, J.C.; Antunes, M.J.M.; de Freitas, V.G.; Moraes, J.A.; Castelo-Branco, M.T.L.; Pérez, C.A.; et al. Manganese systemic distribution is modulated in vivo during tumor progression and affects tumor cell migration and invasion in vitro. Sci. Rep. 2021, 11, 15833. [Google Scholar] [CrossRef]
- Choi, S.; Liu, X.; Pan, Z. Zinc deficiency and cellular oxidative stress: Prognostic implications in cardiovascular diseases. Acta Pharmacol. Sin. 2018, 39, 1120–1132. [Google Scholar] [CrossRef] [Green Version]
- Anonna, S.N.; Ahamed, S.K.; Uddin, M.G.; Adnan, M.T.; Uddin, S.M.N.; Hussain, M.S.; Millat, M.S.; Bulbul, L.; Bhatta, R.; Sarwar, M.S.; et al. A clinical evaluation of the alterations in the level of serum zinc, copper, iron, and manganese in the ischemic heart disease patients of Bangladesh—A case-control study. Heliyon 2020, 6, e05311. [Google Scholar] [CrossRef]
- Mezzaroba, L.; Alfieri, D.F.; Simão, A.N.C.; Reiche, E.M.V. The role of zinc, copper, manganese and iron in neurodegenerative diseases. Neurotoxicology 2019, 74, 230–241. [Google Scholar] [CrossRef]
- Fiore, M.; Barone, R.; Copat, C.; Grasso, A.; Cristaldi, A.; Rizzo, R.; Ferrante, M. Metal and essential element levels in hair and association with autism severity. J. Trace Elem. Med. Biol. 2020, 57, 126409. [Google Scholar] [CrossRef] [PubMed]
- Stanton, J.E.; Malijauskaite, S.; McGourty, K.; Grabrucker, A.M. The metallome as a link between the “omes” in autism spectrum disorders. Front. Mol. Neurosci. 2021, 14, 695873. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.T.; Tan, Y.Q.; Valeri, L.; Lee, J.; Geok, P.P.; Chia, S.E.; Ong, C.N.; Seow, W.J. Association between serum heavy metals and prostate cancer risk—A multiple metal analysis. Environ. Int. 2019, 132, 105109. [Google Scholar] [CrossRef] [PubMed]
- To, P.K.; Do, M.H.; Cho, J.-H.; Jung, C. Growth modulatory role of zinc in prostate cancer and application to cancer therapeutics. Int. J. Mol. Sci. 2020, 21, 2991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nawi, A.M.; Chin, S.-F.; Azhar Shah, S.; Jamal, R. Tissue and Serum Trace Elements Concentration among Colorectal Patients: A Systematic Review of Case-Control Studies. Iran. J. Public Health 2019, 48, 632–643. [Google Scholar]
- Sohrabi, M.; Gholami, A.; Azar, M.H.; Yaghoobi, M.; Shahi, M.M.; Shirmardi, S.; Nikkhah, M.; Kohi, Z.; Salehpour, D.; Khoonsari, M.R.; et al. Trace Element and Heavy Metal Levels in Colorectal Cancer: Comparison between Cancerous and Non-cancerous Tissues. Biol. Trace Elem. Res. 2018, 183, 1–8. [Google Scholar] [CrossRef]
- Zabłocka-Słowińska, K.; Płaczkowska, S.; Prescha, A.; Pawełczyk, K.; Porębska, I.; Kosacka, M.; Pawlik-Sobecka, L.; Grajeta, H. Serum and whole blood Zn, Cu and Mn profiles and their relation to redox status in lung cancer patients. J. Trace Elem. Med. Biol. 2018, 45, 78–84. [Google Scholar] [CrossRef]
- Doble, P.A.; Miklos, G.L.G. Distributions of manganese in diverse human cancers provide insights into tumour radioresistance. Metallomics 2018, 10, 1191–1210. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zhao, H.; Xu, Z.; Cheng, X. Zinc dysregulation in cancers and its potential as a therapeutic target. Cancer Biol. Med. 2020, 17, 612–625. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, X.-W.; Shu, L.-S.; Liu, C.-D. The correlation and role analysis of SLC30A1 and SLC30A10 in cervical carcinoma. J. Cancer 2022, 13, 1031–1047. [Google Scholar] [CrossRef]
- Costello, L.C.; Franklin, R.B.; Zou, J.; Naslund, M.J. Evidence that Human Prostate Cancer is a ZIP1-Deficient Malignancy that could be Effectively Treated with a Zinc Ionophore (Clioquinol) Approach. Chemotherapy 2015, 4, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murali, P.; Johnson, B.P.; Lu, Z.; Climer, L.; Scott, D.A.; Foulquier, F.; Oprea-Ilies, G.; Lupashin, V.; Drake, R.R.; Abbott, K.L. Novel role for the Golgi membrane protein TMEM165 in control of migration and invasion for breast carcinoma. Oncotarget 2020, 11, 2747–2762. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.K.; Malas, K.M.; Tydrick, C.; Siddiqui, I.A.; Iczkowski, K.A.; Ahmad, N. Analysis of Zinc-Exporters Expression in Prostate Cancer. Sci. Rep. 2016, 6, 36772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, M.; Chen, M.; Zhang, R.; Zhang, W.; Wang, C.; Zhang, Y.; Wei, X.; Guan, Y.; Liu, J.; Feng, K.; et al. Manganese is critical for antitumor immune responses via cGAS-STING and improves the efficacy of clinical immunotherapy. Cell Res. 2020, 30, 966–979. [Google Scholar] [CrossRef] [PubMed]
- Toren, A.; Yalon, M.; Dafni, A.; Mehrian-Shai, R. Hgg-04. zinc enhances temozolomide cytotoxicity in pediatric glioblastoma multiforme model system. Neuro-Oncol. 2020, 22, iii344–iii345. [Google Scholar] [CrossRef]
- Toren, A.; Pismenyuk, T.; Yalon, M.; Freedman, S.; Simon, A.J.; Fisher, T.; Moshe, I.; Reichardt, J.K.V.; Constantini, S.; Mardor, Y.; et al. Zinc enhances temozolomide cytotoxicity in glioblastoma multiforme model systems. Oncotarget 2016, 7, 74860–74871. [Google Scholar] [CrossRef]
- Ekoue, D.N.; He, C.; Diamond, A.M.; Bonini, M.G. Manganese superoxide dismutase and glutathione peroxidase-1 contribute to the rise and fall of mitochondrial reactive oxygen species which drive oncogenesis. Biochim. Biophys. Acta Bioenerg. 2017, 1858, 628–632. [Google Scholar] [CrossRef]
- Lossow, K.; Schwarz, M.; Kipp, A.P. Are trace element concentrations suitable biomarkers for the diagnosis of cancer? Redox Biol. 2021, 42, 101900. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, R.; Wei, X.; Lv, M.; Jiang, Z. Metalloimmunology: The metal ion-controlled immunity. Adv. Immunol. 2020, 145, 187–241. [Google Scholar] [CrossRef]
- Fukai, T.; Ushio-Fukai, M. Superoxide dismutases: Role in redox signaling, vascular function, and diseases. Antioxid. Redox Signal. 2011, 15, 1583–1606. [Google Scholar] [CrossRef] [Green Version]
- Gordon, S.J.V.; Fenker, D.E.; Vest, K.E.; Padilla-Benavides, T. Manganese influx and expression of ZIP8 is essential in primary myoblasts and contributes to activation of SOD2. Metallomics 2019, 11, 1140–1153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azadmanesh, J.; Lutz, W.E.; Coates, L.; Weiss, K.L.; Borgstahl, G.E.O. Direct detection of coupled proton and electron transfers in human manganese superoxide dismutase. Nat. Commun. 2021, 12, 2079. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Mackey, M.M.; Diaz, A.A.; Cox, D.P. Hydroxyl radical is produced via the Fenton reaction in submitochondrial particles under oxidative stress: Implications for diseases associated with iron accumulation. Redox Rep. 2009, 14, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Lakes, A.; Dziubla, T. A free radical primer. In Oxidative Stress and Biomaterials; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–33. ISBN 9780128032695. [Google Scholar]
- Henle, E.S.; Luo, Y.; Gassmann, W.; Linn, S. Oxidative damage to DNA constituents by iron-mediated fenton reactions. The deoxyguanosine family. J. Biol. Chem. 1996, 271, 21177–21186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smethurst, D.G.J.; Kovalev, N.; McKenzie, E.R.; Pestov, D.G.; Shcherbik, N. Iron-mediated degradation of ribosomes under oxidative stress is attenuated by manganese. J. Biol. Chem. 2020, 295, 17200–17214. [Google Scholar] [CrossRef] [PubMed]
- Rauen, U.; Springer, A.; Weisheit, D.; Petrat, F.; Korth, H.-G.; de Groot, H.; Sustmann, R. Assessment of chelatable mitochondrial iron by using mitochondrion-selective fluorescent iron indicators with different iron-binding affinities. ChemBioChem 2007, 8, 341–352. [Google Scholar] [CrossRef]
- Zsurka, G.; Peeva, V.; Kotlyar, A.; Kunz, W.S. Is There Still Any Role for Oxidative Stress in Mitochondrial DNA-Dependent Aging? Genes 2018, 9, 175. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Gaidamakova, E.K.; Grichenko, O.; Matrosova, V.Y.; Hoeke, V.; Klimenkova, P.; Conze, I.H.; Volpe, R.P.; Tkavc, R.; Gostinčar, C.; et al. Across the tree of life, radiation resistance is governed by antioxidant Mn2+, gauged by paramagnetic resonance. Proc. Natl. Acad. Sci. USA 2017, 114, E9253–E9260. [Google Scholar] [CrossRef] [Green Version]
- Hernroth, B.; Holm, I.; Gondikas, A.; Tassidis, H. Manganese inhibits viability of prostate cancer cells. Anticancer Res. 2018, 38, 137–145. [Google Scholar] [CrossRef] [Green Version]
- Tholey, G.; Ledig, M.; Mandel, P.; Sargentini, L.; Frivold, A.H.; Leroy, M.; Grippo, A.A.; Wedler, F.C. Concentrations of physiologically important metal ions in glial cells cultured from chick cerebral cortex. Neurochem. Res. 1988, 13, 45–50. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, R.; Li, P.; Huang, F.; Fa, Z.; Chen, L.; Jiang, X. Determination of the detectable concentration of manganese used in neuronal MEMRI and its effect on cortical neurons in vitro. Neurol. Res. 2013, 35, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Guan, Y.; Lv, M.; Zhang, R.; Guo, Z.; Wei, X.; Du, X.; Yang, J.; Li, T.; Wan, Y.; et al. Manganese Increases the Sensitivity of the cGAS-STING Pathway for Double-Stranded DNA and Is Required for the Host Defense against DNA Viruses. Immunity 2018, 48, 675–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milne, D.B.; Sims, R.L.; Ralston, N.V. Manganese content of the cellular components of blood. Clin. Chem. 1990, 36, 450–452. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, S.D.; Bryant, R.G. Manganese-deoxyribonucleic acid binding modes. Nuclear magnetic relaxation dispersion results. Biophys. J. 1986, 50, 669–676. [Google Scholar] [CrossRef] [Green Version]
- Morello, M.; Canini, A.; Mattioli, P.; Sorge, R.P.; Alimonti, A.; Bocca, B.; Forte, G.; Martorana, A.; Bernardi, G.; Sancesario, G. Sub-cellular localization of manganese in the basal ganglia of normal and manganese-treated rats an electron spectroscopy imaging and electron energy-loss spectroscopy study. Neurotoxicology 2008, 29, 60–72. [Google Scholar] [CrossRef]
- Bartelle, B.B.; Mana, M.D.; Suero-Abreu, G.A.; Rodriguez, J.J.; Turnbull, D.H. Engineering an effective Mn-binding MRI reporter protein by subcellular targeting. Magn. Reson. Med. 2015, 74, 1750–1757. [Google Scholar] [CrossRef] [Green Version]
- Kumar, K.K.; Lowe, E.W.; Aboud, A.A.; Neely, M.D.; Redha, R.; Bauer, J.A.; Odak, M.; Weaver, C.D.; Meiler, J.; Aschner, M.; et al. Cellular manganese content is developmentally regulated in human dopaminergic neurons. Sci. Rep. 2014, 4, 6801. [Google Scholar] [CrossRef]
- Weydert, C.J.; Waugh, T.A.; Ritchie, J.M.; Iyer, K.S.; Smith, J.L.; Li, L.; Spitz, D.R.; Oberley, L.W. Overexpression of manganese or copper-zinc superoxide dismutase inhibits breast cancer growth. Free Radic. Biol. Med. 2006, 41, 226–237. [Google Scholar] [CrossRef]
- Batinic-Haberle, I.; Tovmasyan, A.; Huang, Z.; Duan, W.; Du, L.; Siamakpour-Reihani, S.; Cao, Z.; Sheng, H.; Spasojevic, I.; Alvarez Secord, A. H2O2-Driven Anticancer Activity of Mn Porphyrins and the Underlying Molecular Pathways. Oxid. Med. Cell. Longev. 2021, 2021, 6653790. [Google Scholar] [CrossRef]
- Al Haq, A.T.; Tseng, H.-Y.; Chen, L.-M.; Wang, C.-C.; Hsu, H.-L. Targeting prooxidant MnSOD effect inhibits triple-negative breast cancer (TNBC) progression and M2 macrophage functions under the oncogenic stress. Cell Death Dis. 2022, 13, 49. [Google Scholar] [CrossRef]
- El Mchichi, B.; Hadji, A.; Vazquez, A.; Leca, G. p38 MAPK and MSK1 mediate caspase-8 activation in manganese-induced mitochondria-dependent cell death. Cell Death Differ. 2007, 14, 1826–1836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schrantz, N.; Auffredou, M.T.; Bourgeade, M.F.; Besnault, L.; Leca, G.; Vazquez, A. Zinc-mediated regulation of caspases activity: Dose-dependent inhibition or activation of caspase-3 in the human Burkitt lymphoma B cells (Ramos). Cell Death Differ. 2001, 8, 152–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Liu, B.; Yin, X.; Guo, L.; Jiang, W.; Bi, H.; Guo, D. Excessive zinc chloride induces murine photoreceptor cell death via reactive oxygen species and mitochondrial signaling pathway. J. Inorg. Biochem. 2018, 187, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Bozym, R.A.; Thompson, R.B.; Stoddard, A.K.; Fierke, C.A. Measuring picomolar intracellular exchangeable zinc in PC-12 cells using a ratiometric fluorescence biosensor. ACS Chem. Biol. 2006, 1, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Vinkenborg, J.L.; Nicolson, T.J.; Bellomo, E.A.; Koay, M.S.; Rutter, G.A.; Merkx, M. Genetically encoded FRET sensors to monitor intracellular Zn2+ homeostasis. Nat. Methods 2009, 6, 737–740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carter, K.P.; Young, A.M.; Palmer, A.E. Fluorescent sensors for measuring metal ions in living systems. Chem. Rev. 2014, 114, 4564–4601. [Google Scholar] [CrossRef]
- Gee, K.R.; Zhou, Z.-L.; Ton-That, D.; Sensi, S.L.; Weiss, J.H. Measuring zinc in living cells. Cell Calcium 2002, 31, 245–251. [Google Scholar] [CrossRef]
- Andreini, C.; Banci, L.; Bertini, I.; Rosato, A. Counting the zinc-proteins encoded in the human genome. J. Proteome Res. 2006, 5, 196–201. [Google Scholar] [CrossRef]
- Tran, J.B.; Krężel, A. InterMetalDB: A Database and Browser of Intermolecular Metal Binding Sites in Macromolecules with Structural Information. J. Proteome Res. 2021, 20, 1889–1901. [Google Scholar] [CrossRef]
- Ireland, S.M.; Martin, A.C.R. Zincbindpredict-Prediction of Zinc Binding Sites in Proteins. Molecules 2021, 26, 966. [Google Scholar] [CrossRef]
- Peris-Díaz, M.D.; Guran, R.; Domene, C.; de Los Rios, V.; Zitka, O.; Adam, V.; Krężel, A. An Integrated Mass Spectrometry and Molecular Dynamics Simulations Approach Reveals the Spatial Organization Impact of Metal-Binding Sites on the Stability of Metal-Depleted Metallothionein-2 Species. J. Am. Chem. Soc. 2021, 143, 16486–16501. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.W. Regulation of zinc-dependent enzymes by metal carrier proteins. Biometals 2022, 35, 187–213. [Google Scholar] [CrossRef] [PubMed]
- Rana, U.; Kothinti, R.; Meeusen, J.; Tabatabai, N.M.; Krezoski, S.; Petering, D.H. Zinc binding ligands and cellular zinc trafficking: Apo-metallothionein, glutathione, TPEN, proteomic zinc, and Zn-Sp1. J. Inorg. Biochem. 2008, 102, 489–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petering, D.H.; Mahim, A. Proteomic high affinity Zn2+ trafficking: Where does metallothionein fit in? Int. J. Mol. Sci. 2017, 18, 1289. [Google Scholar] [CrossRef] [Green Version]
- Cano-Gauci, D.F.; Sarkar, B. Reversible zinc exchange between metallothionein and the estrogen receptor zinc finger. FEBS Lett. 1996, 386, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Roesijadi, G.; Bogumil, R.; Vasák, M.; Kägi, J.H. Modulation of DNA binding of a tramtrack zinc finger peptide by the metallothionein-thionein conjugate pair. J. Biol. Chem. 1998, 273, 17425–17432. [Google Scholar] [CrossRef] [Green Version]
- Mahim, A.; Karim, M.; Petering, D.H. Zinc trafficking 1. Probing the roles of proteome, metallothionein, and glutathione. Metallomics 2021, 13, mfab055. [Google Scholar] [CrossRef]
- Ryu, R.; Shin, Y.; Choi, J.-W.; Min, W.; Ryu, H.; Choi, C.-R.; Ko, H. Depletion of intracellular glutathione mediates zinc-induced cell death in rat primary astrocytes. Exp. Brain Res. 2002, 143, 257–263. [Google Scholar] [CrossRef]
- Xing, Y.; Wei, X.; Wang, M.-M.; Liu, Y.; Sui, Z.; Wang, X.; Zhang, Y.; Fei, Y.-H.; Jiang, Y.; Lu, C.; et al. Stimulating TRPM7 suppresses cancer cell proliferation and metastasis by inhibiting autophagy. Cancer Lett. 2022, 525, 179–197. [Google Scholar] [CrossRef]
- Kukoyi, B.I.; Costello, L.C.; Franklin, R.B. The effect of exogenous zinc ions on the pattern of oxygen consumption of the hepatic mitochondria of albino rats. Afr. J. Med. Med. Sci. 2004, 33, 361–363. [Google Scholar]
- Costello, L.C.; Guan, Z.; Kukoyi, B.; Feng, P.; Franklin, R.B. Terminal oxidation and the effects of zinc in prostate versus liver mitochondria. Mitochondrion 2004, 4, 331–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simpkins, C.; Balderman, S.; Mensah, E. Mitochondrial oxygen consumption is synergistically inhibited by metallothionein and calcium. J. Surg. Res. 1998, 80, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Chang, I.; Cho, N.; Koh, J.-Y.; Lee, M.-S. Pyruvate inhibits zinc-mediated pancreatic islet cell death and diabetes. Diabetologia 2003, 46, 1220–1227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mercadante, C.J.; Prajapati, M.; Conboy, H.L.; Dash, M.E.; Herrera, C.; Pettiglio, M.A.; Cintron-Rivera, L.; Salesky, M.A.; Rao, D.B.; Bartnikas, T.B. Manganese transporter Slc30a10 controls physiological manganese excretion and toxicity. J. Clin. Investig. 2019, 129, 5442–5461. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Elkoshi, N.; Barber-Zucker, S.; Hoch, E.; Zarivach, R.; Hershfinkel, M.; Sekler, I. Zinc transporter 10 (ZnT10)-dependent extrusion of cellular Mn2+ is driven by an active Ca2+-coupled exchange. J. Biol. Chem. 2019, 294, 5879–5889. [Google Scholar] [CrossRef] [Green Version]
- Fujishiro, H.; Kambe, T. Manganese transport in mammals by zinc transporter family proteins, ZNT and ZIP. J. Pharmacol. Sci. 2022, 148, 125–133. [Google Scholar] [CrossRef]
- Bafaro, E.; Liu, Y.; Xu, Y.; Dempski, R.E. The emerging role of zinc transporters in cellular homeostasis and cancer. Signal Transduct. Target. Ther. 2017, 2, 17029. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Tepaamorndech, S. The SLC30 family of zinc transporters—A review of current understanding of their biological and pathophysiological roles. Mol. Asp. Med. 2013, 34, 548–560. [Google Scholar] [CrossRef]
- Winslow, J.W.W.; Limesand, K.H.; Zhao, N. The functions of ZIP8, ZIP14, and znt10 in the regulation of systemic manganese homeostasis. Int. J. Mol. Sci. 2020, 21, 3304. [Google Scholar] [CrossRef]
- Franz, M.-C.; Simonin, A.; Graeter, S.; Hediger, M.A.; Kovacs, G. Development of the First Fluorescence Screening Assay for the SLC39A2 Zinc Transporter. J. Biomol. Screen. 2014, 19, 909–916. [Google Scholar] [CrossRef] [Green Version]
- Zhao, N.; Zhang, A.-S.; Wortham, A.M.; Jue, S.; Knutson, M.D.; Enns, C.A. The tumor suppressor, P53, decreases the metal transporter, ZIP14. Nutrients 2017, 9, 1335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.-Y.; Jenkitkasemwong, S.; Duarte, S.; Sparkman, B.K.; Shawki, A.; Mackenzie, B.; Knutson, M.D. ZIP8 is an iron and zinc transporter whose cell-surface expression is up-regulated by cellular iron loading. J. Biol. Chem. 2012, 287, 34032–34043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukhopadhyay, S.; Linstedt, A.D. Identification of a gain-of-function mutation in a Golgi P-type ATPase that enhances Mn2+ efflux and protects against toxicity. Proc. Natl. Acad. Sci. USA 2011, 108, 858–863. [Google Scholar] [CrossRef] [Green Version]
- Ohana, E.; Hoch, E.; Keasar, C.; Kambe, T.; Yifrach, O.; Hershfinkel, M.; Sekler, I. Identification of the Zn2+ binding site and mode of operation of a mammalian Zn2+ transporter. J. Biol. Chem. 2009, 284, 17677–17686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishito, Y.; Tsuji, N.; Fujishiro, H.; Takeda, T.-A.; Yamazaki, T.; Teranishi, F.; Okazaki, F.; Matsunaga, A.; Tuschl, K.; Rao, R.; et al. Direct comparison of manganese detoxification/efflux proteins and molecular characterization of znt10 protein as a manganese transporter. J. Biol. Chem. 2016, 291, 14773–14787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Feresin, R.G.; Falcon-Perez, J.M.; Salazar, G. Differential Targeting of SLC30A10/ZnT10 Heterodimers to Endolysosomal Compartments Modulates EGF-Induced MEK/ERK1/2 Activity. Traffic 2016, 17, 267–288. [Google Scholar] [CrossRef] [Green Version]
- Thompson, K.J.; Hein, J.; Baez, A.; Sosa, J.C.; Wessling-Resnick, M. Manganese transport and toxicity in polarized WIF-B hepatocytes. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G351–G363. [Google Scholar] [CrossRef]
- Yamasaki, S.; Sakata-Sogawa, K.; Hasegawa, A.; Suzuki, T.; Kabu, K.; Sato, E.; Kurosaki, T.; Yamashita, S.; Tokunaga, M.; Nishida, K.; et al. Zinc is a novel intracellular second messenger. J. Cell Biol. 2007, 177, 637–645. [Google Scholar] [CrossRef] [Green Version]
- Slepchenko, K.G.; Lu, Q.; Li, Y.V. Zinc wave during the treatment of hypoxia is required for initial reactive oxygen species activation in mitochondria. Int. J. Physiol. Pathophysiol. Pharmacol. 2016, 8, 44–51. [Google Scholar]
- Liu, C.; Jursa, T.; Aschner, M.; Smith, D.R.; Mukhopadhyay, S. Up-regulation of the manganese transporter SLC30A10 by hypoxia-inducible factors defines a homeostatic response to manganese toxicity. Proc. Natl. Acad. Sci. USA 2021, 118, e2107673118. [Google Scholar] [CrossRef]
- Vannini, A.; Volpari, C.; Filocamo, G.; Casavola, E.C.; Brunetti, M.; Renzoni, D.; Chakravarty, P.; Paolini, C.; De Francesco, R.; Gallinari, P.; et al. Crystal structure of a eukaryotic zinc-dependent histone deacetylase, human HDAC8, complexed with a hydroxamic acid inhibitor. Proc. Natl. Acad. Sci. USA 2004, 101, 15064–15069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dowling, D.P.; Gattis, S.G.; Fierke, C.A.; Christianson, D.W. Structures of metal-substituted human histone deacetylase 8 provide mechanistic inferences on biological function. Biochemistry 2010, 49, 5048–5056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Posewitz, M.C.; Wilcox, D.E. Properties of the Sp1 zinc finger 3 peptide: Coordination chemistry, redox reactions, and metal binding competition with metallothionein. Chem. Res. Toxicol. 1995, 8, 1020–1028. [Google Scholar] [CrossRef] [PubMed]
- Carmona, A.; Roudeau, S.; Perrin, L.; Veronesi, G.; Ortega, R. Environmental manganese compounds accumulate as Mn(II) within the Golgi apparatus of dopamine cells: Relationship between speciation, subcellular distribution, and cytotoxicity. Metallomics 2014, 6, 822–832. [Google Scholar] [CrossRef]
- García-Rodríguez, N.; Manzano-López, J.; Muñoz-Bravo, M.; Fernández-García, E.; Muñiz, M.; Wellinger, R.E. Manganese redistribution by calcium-stimulated vesicle trafficking bypasses the need for P-type ATPase function. J. Biol. Chem. 2015, 290, 9335–9347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christenson, E.T.; Gallegos, A.S.; Banerjee, A. In vitro reconstitution, functional dissection, and mutational analysis of metal ion transport by mitoferrin-1. J. Biol. Chem. 2018, 293, 3819–3828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolff, N.A.; Garrick, M.D.; Zhao, L.; Garrick, L.M.; Ghio, A.J.; Thévenod, F. A role for divalent metal transporter (DMT1) in mitochondrial uptake of iron and manganese. Sci. Rep. 2018, 8, 211. [Google Scholar] [CrossRef]
- Jeong, J.; Walker, J.M.; Wang, F.; Park, J.G.; Palmer, A.E.; Giunta, C.; Rohrbach, M.; Steinmann, B.; Eide, D.J. Promotion of vesicular zinc efflux by ZIP13 and its implications for spondylocheiro dysplastic Ehlers-Danlos syndrome. Proc. Natl. Acad. Sci. USA 2012, 109, E3530–E3538. [Google Scholar] [CrossRef] [Green Version]
- Pan, Z.; Choi, S.; Ouadid-Ahidouch, H.; Yang, J.-M.; Beattie, J.H.; Korichneva, I. Zinc transporters and dysregulated channels in cancers. Front. Biosci. 2017, 22, 623–643. [Google Scholar] [CrossRef] [Green Version]
- Costello, L.C.; Franklin, R.B.; Feng, P. Mitochondrial function, zinc, and intermediary metabolism relationships in normal prostate and prostate cancer. Mitochondrion 2005, 5, 143–153. [Google Scholar] [CrossRef] [Green Version]
- Feng, P.; Li, T.-L.; Guan, Z.-X.; Franklin, R.B.; Costello, L.C. Direct effect of zinc on mitochondrial apoptogenesis in prostate cells. Prostate 2002, 52, 311–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franklin, R.B.; Feng, P.; Milon, B.; Desouki, M.M.; Singh, K.K.; Kajdacsy-Balla, A.; Bagasra, O.; Costello, L.C. hZIP1 zinc uptake transporter down regulation and zinc depletion in prostate cancer. Mol. Cancer 2005, 4, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milon, B.C.; Agyapong, A.; Bautista, R.; Costello, L.C.; Franklin, R.B. Ras responsive element binding protein-1 (RREB-1) down-regulates hZIP1 expression in prostate cancer cells. Prostate 2010, 70, 288–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costello, L.C.; Franklin, R.B. A Proposed Efficacious Treatment with Clioquinol (Zinc Ionophore) and Cabergoline (Prolactin Dopamine Agonist) for the Treatment of Terminal Androgen-independent Prostate Cancer. Why and How? J. Clin. Res. Oncol. 2019, 2. [Google Scholar]
- Franklin, R.B.; Zou, J.; Costello, L.C. The cytotoxic role of RREB1, ZIP3 zinc transporter, and zinc in human pancreatic adenocarcinoma. Cancer Biol. Ther. 2014, 15, 1431–1437. [Google Scholar] [CrossRef] [Green Version]
- Cheng, X.; Wang, J.; Liu, C.; Jiang, T.; Yang, N.; Liu, D.; Zhao, H.; Xu, Z. Zinc transporter SLC39A13/ZIP13 facilitates the metastasis of human ovarian cancer cells via activating Src/FAK signaling pathway. J. Exp. Clin. Cancer Res. 2021, 40, 199. [Google Scholar] [CrossRef] [PubMed]
- Jenkitkasemwong, S.; Akinyode, A.; Paulus, E.; Weiskirchen, R.; Hojyo, S.; Fukada, T.; Giraldo, G.; Schrier, J.; Garcia, A.; Janus, C.; et al. SLC39A14 deficiency alters manganese homeostasis and excretion resulting in brain manganese accumulation and motor deficits in mice. Proc. Natl. Acad. Sci. USA 2018, 115, E1769–E1778. [Google Scholar] [CrossRef] [Green Version]
- Himeno, S.; Fujishiro, H. Roles of zinc transporters that control the essentiality and toxicity of manganese and cadmium. Yakugaku Zasshi 2021, 141, 695–703. [Google Scholar] [CrossRef]
- Xu, X.-M.; Wang, C.-G.; Zhu, Y.-D.; Chen, W.-H.; Shao, S.-L.; Jiang, F.-N.; Liao, Q.-D. Decreased expression of SLC 39A14 is associated with tumor aggressiveness and biochemical recurrence of human prostate cancer. OncoTargets Ther. 2016, 9, 4197–4205. [Google Scholar] [CrossRef] [Green Version]
- Thorsen, K.; Mansilla, F.; Schepeler, T.; Øster, B.; Rasmussen, M.H.; Dyrskjøt, L.; Karni, R.; Akerman, M.; Krainer, A.R.; Laurberg, S.; et al. Alternative splicing of SLC39A14 in colorectal cancer is regulated by the Wnt pathway. Mol. Cell. Proteom. 2011, 10, M110.002998. [Google Scholar] [CrossRef] [Green Version]
- Sveen, A.; Bakken, A.C.; Ågesen, T.H.; Lind, G.E.; Nesbakken, A.; Nordgård, O.; Brackmann, S.; Rognum, T.O.; Lothe, R.A.; Skotheim, R.I. The exon-level biomarker SLC39A14 has organ-confined cancer-specificity in colorectal cancer. Int. J. Cancer 2012, 131, 1479–1485. [Google Scholar] [CrossRef] [PubMed]
- Lavilla, I.; Costas, M.; Miguel, P.S.; Millos, J.; Bendicho, C. Elemental fingerprinting of tumorous and adjacent non-tumorous tissues from patients with colorectal cancer using ICP-MS, ICP-OES and chemometric analysis. Biometals 2009, 22, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Juloski, J.T.; Rakic, A.; Ćuk, V.V.; Ćuk, V.M.; Stefanović, S.; Nikolić, D.; Janković, S.; Trbovich, A.M.; De Luka, S.R. Colorectal cancer and trace elements alteration. J. Trace Elem. Med. Biol. 2020, 59, 126451. [Google Scholar] [CrossRef] [PubMed]
- Uchida, A.; Oh-hashi, K.; Kiuchi, K.; Hirata, Y. Manganese regulates caspase-3 gene promoter activity by inducing Sp1 phosphorylation in PC12 cells. Toxicology 2012, 302, 292–298. [Google Scholar] [CrossRef]
- Brito, S.; Lee, M.-G.; Bin, B.-H.; Lee, J.-S. Zinc and its transporters in epigenetics. Mol. Cells 2020, 43, 323–330. [Google Scholar] [CrossRef]
- Yusuf, A.P.; Abubakar, M.B.; Malami, I.; Ibrahim, K.G.; Abubakar, B.; Bello, M.B.; Qusty, N.; Elazab, S.T.; Imam, M.U.; Alexiou, A.; et al. Zinc metalloproteins in epigenetics and their crosstalk. Life 2021, 11, 186. [Google Scholar] [CrossRef]
- Gantt, S.L.; Gattis, S.G.; Fierke, C.A. Catalytic activity and inhibition of human histone deacetylase 8 is dependent on the identity of the active site metal ion. Biochemistry 2006, 45, 6170–6178. [Google Scholar] [CrossRef]
- Rozenberg, J.M.; Bhattacharya, P.; Chatterjee, R.; Glass, K.; Vinson, C. Combinatorial recruitment of CREB, C/EBPβ and c-Jun determines activation of promoters upon keratinocyte differentiation. PLoS ONE 2013, 8, e78179. Available online: https://pubmed.ncbi.nlm.nih.gov/24244291/ (accessed on 14 April 2022).
- Rishi, V.; Bhattacharya, P.; Chatterjee, R.; Rozenberg, J.; Zhao, J.; Glass, K.; Fitzgerald, P.; Vinson, C. CpG methylation of half-CRE sequences creates C/EBPα binding sites that activate some tissue-specific genes. Proc. Natl. Acad. Sci. USA 2010, 107, 20311–20316. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.-G.; Choi, M.-A.; Chae, S.; Kang, M.-A.; Jo, H.; Baek, J.-M.; In, K.-R.; Park, H.; Heo, H.; Jang, D.; et al. Loss of the dermis zinc transporter ZIP13 promotes the mildness of fibrosarcoma by inhibiting autophagy. Sci. Rep. 2019, 9, 15042. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.H.; Kim, J.H.; Lee, J.; Ahn, Y.S. Zinc-induced NF-κB inhibition can be modulated by changes in the intracellular metallothionein level. Toxicol. Appl. Pharmacol. 2003, 190, 189–196. [Google Scholar] [CrossRef]
- Taniguchi, K.; Karin, M. NF-κB, inflammation, immunity and cancer: Coming of age. Nat. Rev. Immunol. 2018, 18, 309–324. [Google Scholar] [CrossRef] [PubMed]
- Uzzo, R.G.; Leavis, P.; Hatch, W.; Gabai, V.L.; Dulin, N.; Zvartau, N.; Kolenko, V.M. Zinc inhibits nuclear factor-κB activation and sensitizes prostate cancer cells to cytotoxic agents. Clin. Cancer Res. 2002, 8, 3579–3583. [Google Scholar] [PubMed]
- Pan, Y.; Huang, J.; Xing, R.; Yin, X.; Cui, J.; Li, W.; Yu, J.; Lu, Y. Metallothionein 2A inhibits NF-κB pathway activation and predicts clinical outcome segregated with TNM stage in gastric cancer patients following radical resection. J. Transl. Med. 2013, 11, 173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anson, K.J.; Corbet, G.A.; Palmer, A.E. Zn2+ influx activates ERK and Akt signaling pathways. Proc. Natl. Acad. Sci. USA 2021, 118, e2015786118. [Google Scholar] [CrossRef] [PubMed]
- Edamatsu, H. Zinc ions negatively regulate proapoptotic signaling in cells expressing oncogenic mutant Ras. Biometals 2022, 35, 349–362. [Google Scholar] [CrossRef]
- Kahen, E.J.; Brohl, A.; Yu, D.; Welch, D.; Cubitt, C.L.; Lee, J.K.; Chen, Y.; Yoder, S.J.; Teer, J.K.; Zhang, Y.O.; et al. Neurofibromin level directs RAS pathway signaling and mediates sensitivity to targeted agents in malignant peripheral nerve sheath tumors. Oncotarget 2018, 9, 22571–22585. [Google Scholar] [CrossRef] [Green Version]
- Kiuru, M.; Busam, K.J. The NF1 gene in tumor syndromes and melanoma. Lab. Investig. 2017, 97, 146–157. [Google Scholar] [CrossRef] [Green Version]
- Naschberger, A.; Baradaran, R.; Rupp, B.; Carroni, M. The structure of neurofibromin isoform 2 reveals different functional states. Nature 2021, 599, 315–319. [Google Scholar] [CrossRef]
- Wan, C.; Ma, X.; Shi, S.; Zhao, J.; Nie, X.; Han, J.; Xiao, J.; Wang, X.; Jiang, S.; Jiang, J. Pivotal roles of p53 transcription-dependent and -independent pathways in manganese-induced mitochondrial dysfunction and neuronal apoptosis. Toxicol. Appl. Pharmacol. 2014, 281, 294–302. [Google Scholar] [CrossRef]
- Kim, D.-S.; Jin, H.; Anantharam, V.; Gordon, R.; Kanthasamy, A.; Kanthasamy, A.G. p73 gene in dopaminergic neurons is highly susceptible to manganese neurotoxicity. Neurotoxicology 2017, 59, 231–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behrend, L.; Mohr, A.; Dick, T.; Zwacka, R.M. Manganese superoxide dismutase induces p53-dependent senescence in colorectal cancer cells. Mol. Cell. Biol. 2005, 25, 7758–7769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rozenberg, J.M.; Zvereva, S.; Dalina, A.; Blatov, I.; Zubarev, I.; Luppov, D.; Bessmertnyi, A.; Romanishin, A.; Alsoulaiman, L.; Kumeiko, V.; et al. The p53 family member p73 in the regulation of cell stress response. Biol. Direct 2021, 16, 23. [Google Scholar] [CrossRef] [PubMed]
- Ofek, P.; Ben-Meir, D.; Kariv-Inbal, Z.; Oren, M.; Lavi, S. Cell cycle regulation and p53 activation by protein phosphatase 2C alpha. J. Biol. Chem. 2003, 278, 14299–14305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanoue, K.; Miller Jenkins, L.M.; Durell, S.R.; Debnath, S.; Sakai, H.; Tagad, H.D.; Ishida, K.; Appella, E.; Mazur, S.J. Binding of a third metal ion by the human phosphatases PP2Cα and Wip1 is required for phosphatase activity. Biochemistry 2013, 52, 5830–5843. [Google Scholar] [CrossRef] [Green Version]
- Jarosz, M.; Olbert, M.; Wyszogrodzka, G.; Młyniec, K.; Librowski, T. Antioxidant and anti-inflammatory effects of zinc. Zinc-dependent NF-κB signaling. Inflammopharmacology 2017, 25, 11–24. [Google Scholar] [CrossRef] [Green Version]
- Sliwinski, T.; Czechowska, A.; Kolodziejczak, M.; Jajte, J.; Wisniewska-Jarosinska, M.; Blasiak, J. Zinc salts differentially modulate DNA damage in normal and cancer cells. Cell Biol. Int. 2009, 33, 542–547. [Google Scholar] [CrossRef]
- Costa, M.I.; Lapa, B.S.; Jorge, J.; Alves, R.; Carreira, I.M.; Sarmento-Ribeiro, A.B.; Gonçalves, A.C. Zinc prevents DNA damage in normal cells but shows genotoxic and cytotoxic effects in acute myeloid leukemia cells. Int. J. Mol. Sci. 2022, 23, 2567. [Google Scholar] [CrossRef]
- Geng, J.; Li, J.; Huang, T.; Zhao, K.; Chen, Q.; Guo, W.; Gao, J. A novel manganese complex selectively induces malignant glioma cell death by targeting mitochondria. Mol. Med. Rep. 2016, 14, 1970–1978. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Guo, W.; Li, J.; Li, X.; Geng, J.; Chen, Q.; Gao, J. Tumor-targeting novel manganese complex induces ROS-mediated apoptotic and autophagic cancer cell death. Int. J. Mol. Med. 2015, 35, 607–616. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Wu, J.; Du, F.; Chen, X.; Chen, Z.J. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 2013, 339, 786–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Liu, W.; Wang, F.; Hayashi, T.; Mizuno, K.; Hattori, S.; Fujisaki, H.; Ikejima, T. DNA damage-triggered activation of cGAS-STING pathway induces apoptosis in human keratinocyte HaCaT cells. Mol. Immunol. 2021, 131, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Flynn, P.J.; Koch, P.D.; Mitchison, T.J. Chromatin bridges, not micronuclei, activate cGAS after drug-induced mitotic errors in human cells. Proc. Natl. Acad. Sci. USA 2021, 118, e2103585118. [Google Scholar] [CrossRef] [PubMed]
- Basit, A.; Cho, M.-G.; Kim, E.-Y.; Kwon, D.; Kang, S.-J.; Lee, J.-H. The cGAS/STING/TBK1/IRF3 innate immunity pathway maintains chromosomal stability through regulation of p21 levels. Exp. Mol. Med. 2020, 52, 643–657. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Ma, Z.; Wang, B.; Guan, Y.; Su, X.-D.; Jiang, Z. Mn2+ Directly Activates cGAS and Structural Analysis Suggests Mn2+ Induces a Noncanonical Catalytic Synthesis of 2′3′-cGAMP. Cell Rep. 2020, 32, 108053. [Google Scholar] [CrossRef]
- Sarhan, J.; Liu, B.C.; Muendlein, H.I.; Weindel, C.G.; Smirnova, I.; Tang, A.Y.; Ilyukha, V.; Sorokin, M.; Buzdin, A.; Fitzgerald, K.A.; et al. Constitutive interferon signaling maintains critical threshold of MLKL expression to license necroptosis. Cell Death Differ. 2019, 26, 332–347. [Google Scholar] [CrossRef]
- Larkin, B.; Ilyukha, V.; Sorokin, M.; Buzdin, A.; Vannier, E.; Poltorak, A. Cutting edge: Activation of STING in T cells induces type I IFN responses and cell death. J. Immunol. 2017, 199, 397–402. [Google Scholar] [CrossRef] [Green Version]
- Ram, D.R.; Ilyukha, V.; Volkova, T.; Buzdin, A.; Tai, A.; Smirnova, I.; Poltorak, A. Balance between short and long isoforms of cFLIP regulates Fas-mediated apoptosis in vivo. Proc. Natl. Acad. Sci. USA 2016, 113, 1606–1611. [Google Scholar] [CrossRef] [Green Version]
- Yi, M.; Niu, M.; Zhang, J.; Li, S.; Zhu, S.; Yan, Y.; Li, N.; Zhou, P.; Chu, Q.; Wu, K. Combine and conquer: Manganese synergizing anti-TGF-β/PD-L1 bispecific antibody YM101 to overcome immunotherapy resistance in non-inflamed cancers. J. Hematol. Oncol. 2021, 14, 146. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, Y.; Li, J.; Park, K.S.; Han, K.; Zhou, X.; Xu, Y.; Nam, J.; Xu, J.; Shi, X.; et al. Amplifying STING activation by cyclic dinucleotide-manganese particles for local and systemic cancer metalloimmunotherapy. Nat. Nanotechnol. 2021, 16, 1260–1270. [Google Scholar] [CrossRef]
- Du, M.; Chen, Z.J. DNA-induced liquid phase condensation of cGAS activates innate immune signaling. Science 2018, 361, 704–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, W.; Lama, L.; Adura, C.; Tomita, D.; Glickman, J.F.; Tuschl, T.; Patel, D.J. Human cGAS catalytic domain has an additional DNA-binding interface that enhances enzymatic activity and liquid-phase condensation. Proc. Natl. Acad. Sci. USA 2019, 116, 11946–11955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kranzusch, P.J.; Lee, A.S.-Y.; Berger, J.M.; Doudna, J.A. Structure of human cGAS reveals a conserved family of second-messenger enzymes in innate immunity. Cell Rep. 2013, 3, 1362–1368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lian, H.; Wei, J.; Zang, R.; Ye, W.; Yang, Q.; Zhang, X.-N.; Chen, Y.-D.; Fu, Y.-Z.; Hu, M.-M.; Lei, C.-Q.; et al. ZCCHC3 is a co-sensor of cGAS for dsDNA recognition in innate immune response. Nat. Commun. 2018, 9, 3349. [Google Scholar] [CrossRef] [PubMed]
- Skrajnowska, D.; Bobrowska-Korczak, B. Role of Zinc in Immune System and Anti-Cancer Defense Mechanisms. Nutrients 2019, 11, 2273. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Du, L.; Li, F.; Deng, Z.; Zeng, S. Intelligent Nanotransducer for Deep-Tumor Hypoxia Modulation and Enhanced Dual-Photosensitizer Photodynamic Therapy. ACS Appl. Mater. Interfaces 2022, 14, 14944–14952. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, L.; Qiang, Z.; Jiang, J.; Zhu, Z.; Ren, J. Enhancing Targeted Cancer Treatment by Combining Hyperthermia and Radiotherapy Using Mn-Zn Ferrite Magnetic Nanoparticles. ACS Biomater. Sci. Eng. 2020, 6, 3550–3562. [Google Scholar] [CrossRef]
- Sun, Y.; Yan, C.; Xie, J.; Yan, D.; Hu, K.; Huang, S.; Liu, J.; Zhang, Y.; Gu, N.; Xiong, F. High-Performance Worm-like Mn-Zn Ferrite Theranostic Nanoagents and the Application on Tumor Theranostics. ACS Appl. Mater. Interfaces 2019, 11, 29536–29548. [Google Scholar] [CrossRef]
- Dippong, T.; Levei, E.A.; Cadar, O. Recent advances in synthesis and applications of mfe2o4 (M = co, cu, mn, ni, zn) nanoparticles. Nanomaterials 2021, 11, 1560. [Google Scholar] [CrossRef]


| Gene Name | Protein Name | Specificity | Type of Transport | Reference |
|---|---|---|---|---|
| SLC30A10 | ZNT10 | Mn2+/Ca2+ exchange, Zn2+ vesicular transport as SLC30A3 heterodimer | Exporter | [95,96] |
| SLC30A3 | ZNT3 | Zn2+ | Exporter | [39,97,98,99] |
| SLC39A14 | ZIP14 | Divalent metal cations Mn2+, Zn2+, Fe2+ | Importer (symport) | [97,100,101,102] |
| SLC39A8 | ZIP8 | Mn, Zn, Fe | Importer (symport) | [97,100,103] |
| TP2C1 | Ca or Mn | Mitochondrial influx | [104] |
| Main Finding | Reference |
|---|---|
| Certain cancers exhibit coordinated changes in Zn2+ and Mn2+ carriers | [40,43] |
| High levels of Mn in cancers are associated with poor survival and low radiosensitivity of tumors, such as for melanoma and glioblastoma | [38] |
| Preclinical models support application of Zn2+ ionophore clioquinol in combination with dopamine agonist for prostate cancer treatment | [41,125] |
| Mn2+ boosts innate and adaptive anti-cancer immune response and boosts PD-1 immunotherapy | [44] |
| Therapeutic activity of YM101 and Mn2+ was demonstrated using mice models of hepatocellular carcinoma, melanoma, colon cancer and breast cancers | [170] |
| Zn2+ enhances temozolomide efficiency in glioblastoma xenograft model | [46] |
| Main Finding | References |
|---|---|
| Mn2+-SOD2 drives H2O2 production in mitochondria in a wide range of extracellular concentrations | [6] |
| Mn2+ at high concentrations induces mitochondrial cell death | [72,151] |
| Zn2+ at high concentrations induces mitochondrial cell death | [13,74] |
| Zn2+ at low concentrations inhibits Mn-induced mitochondrial cell death | [73] |
| Low-molecular-weight complexes of Mn2+ predict cell survival, and double-strand breaks repair efficiency after gamma irradiation | [59] |
| Zn2+ release is required for and precedes ROS generation in mitochondria in response to hypoxia | [21,110] |
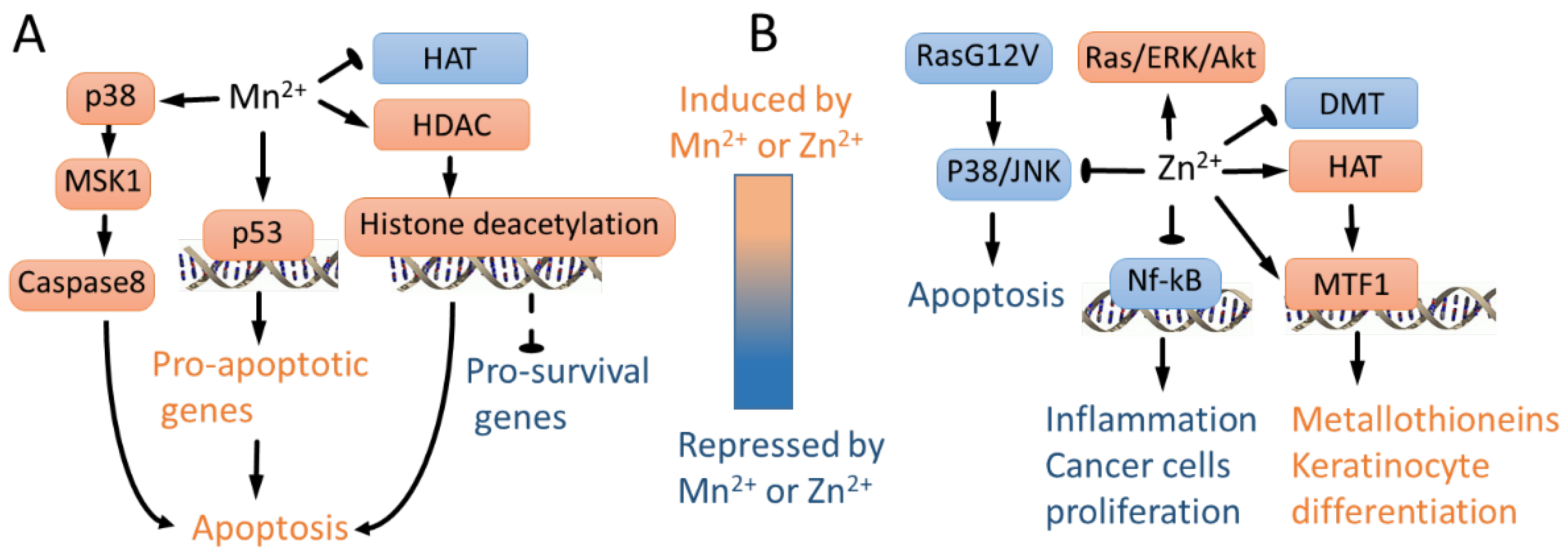
| Mn2+ activates p38/MSK1-regulated apoptosis | [72] |
| Zn2+ inhibits p38 and JNK and represses apoptosis in mutant G12V RAS cells | [147] |
| Zn2+ activates RAS signaling cascade | [146] |
| Mn2+ induces apoptosis or senescence by p53-dependent mechanisms | [151,152,153] |
| Zn2+ represses NF-κB activity and sensitizes prostate cancer cells to cytotoxic agents | [144,145,157] |
| Mn2+ represses histone acetylation by repressing HAT activity and augmenting HDAC, leading to apoptosis | [19] |
| Zn2+ activates HAT and MTF1-mediated transcription, leading to metallothionein induction and keratinocyte differentiation | [22] |
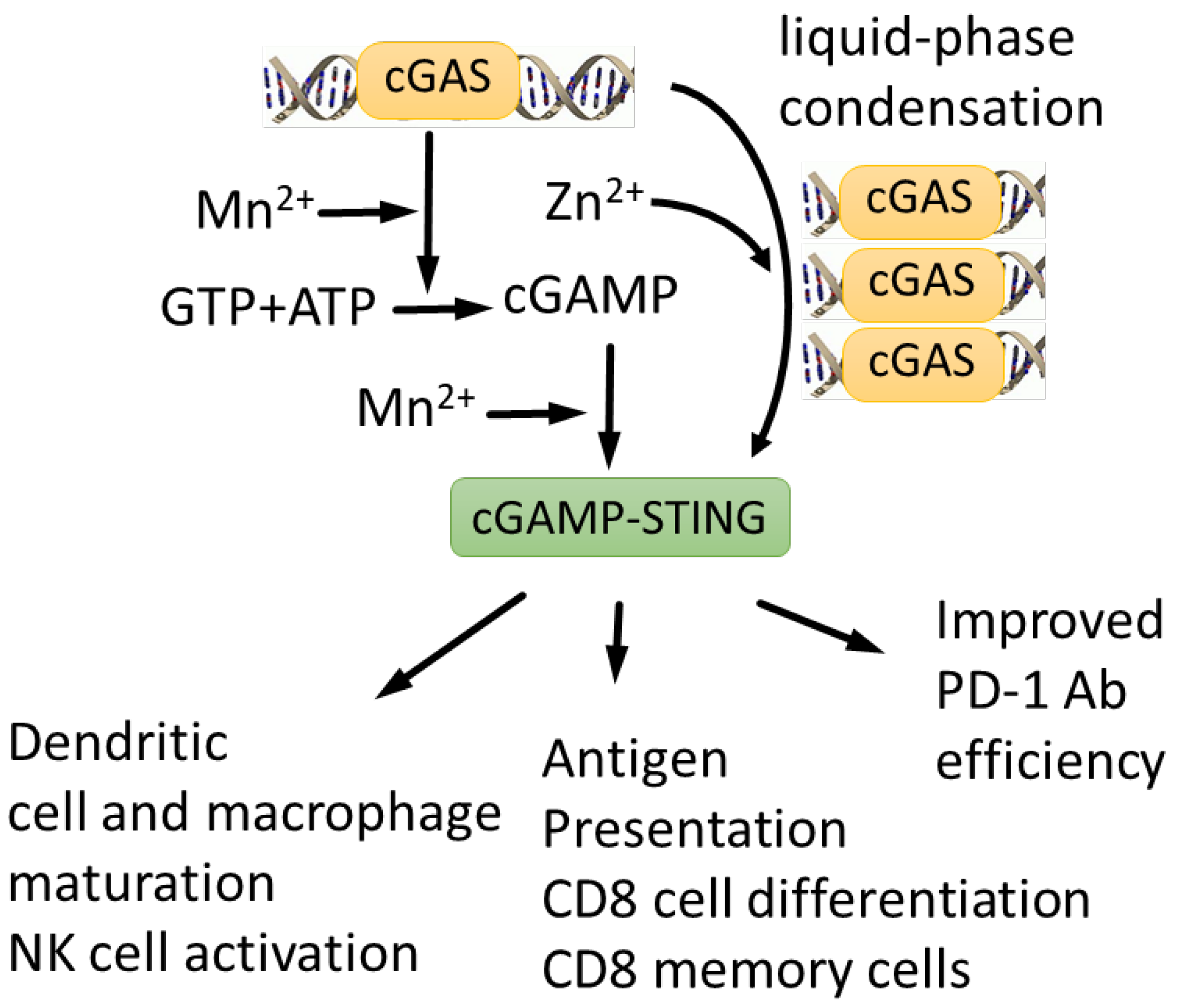
| Mn2+ is indispensable for cGAS-STNG activation and host defense against DNA viruses | [63] |
| Zn2+ coordination is required for cGAS–DNA liquid-phase condensation and cGAMP production | [172,173,174] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rozenberg, J.M.; Kamynina, M.; Sorokin, M.; Zolotovskaia, M.; Koroleva, E.; Kremenchutckaya, K.; Gudkov, A.; Buzdin, A.; Borisov, N. The Role of the Metabolism of Zinc and Manganese Ions in Human Cancerogenesis. Biomedicines 2022, 10, 1072. https://doi.org/10.3390/biomedicines10051072
Rozenberg JM, Kamynina M, Sorokin M, Zolotovskaia M, Koroleva E, Kremenchutckaya K, Gudkov A, Buzdin A, Borisov N. The Role of the Metabolism of Zinc and Manganese Ions in Human Cancerogenesis. Biomedicines. 2022; 10(5):1072. https://doi.org/10.3390/biomedicines10051072
Chicago/Turabian StyleRozenberg, Julian Markovich, Margarita Kamynina, Maksim Sorokin, Marianna Zolotovskaia, Elena Koroleva, Kristina Kremenchutckaya, Alexander Gudkov, Anton Buzdin, and Nicolas Borisov. 2022. "The Role of the Metabolism of Zinc and Manganese Ions in Human Cancerogenesis" Biomedicines 10, no. 5: 1072. https://doi.org/10.3390/biomedicines10051072
APA StyleRozenberg, J. M., Kamynina, M., Sorokin, M., Zolotovskaia, M., Koroleva, E., Kremenchutckaya, K., Gudkov, A., Buzdin, A., & Borisov, N. (2022). The Role of the Metabolism of Zinc and Manganese Ions in Human Cancerogenesis. Biomedicines, 10(5), 1072. https://doi.org/10.3390/biomedicines10051072









