Abstract
A growing interest in the use of a combination of chemosensitizers and cytostatics for overcoming cancer resistance to treatment and the development of their delivery systems has been observed. Resveratrol (Res) presents antioxidant, anti-inflammatory and chemopreventive properties but also limits multidrug resistance against docetaxel (Dtx), which is one of the main causes of failure in cancer therapy with this drug. However, the use of both drugs presents challenges, including poor bioavailability, the unfavourable pharmacokinetics and chemical instability of Res and the poor water solubility and dose-limiting toxicity of Dtx. In order to overcome these difficulties, attempts have been made to create different forms of delivery for both agents. This review is focused on the latest developments in nanoparticles for the delivery of Dtx, Res and for the combined delivery of those two drugs. The aim of this review was also to summarize the synergistic mechanism of action of Dtx and Res on cancer cells. According to recent reports, Dtx and Res loaded in a nano-delivery system exhibit better efficiency in cancer treatment compared to free drugs. Also, the co-delivery of Dtx and Res in one actively targeted delivery system providing the simultaneous release of both drugs in cancer cells has a chance to fulfil the requirements of effective anticancer therapy and reduce limitations in therapy caused by multidrug resistance (MDR).
1. Introduction
According to the World Cancer Report, cancer is the leading cause of premature death in 134 out of 183 countries (in people aged 30–69) and ranks third or fourth in the remaining 45 countries [1]. The clinical efficacy of current treatment methods, such as oncological surgery, chemotherapy and radiotherapy, remains limited, and in the case of tumours that cannot be removed surgically, chemotherapy is often the only treatment option. A major problem with intravenous systemic chemotherapy is non-specific tumour targeting and the difficulty in achieving therapeutic drug levels within the tumour or in the vicinity of the tumour [2]. In the case of paclitaxel (Ptx) administered by intravenous infusion, less than 0.5% of the total dose is locally available within the tumour. In addition, high drug concentrations are observed in healthy tissues, leading to severe side effects and dose-limiting toxicity [3].
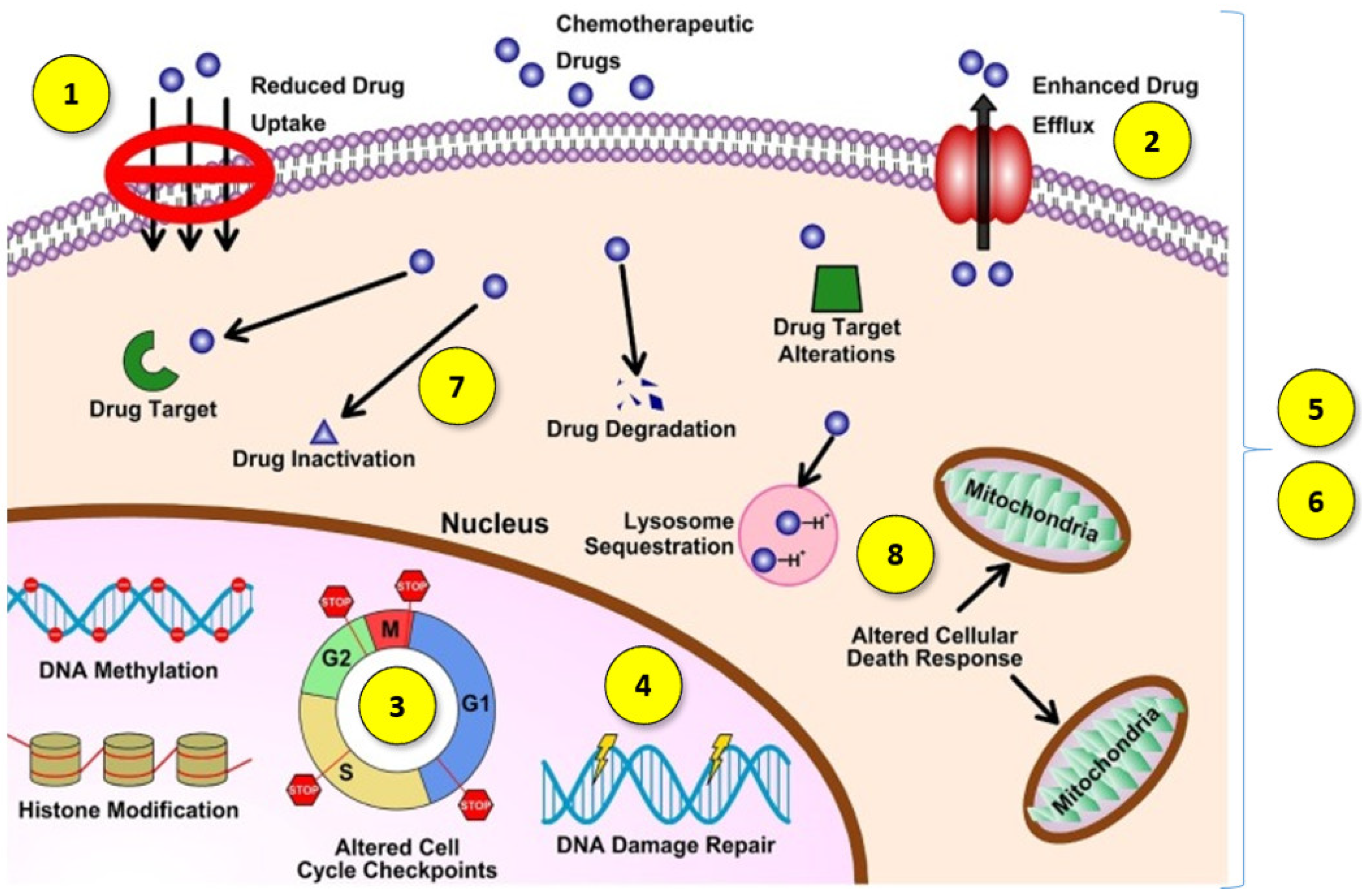
Another challenge in cancer treatment is overcoming multidrug resistance (MDR). MDR is the complex phenomenon of the chemoresistance of neoplastic cells to treatment, which can occur not only as a result of cytostatic drug treatment but can also be a feature of cancer untreated with chemotherapy. Moreover, chemoresistance after the administration of one drug may cause resistance to many other anticancer agents via different structures and mechanisms of action [4]. This is a broad mechanism, which may involve the development of one or more features by cancer cells (Figure 1):
- (1)
- Decreased drug absorption by cancer cells [5];
- (2)
- The increased expression of certain ATP-binding cassette efflux transporters, including P-glycoprotein (P-gp/ABCB1), multidrug resistance protein 1 (MRP1/ABCC1) and BCRP (ABCG2), which lower the cytosolic concentration of the active agents through increased drug transportation outside the cell [4];
- (3)
- The impaired function of pro-apoptotic factors, resulting in cancer cells avoiding programmed death [6];
- (4)
- A better ability to repair damaged DNA [6];
- (5)
- Qualitative or quantitative changes in specific cell targets [5];
- (6)
- Changes that allow cancer cells to tolerate adverse or stressful conditions caused by treatment with antineoplastic agents by transforming them into less effective or inactive metabolites [7];
- (7)
- An increasing in the efficiency of the metabolism and biotransformation of cytostatic drugs, leading to their conversion into metabolites without cytostatic effect [8];
- (8)
- The intracellular and intercellular sequestration of drugs in well-defined organelles away from the cellular target, including the lysosomal compartmentalization of hydrophobic, weakly basic anticancer drugs [9].
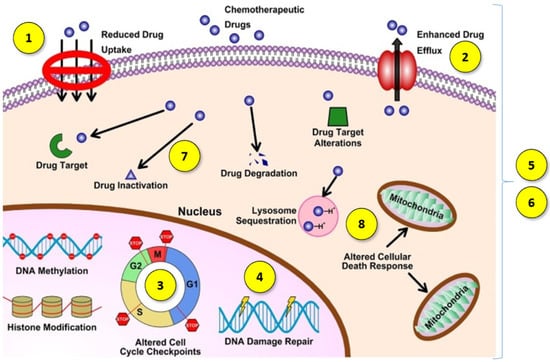
Figure 1.
Diagram of multidrug resistance mechanisms. Reprinted and adapted with permission from Ref. [5] Copyright (2021) Elsevier.
Figure 1.
Diagram of multidrug resistance mechanisms. Reprinted and adapted with permission from Ref. [5] Copyright (2021) Elsevier.
The most commonly used cytostatic drugs, including methotrexate, Ptx, docetaxel (Dtx), vincristine, etoposide, 5-fluorouracil, doxorubicin, daunorubicin, cytarabine and many others, have been found to be resistant to treatment [10]. Therefore, an important goal is to create a therapeutic agent, drug or drug formulation that can overcome these problems and ensure greater clinical effectiveness in anticancer therapy [11]. Currently, effective delivery systems for traditional chemotherapeutic agents as well as combinations of these drugs with chemosensitizing agents (also of natural origin) have been developed. Additionally, the use of naturally occurring plant molecules appears to be a cost-effective way to overcome the resistance of cancer to traditional treatment. However, a considerable challenge in the use of chemosensitizers is the genetic variation between populations, e.g., in gynecological cancer, the multigenic basis of tumours and the accompanying genetic polymorphism that affect the effectiveness of chemosensizitaion and constitutes a significant challenge in the development of an effective treatment [12].
One of the combinations with beneficial therapeutic effects is the co-administration of resveratrol (Res) with docetaxel (Dtx). According to recent outcomes, in addition to the antioxidant, antiinflammatory and chemopreventive properties of Res [13], it also limits multidrug resistance against Dtx [14], which is one of the main causes of failure in cancer therapy with this drug. However, the use of both drugs presents the challenges of poor bioavailability, the unfavourable pharmacokinetics and chemical instability of Res and the poor water solubility and dose limiting toxicity of Dtx. In order to overcome these difficulties, attempts have been made to create different forms of delivery for both agents, which are described below. This review is focused on the latest developments, i.e., published in the last 10 years, in nanoparticles for the delivery of Dtx, Res and for the combined delivery of these two drugs. The aim of this review was also to summarize the synergistic mechanism of action of Dtx and Res on cancer cells. This subject is important and current considering the increasing interest in the use of a combination of chemosensitizers and cytostatics for overcoming cancer resistance to treatment and the development of their delivery systems.
2. Nanoparticles for Drug Delivery Application
The use of nanoparticles (NPs) as drug delivery systems has received much attention in the context of cancer treatment in recent years. NPs allow for the improvement of the pharmacokinetics of poorly soluble hydrophobic drugs and cancer-specific drug delivery by passive or active targeting strategies, which can promote their preferential accumulation in tumour tissues [15]. Nanocarriers are characterized by their nanometric size (<1000 nm), high surface area-to-volume ratio and favourable drug release profiles [16]. Therapeutic agents can either be encapsulated into nanocarriers or covalently bonded or adsorbed onto the surfaces of these carriers [17]. The advantages of NPs include enhancing the safety, pharmacokinetic profiles and bioavailability of the administered drugs, leading to improved therapeutic efficacy compared to conventional therapy. Nanoparticles can be synthesized from various organic or inorganic materials such as lipids, proteins, synthetic/natural polymers and metals. NPs can be classified into several groups, such as polymeric nanoparticles, liposomes, dendrimers, micelles and inorganic NPs, based on the components used for synthesis or the structural aspects of the NPs [17,18,19].
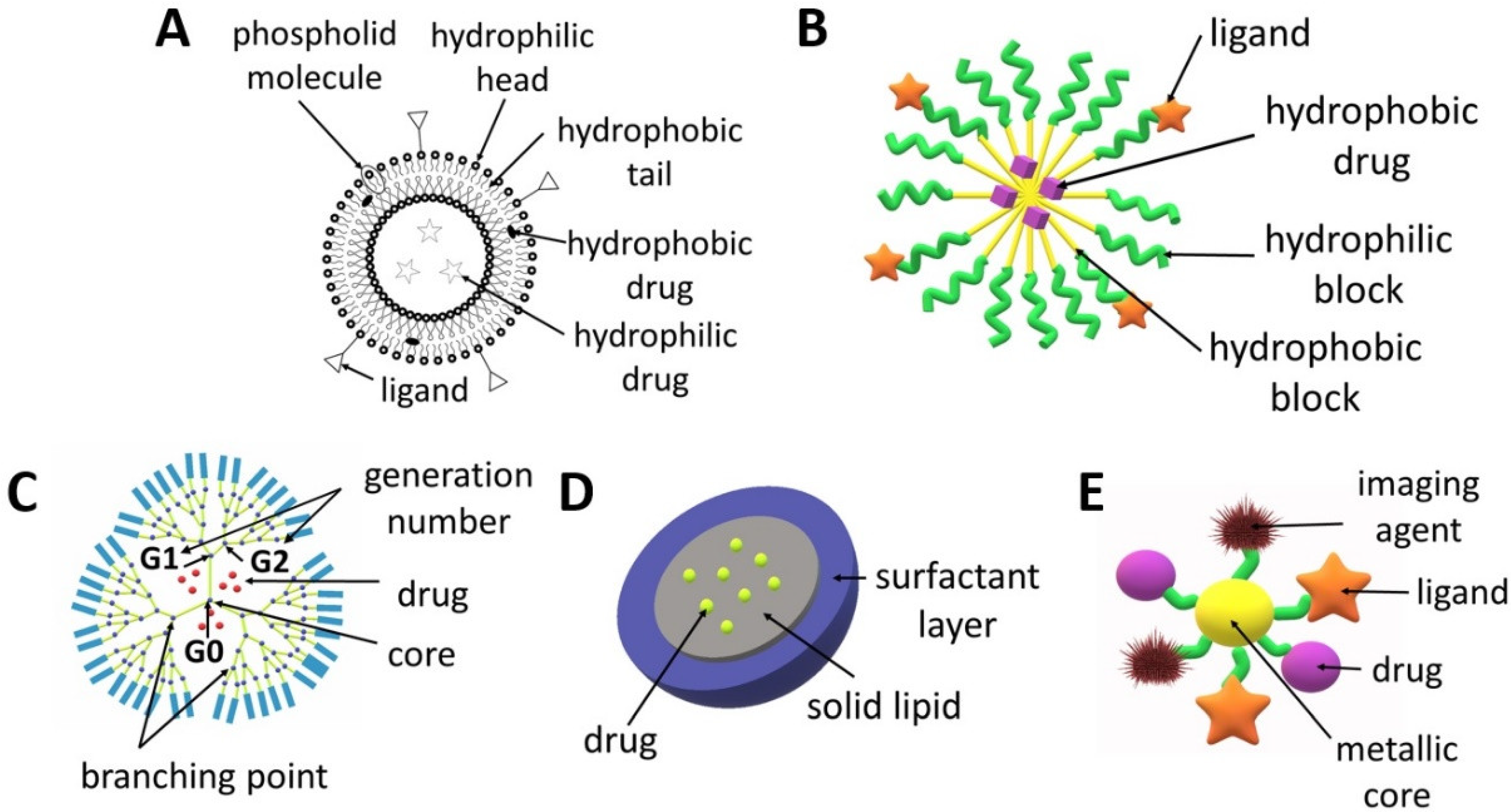
Liposomes (Figure 2A) are spherical structures made of phospholipids and cholesterol that form a bilayer, which enclose a central space for the encapsulation of various molecules ranging from small to large in terms of molecular weight such as DNA or drugs. The main advantages of liposomes include a high drug loading capacity, the possibility of the simultaneous delivery of hydrophilic and hydrophobic active agents and the fact that the entrapment of molecules in liposomes increases their circulation time in the blood [20]. The structure of liposomes can be easily modified in order to obtain the expected therapeutic effect [21]. In addition to an active targeting strategy which uses ligands (both small molecule ligands and antibodies), liposomes can be designed in such a way so that the drug is released in a specific tumour microenvironment or as a result of strictly defined conditions, e.g., under the influence of light, enzymes, pH or ultrasound [22]. The possibility of using various strategies to target liposomes at cancer cells reduces the risk of the negative side effects of chemotherapy on healthy cells. The choice of the preparation method for liposomes is determined by the desired properties of the liposomes (e.g., size, half-life), repeatability of the process, cost, properties of the liposome components used for synthesis, properties of the encapsulated drugs, type of solvent and liposome application processes. The most frequently used technique for liposome production is thin-film hydration, which involves dissolving lipids in an organic solvent followed by its evaporation and then the dispersing of the obtained film in water. The disadvantages of this method are the low efficiency in terms of drug encapsulation and the obtaining of large and heterogeneous particles that require further processing. Higher encapsulation efficiency can be ensured by the reverse-phase evaporation, solvent injection and dehydration–rehydration techniques. Typically, the liposomes require post-production treatment, e.g., sonication, extrusion and high-pressure homogenization. A more advanced method for obtaining liposomes is the microfluidic-based technique, which consists of the controlled mixing of the components and their flow through tubes with a defined diameter from tens to hundreds of micrometres. This technique enables precise control with respect to their size and size distribution. This method can be combined with in-line sample purification and drug loading to develop a fast, one-step process that produces high concentrations of reproducible drug-loaded liposomes with high efficiency [20,23,24,25]. An interesting type of liposomes are magnetoliposomes, which possess a magnetic core surrounded by a double lipid layer. Due to this structure, liposomes can be excited by magnetic radiation inducing local hyperthermia within the tumour [26,27]. There are numerous reviews focusing on trends in the design and use of liposomes for tumour targeting [20,24,25,28,29], comparison of liposomes and polymersomes [30,31] or localized delivery of liposomal formulations of drugs, which have been investigated pre-clinically and clinically in the last ten years [32].
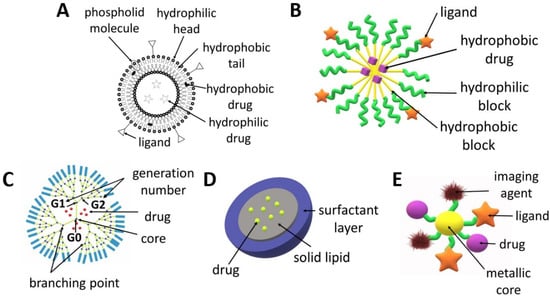
Figure 2.
Scheme of the structures of drug delivery systems: (A) liposome, (B) micelle, (C) dendrimer, (D) solid lipid nanoparticle, (E) metallic nanoparticle.
Dendrimers (Figure 2C) can be made of various compounds, e.g., poly(propylene imine) (PPI), poly(L-lysine), poly(amidoamine) (PAMAM) and PAMAM-organosilicon (PAMM-OS). They possess a predictable shape, size, molecular weight and structure, which allows for the design of carriers with specific properties (e.g., lipophilicity, affecting the penetration through cell membranes) or the modification of adjustable ends, which allows to obtain dendrimers with specific targeting cancer cells and improved safety profile [33]. Dendrimers can increase the stability, solubility and bioavailability of active agents [34]. They can be used to deliver drugs, genes or contrast agents. Dendrimers are obtained as a result of a sequence of reactive steps through a step-by-step controlled synthesis related to molecular chemistry and polymer chemistry. A repeating structure of monomers is used, creating successive generations and an extensive structure of dendrimers. The synthesis of dendrimers involves divergent or convergent growth methods. In the convergent growth method, the synthesis commences with the component elements (branches), which are then combined into a single dendrimer structure. Due to the complexity of dendrimer synthesis processes and their associated costs and structural control problems, accelerated methods of dendrimer synthesis were developed, such as the double exponential growth technique, double-stage convergent method, hypercore approach, hypermonomer method or the branched monomer approach [33,35,36,37]. The properties, structure, synthesis and use of dendrimers were characterized in several recent review articles [35,38,39,40].
Polymeric micelles (Figure 2B) are small (50–200 nm), core-shell structures which ensure the extended circulation of drugs by avoiding uptake by the reticuloendothelial system. They are obtained from various kinds of amphiphilic polymers, e.g., copolymers of poly(ethylene glycol) (PEG), poly(lactide) (PLA) or poly(ε-caprolactone) (PCL) [41]. The micelles are composed of a hydrophilic shell and a hydrophobic core. Micelles obtained from amphiphilic block copolymers are more stable and ensure more versatility compared to lipid nanoparticles [42]. Surface modifications of polymeric micelles can also provide active targeting to cancer cells. Additionally, the release of drugs from micelles can be activated by the tumour microenvironment (pH, enzymes, temperature) or by external factors (e.g., heat, light, ultrasound and the magnetic field) [43,44,45]. Polymeric micelles are the most frequently obtained by means of three methods: direct dissolution, solvent evaporation or dialysis [46]. The choice of method for forming micelles from block copolymers depends on the solubility of the copolymer. If the copolymer is relatively water soluble, micelles can be formed by using the direct dissolution method, in which the copolymer is simply added to the aqueous media at a concentration above the critical micellar concentration and the drug is allowed to partition into the core of the micelles [47,48]. For more hydrophobic copolymers, the dialysis or film hydration methods can be utilized. Film hydration involves the dissolution of the copolymer and drug in a volatile solvent, which is then evaporated to leave a film in the bottom of a vial. Buffer or water is then added under heated conditions and agitated to dissolve the polymer film [49,50]. In dialysis, the copolymer and drug are solubilized in a water-miscible organic solvent followed by dialysis against aqueous media to remove the solvent, with the micelles being formed with the addition of water. The co-solvent evaporation procedure involves the dissolution of the copolymer in a volatile, non-water-miscible organic solvent, with this being added into a rapidly stirred aqueous media, with or without a surfactant, followed by the evaporation of the solvent [47,51]. In this technique, the drug may be loaded during the formation of micelles or via a two-step process when the drug is loaded into the aqueous micellar solution [52]. The recent advances in polymeric micelles have been described in numerous articles focused on their design, characterization and biological significance [53], anticancer drug delivery [54,55], the multimolecular interactions between block copolymers and the loaded drugs, proteins or nucleic acids [56], the stability of micelles [57], techniques for their characterization and assessment in biorelevant conditions [58] and the stimuli-sensitivity of micelles [59,60,61].
Metal NPs (Figure 2E) have also garnered specific attention due to their potential to serve as multipurpose agents. Metallic and magnetic NPs can be used in magnetic resonance imaging and photothermal therapy [62,63]. They are regarded as one of the smallest nanocarriers because their diameter usually does not exceed 50 nm. AuNPs can be obtained via chemical, physical and biological synthesis. Metallic NPs can be surface-functionalized with a drug, ligand, protein, peptide, antibody, contrast agent, enzyme or nucleotide, which offers a wide range of clinical possibilities. The great advantage of these nanocarriers is the ability to control drug release depending on environmental factors, e.g., pH. Gold, silver, iron and/or iron oxide, zinc, titanium, cerium oxide, nickel, copper, magnesium, barium, calcium and bismuth-based metal NPs have been reported for cancer treatment [62,64].
Solid lipid nanoparticles (SLNPs), which are characterized by a size of 50–500 nm, are obtained from lipids that remain solid at room temperature and at human body temperature. They are composed of a lipid monolayer enclosing a solid lipid core (Figure 2D). SLNPs are biodegradable, biocompatible and non-toxic. Two of the most popular methods of obtaining SLNCs are the hot homogenization technique and the solvent emulsification and diffusion method [65]. Although SLNPs can transport both hydrophobic and hydrophilic agents, they are especially useful in the delivery of hydrophobic drugs due to their high affinity to the core [25]. The feature that distinguishes SLNPs from non-lipid nanocarriers is their easy uptake by cells through the lipid bilayer. They are a good nanodevice for the delivery of inhaled and transdermal drugs [66,67].
A number of these delivery systems have already been approved by the US Food and Drug Administration (FDA) for the treatment of cancer in humans with various drugs. The examples include: a nanoparticle combination of Ptx with albumin (Abraxane®) [68], pegylated liposomes with Ptx (Caelyx® (Europe), Doxil® (US)) [69], non-pegylated liposomes with Ptx (Myocet®) [70] or a pegylated form of cytarabine used in the intrathecal treatment of meningitis in the course of lymphoma (DepoCyte®). However, there is still no effective delivery system for Dtx and Res registered as a medical product, although numerous clinical trials are being conducted.
3. Docetaxel
3.1. Docetaxel as an Anticancer Drug
Dtx is a chemotherapeutic agent which is used in the treatment of a variety of cancers in monotherapy and combination therapy. Dtx has demonstrated antitumour activity against recurrent ovarian cancer, non-small cell lung cancer, breast cancer, squamous cell carcinoma of the head and neck and gastric cancer [71].
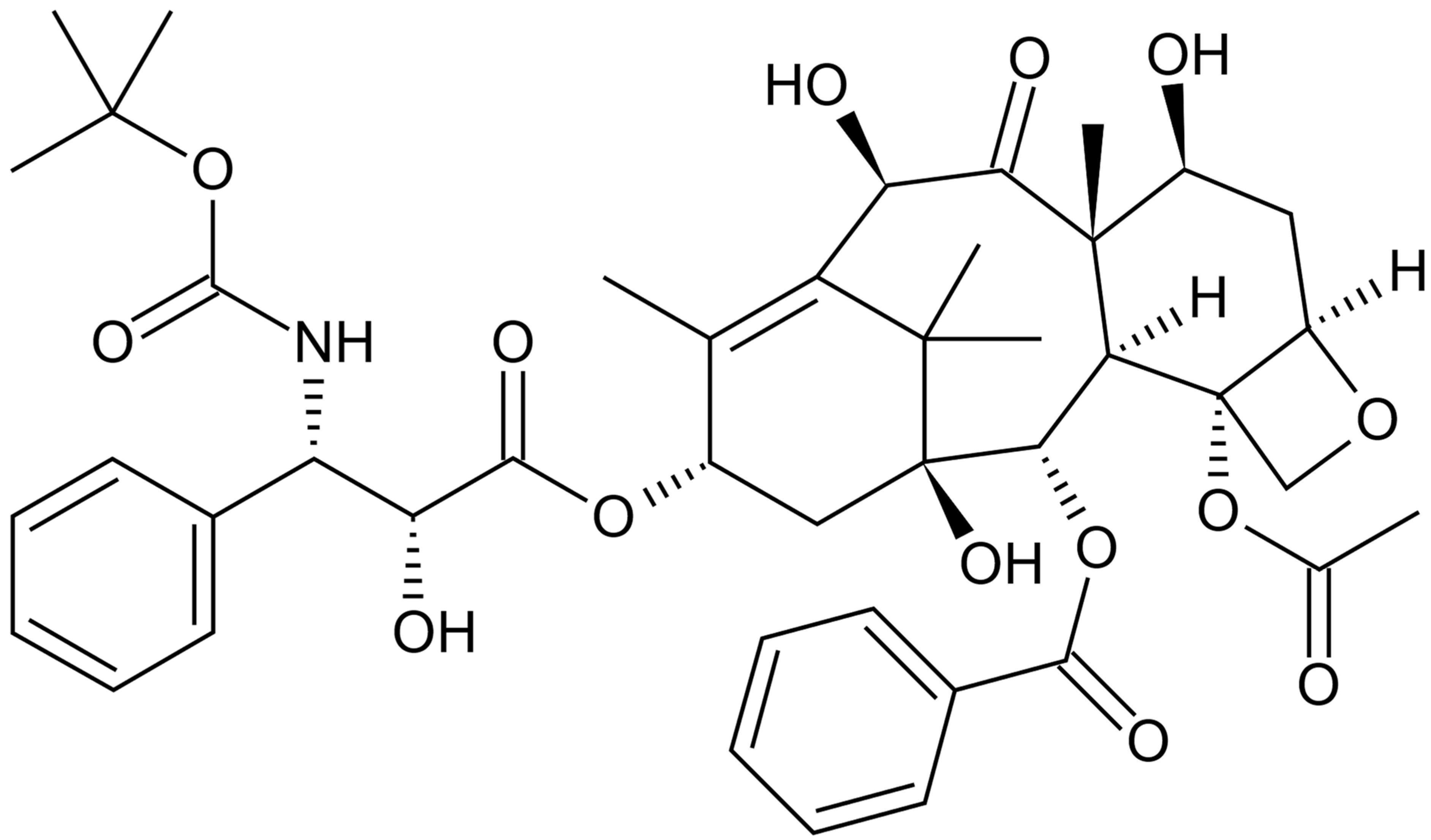
Dtx (Figure 3) is a cytotoxic taxane, semisynthetic product obtained by the esterification of 10-deacetylbaccatin III, which has been isolated from the needles of Taxus baccata since 1981. After intravenous administration, Dtx has a large volume of distribution, binds to plasma proteins and is metabolized by the cytochrome CYP3A4 [72]. Dtx is an antimicrotubular agent that mainly exerts a cytotoxic effect by disrupting the microtubule network in cells that is essential for mitotic and interphase cellular functions (it supports and stabilizes the formation of microtubules and prevents the depolymerization of microtubules), thus inhibiting proper cell division [73].
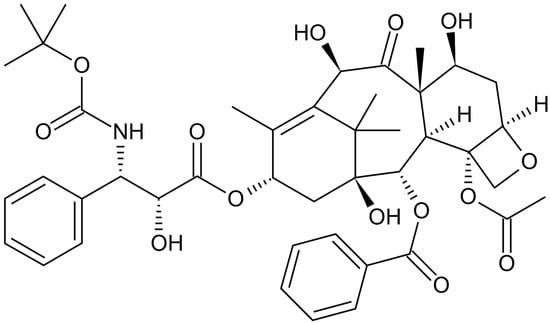
Figure 3.
Chemical structure of docetaxel (Dtx).
Dtx toxicity and its systemic side effects are one of the serious problems that limit the effectiveness of treatment in humans. Neutropenia is one of the most common adverse reactions, occurring in 50–80% of patients treated with the basal dose of 100 mg/m2. In addition, hypersensitivity reactions (HSRs) characterized by hypotension, bronchospasm and/or generalized rash/erythema, skin toxicity associated with a risk of major infection, fluid retention, hypoalbuminemia and cardiac, renal or hepatic dysfunction are common. Dtx can also cause neurotoxicity (motor and sensory neuropathies: 40%), asthenia (60–70%), stomatitis and diarrhoea. Often, during therapy, it is necessary to reduce the Dtx dose to 30–75 mg/m2 (in 5–25%) due to toxicity, despite the use of premedication with corticosteroids (dexamethasone 8 mg, betamethasone 8 mg or prednisolone 50 mg) and the prophylactic use of granulocyte colony-stimulating factors (G-CSF) [73,74,75].
The second major problem associated with the effective action of Dtx against cancer cells is the emergence of treatment resistance, which results in a decrease in the anticancer effect. The first possible resistance mechanism is related to the altered expression of the β-tubulin isotype. Isotype I is comprised of 80–99% cellular β-tubulin in benign cells. On the other hand, an increase in the amount of the β-III isotype increases the dynamic instability of microtubules, impairs the rate of microtubule assembly and increases resistance to taxanes. The second Dtx resistance mechanism follows the classical pattern of MDR and may be reversible with appropriate treatment. Another source of Dtx resistance may be an increase in caveolin-1 expression, which is the main component of membrane vesicles involved in small molecule transmembrane transport and intracellular signalling [74].
3.2. Docetaxel Drug Delivery Systems
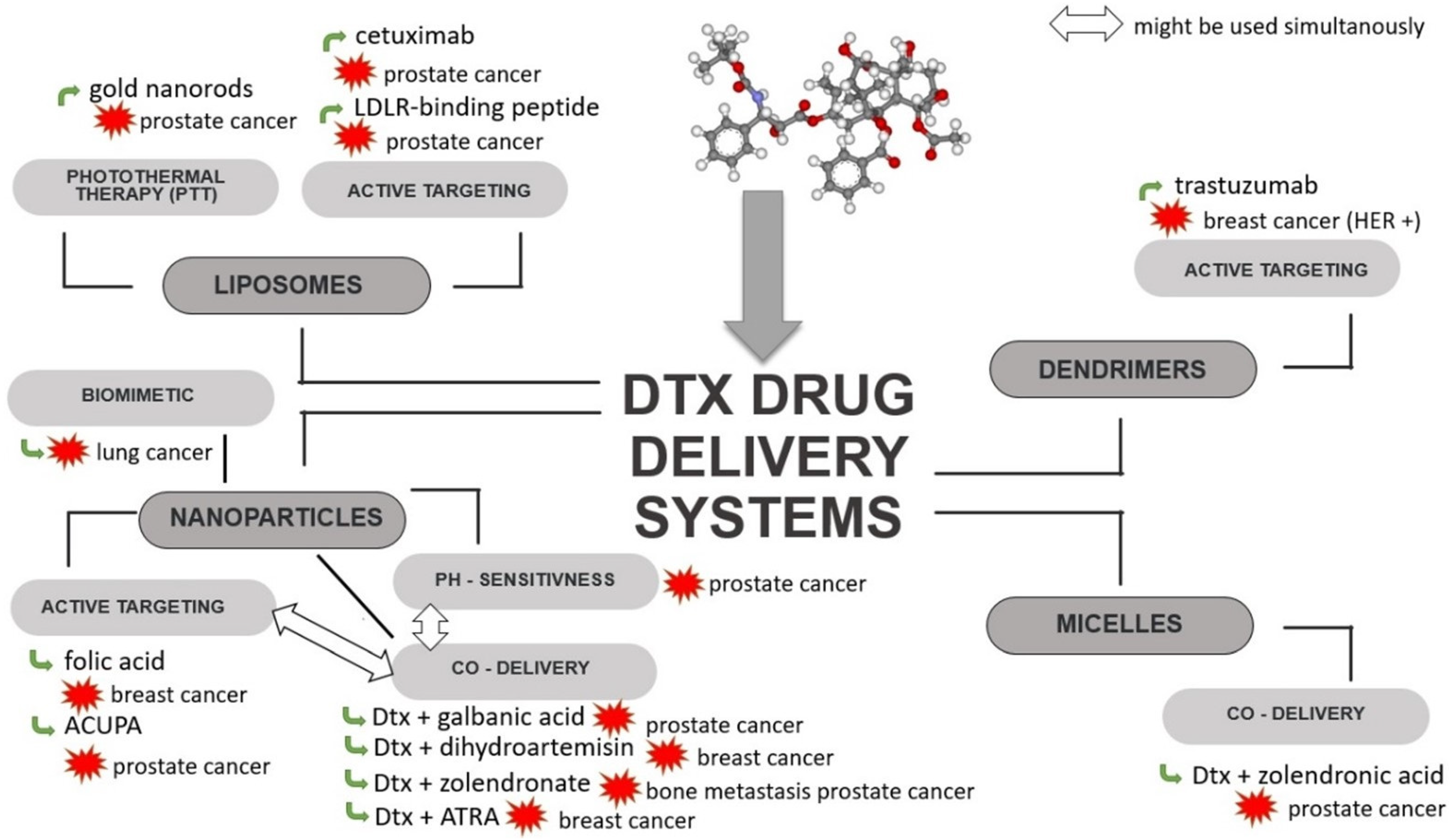
In order to overcome the limitations associated with Dtx, such as its dose-limited systemic toxicity, the effect of tumour drug resistance or the problem of delivering the therapeutic concentration directly to the tumour, efforts are being made to create an advanced delivery system targeting tumour cells. To achieve this goal, several kinds of Dtx carriers have been developed, such as liposomes [76,77,78,79], solid lipid nanoparticles (SLNPs) [80,81,82,83], dendrimers [84,85,86], nanoparticles (NPs) [87,88,89,90,91,92,93,94,95,96] and micelles [97] (Figure 4, Table 1), all of which will be described in this section.
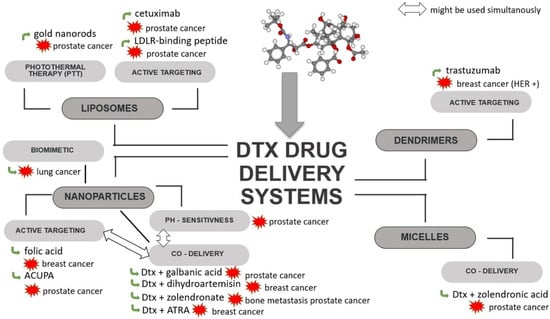
Figure 4.
Scheme of strategies used for Dtx-loaded delivery systems. ACUPA—((S)-2-(3-((S)-5-amino-1-carboxypentyl) ureido) pentanedioic acid; ATRA—all-trans-retinoic acid; Dtx—docetaxel; HER—human epidermal growth factor receptor; LDLR—low-density lipoprotein receptor.

Table 1.
Examples of Dtx–loaded delivery systems for anticancer therapy.
Multifunctional liposomes have been developed for the delivery of Dtx and modified with RLT (low-density lipoprotein receptor (LDLR)—binding peptide), PEG and gold nanorods (GNRs). These liposomes can be used for combined chemo- and photo-thermal therapy (PTT) of cancer. Decoration with RLT allows for the active targeting of tumour cells with the overexpression of LDLRs, which is associated with an increased demand for fatty acids by rapidly dividing cells. With the use of GNRs and near-infrared radiation (NIR), which has ability to penetrate deep tissue, local heating occurs, causing cell damage and death as a result of necrosis or apoptosis [76].
Immunoliposomes targeted with epidermal growth factor receptor (EGFR) and loaded with Dtx have been tested against prostate cancer. Cetuximab, which was used to increase the cellular uptake of liposomes, is an anti-EGFR chimeric-murine monoclonal antibody, a biological drug that competitively binds to the EGFR on EGFR-positive cancer cells (e.g., DU145). It has been observed that cetuximab-modified liposomes show increased cellular uptake and cell selectivity compared to non-targeted liposomes [77]. Also, liposomes functionalized with transferrin (TF) developed for the delivery of Dtx may show advantages in the treatment of prostate cancer. An in vitro cytotoxicity test showed higher toxicity in TF-modified liposomes compared to unmodified nanoparticles. Moreover, modification with transferrin resulted in a slow and sustained release of Dtx, most likely through liposome stabilization [79]. Another example of Dtx immunoliposomes targeted by a surface monoclonal antibody are trastuzumab liposomes prepared to target breast cancer cells overexpressing HER-2 receptors. The increased efficacy of trastuzumab-functionalized and encapsulated Dtx liposomes compared to free Dtx and non-Dtx liposomes with trastuzumab was demonstrated against breast cancer cells (MDA-MB-453 and SKBR3) [105]. However, the potential of EGFR- and TF-functionalized liposomes require further study to confirm their effectiveness in vivo.
An example of dendrimers that actively target tumour cells is the system obtained by the conjugation of PAMAM with trastuzumab and Ptx or Dtx. The developed dendrimers showed high antitumour efficacy against SKBR-3 cells that are characterized by the overexpression of HER receptors and low toxicity against HER-negative tumour cells [86].
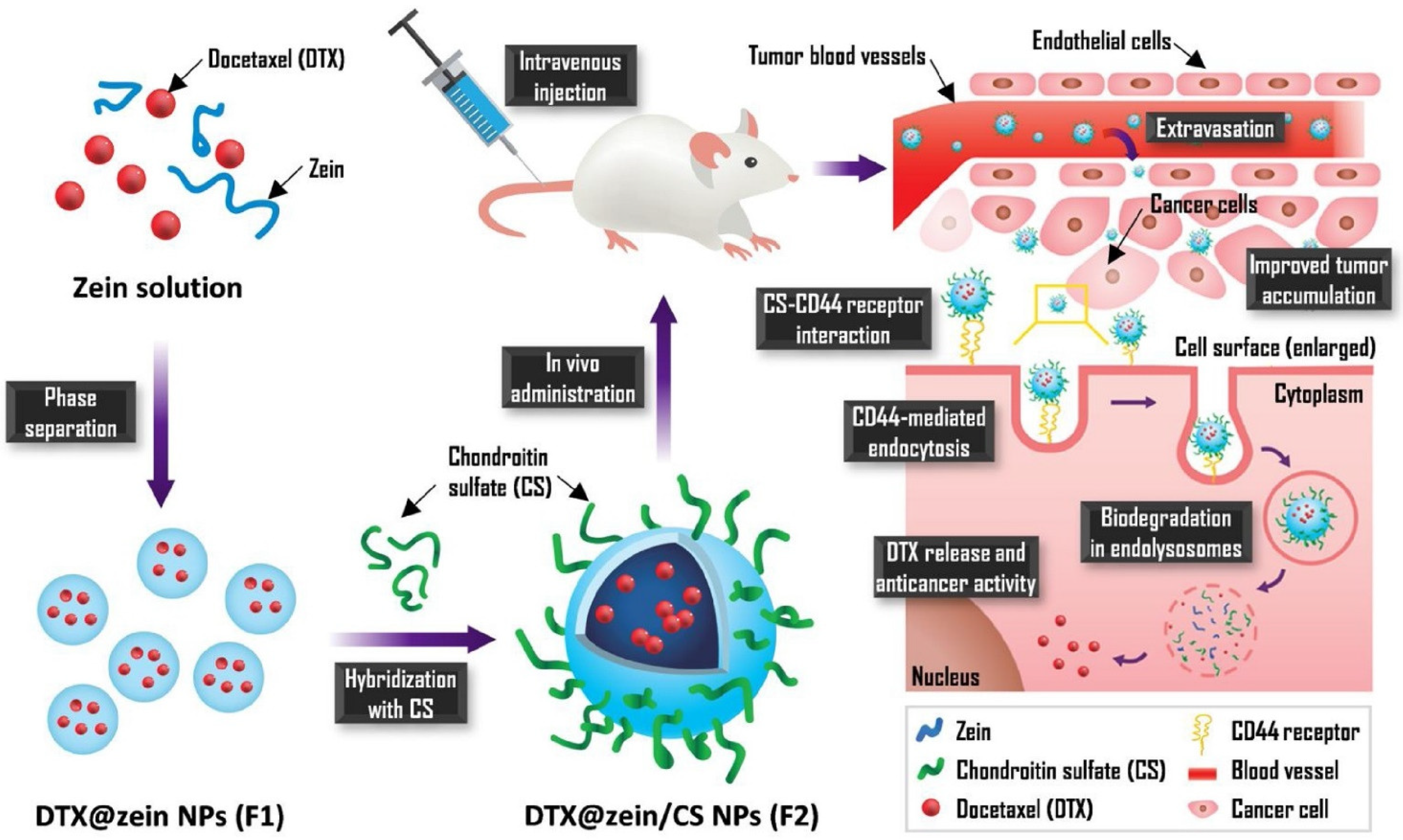
A recently published study on nanoparticles obtained from chondroitin sulphate (CS) and zein and loaded with Dtx indicated very favourable properties and antitumour effects in comparison to Taxotere®. The NPs enabled a 23% increase in cellular uptake and 35.5 times greater accumulation in the tumour. Zein/CS NPs were obtained using the solvent displacement method followed by CS hybridisation during the self-assembly process via electrostatic interaction. After intravenous administration, the NPs are expected to pass beyond the blood vessel and reach cancer cells due to interaction between CS and the CD44 receptor. Receptor-mediated endocytosis is responsible for improving cellular uptake efficiency. The mechanism of action of these NPs is shown in Figure 5 [99].
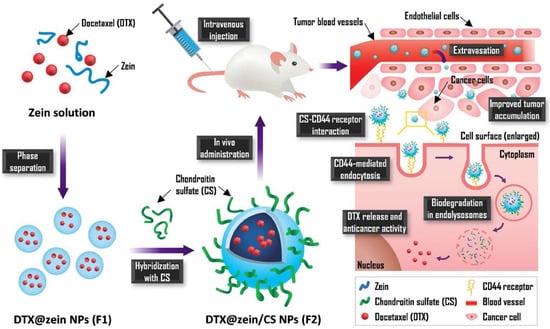
Figure 5.
Scheme for the preparation Dtx-loaded CS-hybridized zein NPs and their mechanism of action. Reprinted with permission from Ref. [99]. Copyright (2021) Elsevier.
Dtx-loaded nanoparticles obtained from poly(lactide-co-glycolide) (PLGA) and modified with folic acid (FA) were developed by Poltavets et al. An in vitro study demonstrated the ability of the particles to overcome MDR and their higher cytotoxicity against cells overexpressing folic acid alpha receptor (FRα) compared to the free drug. The efficacy of these nanoparticles has also been confirmed in vivo [14]. Functionalization with folic and was also used in gold nanoparticles (Au-NPs) coupled to Dtx. An in vitro study of Au/NPs/FA/Dtx demonstrated that such particles achieve 50% higher cytotoxicity compared to free Dtx [98].
Biomimetic nanoparticles developed for the targeted delivery of Dtx to lung cancer combined the latest advances in nanomedicine with naturally occurring cell membranes to increase the antitumour activity of the cytostatic drug. They were obtained by the incorporation of PLGA NPs loaded with Dtx into platelet membrane (PM). Coating the surface of nanoparticles with PM is advantageous because of their subsequent immune escape ability and cancer targeting properties, resulting in enhanced tumour growth suppression. An in vitro study demonstrated that PM-coated NPs loaded with Dtx have an ability to inhibit cell growth at a concentration range of 0.31–10.00 μg/mL, which was also confirmed in vivo [104].
3.2.1. Carriers for Co-Delivery of Dtx with Another Drug
In addition to the delivery strategies of Dtx described above, more advanced delivery systems have also been developed, e.g., ligand-targeted NPs for the co-delivery of Dtx and another anticancer agent. An example are NPs co-loaded with Dtx and galbanic acid (Gba) for targeting prostate-specific membrane antigen (PSMA)-positive cancer cells. The NPs were obtained from poly(lactide)-co-poly(ethylene glycol) (PLA-PEG) modified with ((S)-2-(3-((S)-5-amino-1-carboxypentyl)ureido) pentanedioic acid (ACUPA), which is an inhibitor targeting PSMA [103]. It should be noted that similar polymer nanoparticle systems, including those targeting PSMA, have been the subject of promising clinical trials, as described in Section 3.2.1.
pH-sensitive NPs for the simultaneous delivery of Dtx and dihydroartemisin (Dha) have been investigated for the treatment of transient breast cancer. The mechanism of synergistic action of Dtx with Dha was explained by induction of apoptosis mediated by mitochondria. As a result of the simultaneous action of both active agents, p53 expression and ROS levels are increased and the release of cytochrome C to the cytoplasm is promoted, which activates caspase 3 [102]. Another pH-sensitive nanoparticle developed for the co-delivery of zoledronate (Zol) and Dtx are bone-targeted calcium phosphate-polymer hybrid NPs, which were tested against prostate cancer bone metastasis. CaP (calcium phosphate) was used to obtain the pH-sensitivity of the micelles. Good antitumour properties were confirmed in the nude mice model because the use of high doses of Dtx/CaP/HP caused more a significant decrease in tumour volume compared to free Dtx and Zol [101].
A co-delivery system for Dtx and all-trans-retinoic acid (ATRA) was obtained from the poly(β-amino ester) (PBAE). ATRA, a derivative of vitamin A, is effective against some cancer cell lines; however, due to its low water solubility, low plasma concentration and side effects, its clinical use has been limited. Moreover, it was found that in combination with traditional chemotherapeutic agents, ATRA exhibits antitumour activity against metastatic breast cancer. An in vitro study of ATRA-PBAE-NPs revealed not only a successful pH sensitive drug release and the synergistic cytotoxic activity of ATRA and Dtx but also their antiangiogenic properties [100].
3.2.2. Clinical Trials of Docetaxel Nanoparticles
Treatment with Dtx has been the subject of numerous clinical trials and more than 1700 of these can be found in the ClinicalTrials.gov (provided by the US National Library of Medicine) register. Among the many research studies of the use of the traditional intravenous form of Dtx, there are several clinical trials that have evaluated NPs as delivery systems of Dtx: five clinical studies involving the use of the BIND-014, three testing results of LE-DT treatment, one regarding the application of Dtx-PNP (a polymeric nanoformulation of Dtx) and two evaluating Cripec (Table 2). LE-DT is a liposome-entrapped Dtx designed by NeoPharm and studied for the treatment of solid tumours (pancreatic cancer and prostate cancer). A phase one study (NCT01151384) of LE-DT showed that the use of this drug formulation had clinical benefits for 41% of patients [106]. Phase two studies were launched, and one of them, on metastasis in pancreatic cancer, was completed in 2012, with the second one, which was withdrawn in 2010, testing LE-DT in prostate cancer. Another example of a carrier developed for Dtx that has reached phase two is BIND-014, which is composed of Dtx encapsulated in a polymer core made of a hydrophobic poly(lactide) surrounded by a hydrophilic poly(ethylene glycol) conjugated with a small molecule of PSMA-targeting ligands. In the first phase of clinical trials, BIND-014 showed satisfactory properties. In NCT01300533, a clinical study was conducted in 52 patients with various types of neoplasms to determine the pharmacokinetics, safety profile and antitumour activity of BIND-014. It was observed that the BIND-014 was well tolerated by patients, had dose-dependent linear pharmacokinetics and a prolonged circulation time. The BIND-014 accumulated in a variety of cancer cells independently of PSMA detection. However, due to difficulties in phase two related to a low response to treatment (NCT02479178) and other factors related to the manufacturer, this research on BIND-014 was suspended [107,108].

Table 2.
Clinical trials of Dtx-loaded NPs based on https://clinicaltrials.gov/ (accessed on 10 February 2021).
Another example of polymeric nanoformulation for the delivery of Dtx is Dtx-PNP, which has been tested preclinically in the orthotopic mouse model and was shown to be more effective in inhibiting tumourigenesis than free Dtx or gemcitabine. The NCT01103791 study was conducted in 19 patients with various types of cancer (e.g., colon, rectum, cervix, breast, bladder, kidney, pancreas cancer) and in various doses ranging from 20 to 75 mg/m2. The goal of this study was to find the maximum tolerated dose (MTD) and dose limiting toxicity (DLT). The maximum dose of Dtx-PNP was found to be 75 mg/m2, despite the fact that all patients developed a symptom of neutropenia, which was normalized within 7 days. In addition, it was found that the partial remission was at the level of 22%. However, these clinical trials involved a small control group, and further research is necessary [87]. Recently, in December 2020, a phase two clinical trial (NCT03742713) of Cripec® in ovarian cancer treatment was completed; however, the results have not yet been presented. Cripec® is novel formulation that consists of Dtx-entrapped core-cross-linked polymeric micelles (Dtx-CCL-PMs). It was developed by Cristal Therapeutics via the conjugation of Dtx to linker and subsequent covering with polymer to achieve a stable nanoparticle. An in vitro and in vivo study showed that Cripec® is more safe and efficient than free Dtx in breast cancer treatment [109,110].
3.3. Summary
Dtx is a semisynthetic anticancer mitotic chemotherapy drug used against a wide range of solid tumours, including breast, lung, head, prostate, neck, non-small cell lung and ovarian cancer. However, due to the limitations of Dtx, such as its low water solubility, systemic toxicity and severe allergic reactions, various kinds of NPs have been formulated for Dtx delivery, such as liposomes, dendrimers, micelles and SLNPs (Table 1). The achievements of the last 10 years clearly demonstrate the prevalence of advanced systems designed for the targeted delivery of Dtx. For this purpose, different kinds of ligands have been tested, e.g., RLT, TF and EGFR, and these tests demonstrated that functionalized NPs have a higher efficiency than non-targeted delivery systems. Another direction of the research has been focused on the development NPs suitable for the delivery of combinations of docetaxel and other drugs (e.g., Gba, Dha, Zol and ATRA), and these have exhibited enhanced cytotoxicity against cancer cells compared to the single drug. The effectiveness of the carriers of Dtx and Dtx with other drugs developed to date is frequently supported by solid experimental data obtained from in vitro and in vivo studies. It is very promising that some of the NPs have already reached the clinical study stage (Table 2); however, their number is still very limited.
4. Resveratrol
4.1. Resveratrol as an Active Agent
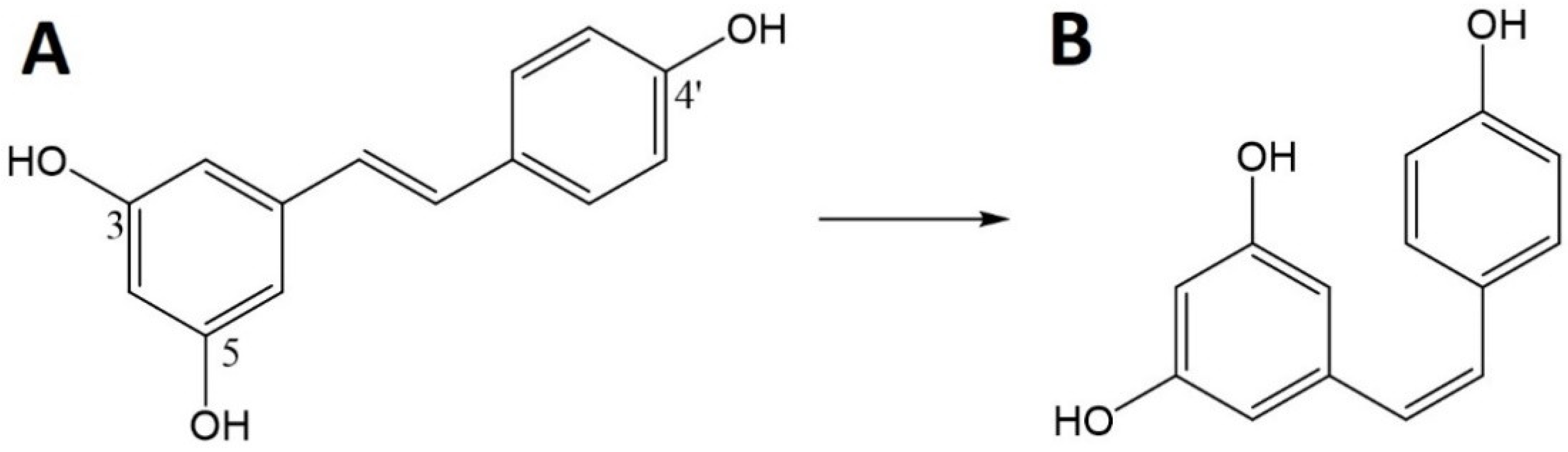
Res (trans-3,4′,5,-trihydroxystilbene) is a natural polyphenolic molecule. This polyphenolic stilbene structure has two aromatic rings connected by an ethylene bridge and three hydroxyl groups [111,112].
Res can exist in the form of two isomers (Figure 6), cis and trans. In nature, the trans isomer is common and the less stable cis isomer is formed from trans-Res by the action of light, UV and heat [113]. However, even the more stable trans-isomer can be easily degraded by numerous factors, both physical and chemical, including temperatures above 37 °C, pH above 6.8 and exposure to light [112]. Res is also classified as a phytoestrogen because it can bind to estrogen receptors [111]. Historically, Res has been associated with Asian medicine and red wine, and then linked with the so-called the French paradox that cardiovascular diseases and hypercholesterolemia were less frequent in the French population. It has now been found that Res is present in many plants, e.g., grapes (Vitis vinifera), mulberries (Morus sp.) and peanuts (Arachis hypogaea) [112,114]. Moreover, research confirms the presence of its high content in the Itadorii plant and tea [115].
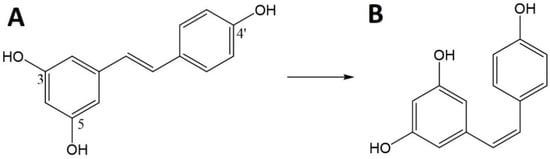
Figure 6.
Chemical structure of resveratrol (Res)—trans (A) and cis (B).
Numerous studies confirm the chemoprotective [115,116], chemopreventive [117,118,119] and chemotherapeutic properties of Res. The chemotherapeutic effect of Res covers a broad spectrum of therapeutic effects such as antiviral [120], antifungal and antibacterial [121], antiaging, antioxidant and anti-inflammatory activity [122]. Additionally, it has a cardioprotective and analgesic effect [123] or can be used as an antiobesity agent [124]. Res also plays a role in the treatment of neurological diseases, such as Alzheimer’s [125] or Parkinson’s [126].
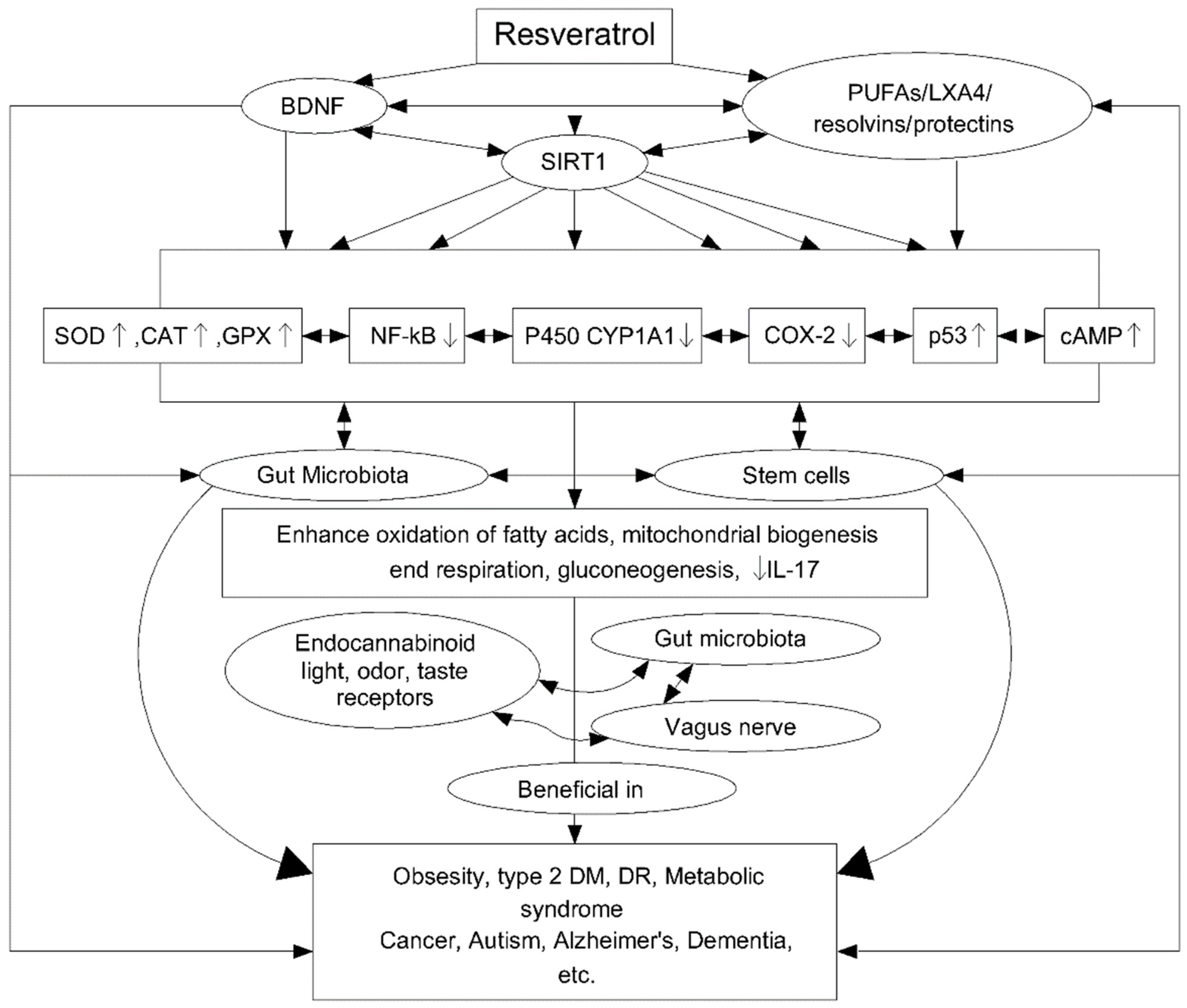
The antioxidant properties of Res are related to the ability to regulate many signalling pathways (Figure 7). Studies have been carried out on peripheral blood mononuclear cells (PBMC) to assess the antioxidant properties of Res under oxidative stress in the cells of middle-aged and elderly people. The Res resulted in a significant reduction in reactive oxygen species (ROS) in both age groups, but with a better performance in the middle-aged group (92.6%) than in the older group (76.7%). The effect of Res on signal pathways was also studied, finding an effect not only on AMP-activated protein kinase (AMPK) and the sirtuins signalling pathway (SIRT1) but also on p38-Mitogen activated protein kinase (MAPK) and PKA and AkT/PKB pathways [122]. In turn, the anti-inflammatory properties of Res are associated with the regulation of cytochrome P450 (CYP1A1), p53, cyclooxygenase enzymes (COX), transcription factor NF-kB, Fas/Fas ligand mediated apoptosis, mTOR and cyclins, various phosphor-diesterases which have an impact on cAMP level and modulate the activity of the Epac1/CaMKKb/AMPK/SIRT1/PGC-1a pathway. This, in turn, affects the oxidation of fatty acids, mitochondrial respiration and their biogenesis and gluconeogenesis. The anti-inflammatory effect of Res is additionally related to its ability to trigger apoptosis in activating T lymphocytes and to inhibit tumour necrosis factor (TNF-α), interleukin-17 (IL-17), the expression of hypoxia-induced factor (HIF-1α) and vascular endothelial growth factor (VEGF) [127].
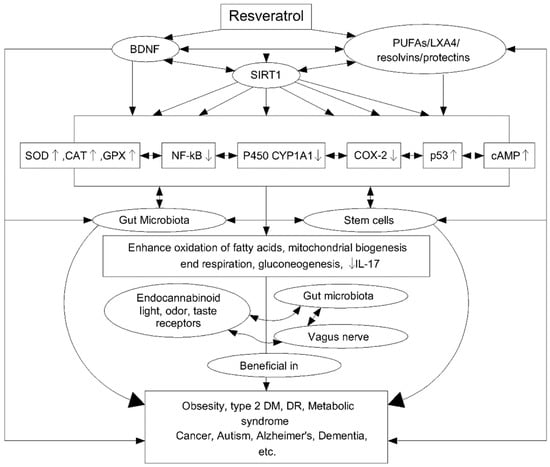
Figure 7.
Scheme of resveratrol (Res) cellular action and its further impact. Reprinted and adapted with permission from Ref. [127]. Copyright (2020) Elsevier.
Furthermore, several reports have indicated potential antiproliferative or anticancer effects of Res per se against many types of human cancers [128], and the first report mention its potential anticancer activity was published in 1997 [129]. The cancer preventive effect of Res has mainly been attributed to its antioxidant and anti-inflammatory/immunoregulatory profiles. It has been reported that Res can downregulate the expression of multidrug-resistant genes encoded for P-gp and inhibit the mammalian target of rapamycin via pyruvate kinase isoenzyme type M2, thereby preventing cancer cell metabolism. Res has shown promising cytotoxic effects on a wide range of solid tumour cells, e.g., breast, liver, prostate, colorectum, pancreas, lung and gliomas [111,114,115].
However, despite high interest in Res as an anticancer drug, the complete mechanism of anticancer activity has not been elucidated and research is continuing. A cytotoxicity study of Res was performed in vitro on melanoma lung metastasis cells and in vivo on a mice model. The results showed that the number of melanoma lung metastasis cell colonies in mice treated with Res was significantly lower. Moreover, 70% of the Res-treated animals survived for 1 month, while all the controls were dead by then [130].
The cytotoxicity of Res has also been proved also in a study of cell cycle arrest and inducing apoptosis of breast cancer cells. Transcriptome and qRT-PCR data showed that among 330 assessed genes, 103 were upregulated after the administration of Res and 227 were downregulated. In addition, Res has been shown to arrest cells in the S phase, to reduce proliferation and to induce apoptosis. The process was dose- and time-dependent [131]. The effect of modulated electro-hyperthermia (mEHT) with the administration of Res and curcumin (Cur) via colorectal cancer as well as the impact of such a procedure on the cell cycle was also studied. By using mEHT and disrupting the cell membrane structure, the cellular uptake of Res and Cur increased, causing cell cycle arrest and the apoptosis of CT26 cells. Moreover, CT26 tumour growth in BALB/c mice was significantly inhibited [132].
4.2. RES-Loaded Nanoparticles
Despite its broad healing properties, Res has several disadvantages that make it difficult to use in medicine. First of all, it is poorly soluble in water and decomposes quickly under the influence of light, oxygen and oxidative enzymes. Due to the fact that it is a sensitive molecule, the trans isomer is easily converted into cis—with much weaker activity. Another obstacle is the rapid metabolism and elimination of Res and its conjugation with sulphates and glucuronic acid, which prevents it from exerting its effect. All the above-mentioned features result in the low bioavailability of Res and mean that its administration does not bring about the expected therapeutic effects [18]. This is the reason behind the study of the development of delivery systems for Res that will improve its biodistribution. The encapsulation of Res in NPs enables it to be protected from decomposition by physicochemical factors, increase its uptake by cells as well as ensure the controlled site or time of release from the carrier, and, thus, this increases the bioavailability of Res. Therefore, this part of the review focuses on the presentation of Res-loaded NPs tested for cancer treatment (Table 3).

Table 3.
The examples of Res–loaded delivery systems for anticancer therapy.
The dosage form that can protect Res from a loss in stability and improve its antioxidant activity may be zein NPs made from a combination of bovine serum albumin (BSA) and conjugates of BSA and caffeic acid (CA). The increase in the stability and antioxidant activity of Res encapsulated in zein-BSA NPs and zein-BSA-CA NPs was observed in comparison to free Res and Res-loaded zein-NPs, suggesting that the developed NPs may be used in the food industry for effective Res supplementation [142].
For medical use, to enhance the low bioavailability (<1%), solubility and stability of Res, Res-loaded NPs were obtained from chitosan (CS) and poly(γ-glutamic acid) (γ-PGA) (Res-chitosan/γ-PGA NPs) using the ionic gelation method. The increased uptake of Res-CS/γ-PGA NPs by colon carcinoma cells in comparison to non-encapsulated Res was observed [143].
Res-loaded gold NPs (Res-AuNPs) were developed and studied for breast cancer treatment. It was found that the level of matrix metalloproteinases (MMPs) and cyclooxygenase-2 (COX-2) facilitating tumour angiogenesis as well as the nuclear transcription factor-κB (NF-κB) and activator protein-1 (AP-1) that promote cancer invasiveness through gene regulation were lowered after the use of Res-AuNPs [133].
Res-loaded NPs have been also developed from poly(ε-caprolactone) (PCL) and tested against murine melanoma. The results of an in vivo study showed that Res-encapsulated PCL-NPs reduced the volume of tumours significantly compared to the control group and free Res. Moreover, they increased necrosis and inflammation in the cancer area [134]. Also, poly(lactide-co-glycolide) (PLGA) was used to produce Res-loaded NPs. The chemopreventive effect of Res-PLGA NPs on prostate cancer cells was investigated. Although the chemopreventive properties of free Res on tumour cells has been the subject of clinical trials, a satisfactory effect has not been achieved, probably due to the poor bioavailability, solubility and stability of Res. As expected, the encapsulation of Res in PLGA resulted in the improved availability of Res, which exhibited cytotoxicity against prostate cancer cells without any undesirable effects on normal cells, even at high doses of Res [136].
Res-loaded SLNPs (Res-SLNPs) were analysed for the delivery of Res to human breast cancer. An in vitro study showed a greater number of the tested cells retained in the G0/G1 phase compared to free Res and reduced cell migration and invasion. In addition, the Res-SLNPs increased the expression level of Bax and decreased the level of Bcl-2, which plays important role in apoptosis [140].
FA-conjugated NPs obtained from HSA and loaded with Res were prepared and evaluated under in vitro and in vivo conditions. It was confirmed that FA-HSA-Res NPs have the ability to continuously and slowly release the drug. Compared to unmodified Res, FA-HSA-Res NPs increased the bioavailability of Res and inhibited the proliferation of neoplastic cells to a greater degree, which was also confirmed in vivo [138].
4.3. Resveratrol Clinical Trials
Res is the subject of numerous clinical trials in a wide variety of diseases, including cancer. The NCT00256334 phase one study of the effectiveness of Res in the treatment of colon cancer has already been completed. The 3 years-trial involved 11 patients. It was shown that oral Res supplementation may play a role in the prevention of colon cancer in humans [144]. The subsequent phase one study NCT00578396 on excised colorectal tumour cell lines involved the administration of mitomycin C and Res. These studies have demonstrated the increased upregulation of p21 (WAF1/CIP1) in cells treated with both drugs compared to cells treated with only one active agent. Two other clinical trials (phase one) on colorectal cancer (NCT00920803 and NCT00433576) have been conducted; however, the post-study information for these studies is poor [145]. The FDA has also registered a clinical trial for gastrointestinal tumours (NCT01476592). During this trial, the influence of Res on notch-1-signaling, effect on patient treatment and patient toleration on Res administration was investigated. It was shown that the administration of Res increased cleaved caspase-3 level in cancerous tissue, which means that the apoptosis in cancer cells was higher [145]. In phase two of the NCT00920556 clinical trial, a Res with bortezomib treatment for multiple myeloma was tested. The 24 patients involved in this study received 5 g of SRT501 (Res) orally daily before breakfast on 20 days of a 21-day chemotherapy cycle for 12 months. However, the obtained data showed an unacceptable safety profile, with no improvement in treatment effectiveness [146]. Another clinical study (NCT01370889) was focused on the effects of Res on the endocrine management of sex hormones in postmenopausal women with breast cancer and obesity (BMI > kg/m2). Measurements of the levels of estradiol, estrone and testosterone in the blood showed no changes, but the administration of Res caused a 10% increase in the concentration of sex steroid hormone-binding globulin (SHBG) [147]. It is worth noting that many studies are being conducted not only with respect to the treatment of disease entities but also in order to investigate the preventive effect of using Res or products containing Res, including in children.
4.4. Summary
Among active natural compounds, polyphenols are becoming highly popular because of their antioxidant, anti-inflammatory, antiaging and anticancer properties. Res is a polyphenolic stilbene derivative that has been shown to have promising cytotoxic effects on a wide range of solid tumour cells, e.g., breast, liver, prostate, colorectum, pancreas, lung and gliomas. However, due to the poor solubility of Res in water, its decomposition under the influence of light, oxygen and oxidative enzymes and the rapid metabolism and elimination of Res, more effective delivery systems are being developed. The several kinds of carriers have been studied for the encapsulation of Res include Res-zein-NPs, AuNPs, PCL-NPs, PLGA NPs, SLNPs and FA-HSA NPs (Table 3). Although most of them have been limited to in vitro study, it has been observed that the encapsulation of Res in NPs enables its protection from decomposition by physicochemical factors, an increase its uptake by cells as well as the controlled site or time of release from the carrier to be ensured, and, thus, the bioavailability of Res can be increased.
5. Co-Delivery of Docetaxel and Resveratrol
It is considered that co-encapsulation of a cytostatic drug with a natural chemosensitizer in the same delivery system can be an effective tool against cancer. This concept may not only combine the healing properties of two active agents but also enable a synergistic effect of action. The beneficial effect of the simultaneous use of a chemotherapeutic agent with a chemosensitizer is a reduction in the MDR effect, which often adversely affects the effectiveness of traditional chemotherapy. On the other hand, a wide range of expected properties, e.g., enhanced bioavailability, protection against biotransformation and controlled release or active targeting can be obtained by using the appropriate materials to form NPs. Moreover, using natural compounds in drug delivery systems may be one of the ways to overcome high costs and the time involved to undertake new drug research.
The aim of this part of the review was to explain the synergistic effects of Res and Dtx and to present the latest nanomedical achievements, in which NPs for the simultaneous delivery of these drugs have been developed.
5.1. Synergistic Effect of Docetaxel with Resveratrol
One of the earliest in vivo analyses of the pharmacokinetics of simultaneously administered trans-Res and Dtx was conducted in 2009 in mice implanted with a Dtx-resistant prostate carcinoma cell line (DU-145). Dtx and Res were administrated subcutaneously via Alzet osmotic pumps for 1 month. Data showed enhanced tumour regression and lower tumour angiogenesis compared to the control group treated only with Dtx [148]. Subsequently, it was found that Res can chemosensitize HER-2 positive breast cancer cells for Dtx action and overcome cancer resistance for its treatment by affecting the Akt axis. It was assessed that HER-2 overexpression through the activation of PI3K/Akt and upregulation of survivin allows cancer cells to escape from cytotoxic effects, e.g., Dtx through early mitotic exit and by activating the multi-drug efflux pump [149].
Further studies showed that Res enhances the cytotoxic properties of Dtx by increasing its intracellular level due to P-gp inhibition and the downregulation of MDR1 [71,128]. The combination of Res and Dtx up-regulates pro-apoptotic genes, provides a synergistic anticancer effect and generates an enhanced cancer treatment effect compared to the single drug. Dtx in combination with Res causes the significant downregulation of BCL-2, BCL-XL and MCL-1 (anti-apoptotic markers), upregulates BAX, BAK and BID (apoptotic markers) and cleaves PARP. Furthermore, the combination of Dtx and Res suppresses the expression of NF-kB p65, which increase the level of inflammatory markers, e.g., COX-2. Moreover, Dtx with Res decreases the levels of survivin protein, part of the inhibitor of apoptosis (IAP) protein family. It is found that a higher survivin level occurs in most cancers, with blocking the cell cycle arrest and restraining apoptosis by binding to caspase 3 and 7 [150,151]. In PCa cells, the combination of Dtx and Res has been shown to block the cell cycle by influencing key regulators and promoting apoptosis. The interaction was found to occur through a p53-dependent and p53-independent mechanism. In turn, the p53 protein is a known inhibitor of CDKs and affects the p21WAF1/CIP1 and p27KIP pathways, which play a significant role in apoptosis by stimulating pro-apoptotic proteins and inhibiting those that block apoptosis [150]. Additionally, one of the newest findings indicated that a combination of Res and Dtx has also an effect on ROS-induced mitochondrial dysfunction and DNA damage [152].
Nevertheless, an explanation for the mechanism of synergistic action of Dtx and Res is still under investigation and has not yet been fully elucidated. Regardless of this, their synergistic effect has been confirmed and is reflected in the increased toxicity of Dtx administered with Res, in comparison to the effect of free Dtx, as well as in the fact that the co-administration of Dtx with Res enables to overcome cell drug resistance, leading to cell death.
5.2. Nanoparticles for Co-Delivery of Docetaxel and Resveratrol
The co-encapsulation of several drugs into the same delivery system enables their simultaneous intracellular release and action. The co-administration of two or more pharmacologically active agents with different mechanisms of action (combination therapy) is recognized as more efficient compared to conventional therapy based on a single therapeutic agent [153,154,155].
Several studies have been carried out to evaluate the synergistic effects of Res and Dtx loaded in a nanoparticle delivery system (Table 4). The effect of Res and Dtx co-encapsulated in polymeric micelles prepared from methoxyl poly(ethylene glycol)-poly(D,L-lactide) (mPEG-PDLA) was analysed. It was found that the 1:1 ratio of Dtx and Res is the most favourable for the synergistic effect of both compounds on MCF-7 cells. The loading properties of Dtx and Res were comparable, and their release was rapid for the first 12 h (80% of drugs released until 72 h). The cytotoxic effect of micelles loaded with Dtx and Res was significantly higher compared to free drugs and single-drug loaded micelles [71].

Table 4.
Examples of Res and Dtx-loaded delivery systems for anticancer therapy.
Planetary ball milled (PBM) NPs functionalized with folic acid and co-loaded with Dtx and Res were developed for the treatment of drug resistance in prostate cancer cells. After preparation in a milling jar, the NPs containing Dtx and Res were coated with a FA-PCL-PEG copolymer. This innovative technique for the formation of nanoparticles loaded with a cytotoxic drug has been described in US Patent 8,231,907. This method uses a milling jar containing heat-absorbent zirconium oxide planetary milling balls. The milling balls rotate about their own axis and in the opposite direction around the common axis of the chamber wheel. Through the appropriate use of controlled centrifugal forces as well as the regulation of the length and number of cycles and the number of zirconium oxide balls, NPs with the desired properties are created [151,157]. Cytotoxicity and cell viability was tested on PC3 cells and a cell line resistant to Dtx (PC3-R). Time and dose-dependent Dtx re-sensitization after the administration of FA-Dtx+Res-PBM NPs was observed. The IC50 of Dtx and Res was reduced to a greater degree after treatment with FA-Dtx + Res-PBM NPs compared to free drugs [151].
Another ligand used to target NPs co-loaded with Dtx and Res is EGFR. Core-shell lipid-polymer hybrid NPs (LPNs) conjugated to EGFR were developed for the treatment of non-small cell lung cancer (NSCLC). LPNs are considered to be appropriate carriers for creating complex targeted delivery systems due to the fact that they combine the biosafety of liposomes and the versatility of polymeric materials. An in vitro study showed that the cellular uptake of EGFR conjugated LNPs was higher in HCC827 cells with a positive mutation (EGFR M+). The EGFR-RES+Dtx-LPNs exhibited a higher level of efficiency than single drug loaded NPs, which proves the synergistic effect of Dtx and Res. Also, in vivo tests revealed a the greater accumulation of EGFR-Res+Dtx–LPNs in neoplastic and lung tissues [156].
The results of the studies described above are promising; however, their cytostatic potential, efficiency and therapeutic effects require further analysis and, subsequently, confirmation in clinical trials.
5.3. Summary
It is considered that the co-encapsulation of cytostatic drug with natural chemosensitizer in the same delivery system can be effective tool against cancer. The synergistic effect of Dtx and Res has been confirmed and is reflected in the increased toxicity of Dtx administered with Res in comparison to the effect of free Dtx. Moreover, the beneficial effect of the simultaneous administration of Dtx as a chemotherapeutic agent with a chemosensitizer (Res) enables the MDR effect, which often adversely affects the effectiveness of traditional chemotherapy, to be overcome. These encouraging results are the driving force behind the development of nanocarriers for the co-delivery of Dtx and Res. This is a novel approach, so there are only few published reports on co-delivery systems for Dtx and Res (Table 4). The results show the synergistic effect of the drugs, which provides a rationale for further research.
6. Challenges and Opportunities
The use of NPs as anticancer drug delivery systems is currently one of the most important research areas in cancer treatment and provide promising alternatives to the limitations of conventional chemotherapy. According to the presented results, NPs may improve biodistribution, enhance cytotoxicity against cancer cells, reduce the side effects of commonly used cytostatic drugs, such as Dtx, and may be also used for active agents of natural origin, such as Res. The increasing interest in using the combination of Res as a chemosensitizer with Dtx (cytostatic drug) for overcoming cancer resistance to treatment has also become a driving force behind the development of their delivery systems. The presented literature published within last decade shows great progress in the development of nano-delivery systems for Dtx and Res. Although the developed strategies have become more and more advanced, further effort is required in the development of a carrier, which, in addition to providing satisfactory results in laboratory tests, would also allow for clinical translation. The data published data so far on carriers enabling the co-delivery of Dtx and Res are limited. This subject is expected to be explored in the near future. Several problems require special attention, such as the development optimal nanocarriers for co-loading Dtx and Res and the verification of their usefulness under in vitro and in vivo environments involving various kinds of cancers. The development of an optimal nanocarrier may present a significant challenge, especially taking into account that drugs should be encapsulated according to the particular ratio that shows the highest synergistic effect and that drugs may not have the same affinity for loading into the carrier. Different factors, such as drug chemistry or molar mass, may affect a drug’s encapsulation efficiency. Polymeric drug delivery systems should be characterized comprehensively, including related inter- and intramolecular interactions. It is commonly described in the literature that polymer–drug interactions or drug–drug interactions influence the rate of drug diffusion and the degradation of kinetics [158,159].
Undoubtedly, finding a system that might respond to intra- and inter-tumoural variability remains a significant challenge. Neoplasms have a large variety of microenvironments, and there is significant heterogeneity with respect to the molecular, pathologic and clinical features of each tumour type, both of which present huge challenges for modern medicine.
7. Conclusions
Cancer remains one of the most common causes of death in the world. However, the effectiveness of the available treatments is still limited. Dtx is a chemotherapeutic agent commonly used in the treatment of a variety of cancers in monotherapy and combination therapy. However, its toxicity and systemic side effects are one of the serious problems that limit the effectiveness of treatment in humans. Therefore, various kinds of NPs have been studied for improving the bioavailability of Dtx and its therapeutic effects. Also, there is an increasing interest in using combinations of Dtx with other drugs to obtain a synergistic effect or chemosensitization. One of the combinations with beneficial therapeutic effects is the use of Res with Dtx. Res presents antioxidant, antiinflammatory and chemopreventive properties, but also limits multidrug resistance against Dtx, which is one of the main causes of failure in cancer therapy with this drug. However, the use of both drugs presents challenges, including poor bioavailability, the unfavourable pharmacokinetics and chemical instability of Res and the poor water solubility and dose limiting toxicity of Dtx. In order to overcome these difficulties, attempts have been made to create different forms of delivery for both agents. The review has presented the progress achieved in this regard in the last 10 years in delivery systems developed for the administration of a single drug (Dtx or Res) and the co-administration of these two active agents. It has been presented that various kinds of carriers have been considered for the delivery of Dtx and Res, such as liposomes, micelles, dendrimers, SLNPs and metallic NPs. The published data indicate the high efficiency of advanced NPs, which, in addition to their drug release effect, possess additional properties, e.g., pH sensitivity or active targeting abilities, and this line of research is expected to dominate in future. The published results indicate that the selective surface modifications of the carriers are highly effective in cancer chemotherapy. It has been shown that controlled delivery systems containing a single drug (Dtx or Res) or combinations of two drugs present greater efficiency in cancer treatment compared to free drugs. Also, treatment with a combination of two drugs with a synergistic effect presents higher cytotoxicity against cancer cells than a single drug. Based on the presented literature, it can be concluded that the co-delivery of Dtx and Res in an actively targeted transport system providing the simultaneous release of both drugs in cancer cells has a chance to fulfil the requirements of effective anticancer therapy and reduce limitations in therapy caused by MDR. Thus, there is a rationale for continued research on the development of advanced nanocarriers based on the active targeting of cancer cells by ligand-receptor recognition.
Author Contributions
Conceptualization: K.J., M.J.; writing—original draft preparation: M.J., K.J., A.B., D.W. and J.K.; writing—review and editing: M.J., K.J. and A.B.; funding acquisition, K.J. All authors have read and agreed to the published version of the manuscript.
Funding
This work was created as a result of the research project No. 2019/34/E/NZ7/00010 financed by the National Science Centre.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| 4T1 cells | murine mammary carcinoma cells |
| γ-PGA | poly(γ-glutamic acid) |
| A549 cells | human epithelial carcinoma cell line |
| ACUPA | ((S)-2-(3-((S)-5-amino-1-carboxypentyl) ureido) pentanedioic acid |
| AMPK | AMP-activated protein kinase |
| AP-1 | activator protein-1 |
| ATRA | all-trans-retinoic acid |
| Au | gold |
| B16F10 cells | melanoma lung metastasis cell line |
| B6 cells | melanoma lung metastasis cell line |
| A375 cells | melanoma lung metastasis cell line |
| BSA | bovine serum albumin |
| BT-474 cells | human breast cancer cell line |
| CA | caffeic acid |
| Caco-2 cells | colon carcinoma cell line |
| Cap | calcium phosphate |
| CB | carbon black |
| CHOL | cholesterol |
| cho-CpG | cholesterol-modified Toll-like receptor 9 (TLR9) agonist oligonucleotide |
| COX | cyclooxygenase enzymes |
| COX-2 | cyclooxygenase-2 enzymes |
| CS | chitosan |
| CRPC cells | castration-resistant prostate cancer cell line |
| CT26 cells | murine colorectal carcinoma cell line |
| Cur | curcumin |
| DA | deoxycholic acid |
| DDS | drug delivery system |
| DGC | N-deoxycholic acid glycol chitosan |
| Dha | dihydroartemisin |
| DLT | dose limiting toxicity |
| DSPE | 1,2-distearoyl-sn-glycero-3-phosphorylethanolamine |
| DSPE-PEG2000 | 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(poly(ethylene glycol)-2000] |
| Dtx | docetaxel |
| Dtx-CCL-PMs | docetaxel-entrapped core-cross-linked polymeric micelles |
| DU-145 cells | docetaxel-resistant prostate carcinoma cell line |
| EGFR | epidermal growth factor receptor |
| eSM | egg sphingomyelin |
| FA | folic acid |
| FDA | Food and Drug Administration |
| FRα | folic acid α receptor |
| fWGA | fluorescein-labelled wheat germ agglutinin |
| G-CSF | granulocyte colony-stimulating factors |
| Gba | galbanic acid |
| GNRs | gold nanorods |
| GX1 | gastric cancer angiogenesis marker peptide |
| H520 cell line | human lung squamous carcinoma cell lines |
| HA | hyaluronic acid |
| HCC827 cells | non-small cell lung cancer cell line |
| hCMEC/D3 cells | brain microvascular endothelial cell line |
| HepG2 cells | human hepatocellular carcinoma cell line |
| HIF-1α | hypoxia-induced factor |
| HSA | human serum albumin |
| HSPC | hydrogenated phosphatidylcholine |
| HSR | hypersensitivity reaction |
| HT-29 cells | human colorectal adenocarcinoma cell line |
| HUVEC cells | human umbilical vein endothelial cell line |
| IL-17 | interleukin-17 |
| LNCaP | prostate cancer cells |
| LPNPs | lipid-polymer nanoparticles |
| MAPK | p38-Mitogen activated protein kinase |
| MC-38 cells | murine colon adenocarcinoma cell line |
| MCF-7 | human breast carcinoma cell line |
| MCF-10A cells | non-malignant breast epithelial cell line |
| MDA-MB-231 cells | human breast adenocarcinoma cell line |
| MDA-MB-453 cells | human breast adenocarcinoma cell line |
| MDR | multidrug resistance |
| mEHT | modulated electro-hyperthermia |
| MMPs | metalloproteinases |
| mPEG-PDLA | methoxyl poly(ethylene glycol)-poly(D,L-lactide) |
| MTD | maximum tolerated dose |
| Myrj52 | polyoxyethylene (40) stearate |
| NCIH2135 cells | non-small cell lung cancer cell line |
| NF-κB | nuclear transcription factor-κB |
| NIR | near-infrared radiation |
| NPs | nanoparticles |
| NSCLC | non-small cell lung cancer |
| P-gp | P-glycoprotein |
| PAMAM | poly(amidoamine) |
| PAMAMOS | PAMAM-organosilicon |
| PBAE | poly (β-amino ester) |
| PBM | planetary ball milled |
| PBMC | peripheral blood mononuclear cells |
| PBS | phosphate-buffered saline |
| PC-3 cells | human caucasian prostate adenocarcinoma cell line |
| PC-3–R cells | resistance human caucasian prostate adenocarcinoma cell line |
| PCL | poly(ε-caprolactone) |
| PEG | poly(ethylene glycol) |
| PDLA | poly(D,L-lactide) |
| PLA | poly(lactide) |
| PLGA | poly(lactide-co-glycolide) |
| PLGA-ATRA | poly(lactide-co-glycolide) all-trans-retinoic acid |
| PM | platelet membrane |
| PNT2 cells | normal human prostate cell line |
| PPI | poly(propylene imine) |
| PTT | photo-thermal therapy |
| Ptx | paclitaxel |
| Res | resveratrol |
| Res-AuNPs | Res-loaded gold nanoparticles |
| RLT | low-density lipoprotein receptor (LDLR)-binding peptide |
| ROS | reactive oxygen species |
| SA | stearic acid |
| SHBG | sex steroid hormone-binding globulin |
| SIRT1 | sirtuins signalling pathway |
| SKBR-3 cells | human breast adenocarcinoma cell line |
| SPC | phosphatidylcholine |
| SLNPs | solid lipid nanoparticles |
| STTP | chitosan and sodium tripolyphosphate |
| TF | transferrin |
| TNF-α | tumour necrosis factor |
| VEGF | vascular endothelial growth factor |
| Zol | zoledronate |
References
- IARC. World Cancer Report 2020; IARC: Lyon, France, 2020. [Google Scholar]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Wolinsky, J.B.; Colson, Y.L.; Grinstaff, M.W. Local drug delivery strategies for cancer treatment: Gels, nanoparticles, polymeric films, rods, and wafers. J. Control. Release 2012, 159, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xu, H.; Ashby, C.R., Jr.; Assaraf, Y.G.; Chen, Z.-S.; Liu, H.-M. Chemical molecular-based approach to overcome multidrug resistance in cancer by targeting P-glycoprotein (P-gp). Med. Res. Rev. 2021, 41, 525–555. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Dong, S.; Zhao, S.-C.; Liu, K.; Tan, Y.; Jiang, X.; Assaraf, Y.G.; Qin, B.; Chen, Z.-S.; Zou, C. Novel nanomedicines to overcome cancer multidrug resistance. Drug Resist. Updates 2021, 58, 100777. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Wu, Z.-X.; Assaraf, Y.G.; Chen, Z.-S.; Wang, L. Overcoming anti-cancer drug resistance via restoration of tumor suppressor gene function. Drug Resist. Updates 2021, 57, 100770. [Google Scholar] [CrossRef]
- Merlos Rodrigo, M.A.; Jimenez Jimemez, A.M.; Haddad, Y.; Bodoor, K.; Adam, P.; Krizkova, S.; Heger, Z.; Adam, V. Metallothionein isoforms as double agents—Their roles in carcinogenesis, cancer progression and chemoresistance. Drug Resist. Updates 2020, 52, 100691. [Google Scholar] [CrossRef]
- McLellan, L.I.; Wolf, C.R. Glutathione and glutathione-dependent enzymes in cancer drug resistance. Drug Resist. Updates 1999, 2, 153–164. [Google Scholar] [CrossRef]
- Zhitomirsky, B.; Assaraf, Y.G. Lysosomes as mediators of drug resistance in cancer. Drug Resist. Updates 2016, 24, 23–33. [Google Scholar] [CrossRef]
- Wang, J.-Q.; Yang, Y.; Cai, C.-Y.; Teng, Q.-X.; Cui, Q.; Lin, J.; Assaraf, Y.G.; Chen, Z.-S. Multidrug resistance proteins (MRPs): Structure, function and the overcoming of cancer multidrug resistance. Drug Resist. Updates 2021, 54, 100743. [Google Scholar] [CrossRef]
- Vasan, N.; Baselga, J.; Hyman, D.M. A view on drug resistance in cancer. Nature 2019, 575, 299–309. [Google Scholar] [CrossRef]
- Sharma, I.; Hannay, N.; Sridhar, S.; Ahmad, S.; Basha, R. Chapter 15—Future perspectives and new directions in chemosensitizing activities to reverse drug resistance in gynecologic cancers: Emphasis on challenges and opportunities. In Cancer Sensitizing Agents for Chemotherapy; Basha, R., Ahmad, S., Eds.; Academic Press: Cambridge, MA, USA, 2021; Volume 17, pp. 339–355. ISBN 24683183. [Google Scholar]
- Ren, B.; Kwah, M.X.-Y.; Liu, C.; Ma, Z.; Shanmugam, M.K.; Ding, L.; Xiang, X.; Ho, P.C.-L.; Wang, L.; Ong, P.S.; et al. Resveratrol for cancer therapy: Challenges and future perspectives. Cancer Lett. 2021, 515, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Poltavets, Y.I.; Zhirnik, A.S.; Zavarzina, V.V.; Semochkina, Y.P.; Shuvatova, V.G.; Krasheninnikova, A.A.; Aleshin, S.V.; Dronov, D.O.; Vorontsov, E.A.; Balabanyan, V.Y.; et al. In vitro anticancer activity of folate-modified docetaxel-loaded PLGA nanoparticles against drug-sensitive and multidrug-resistant cancer cells. Cancer Nanotechnol. 2019, 10, 2. [Google Scholar] [CrossRef]
- Kumari, P.; Ghosh, B.; Biswas, S. Nanocarriers for cancer-targeted drug delivery. J. Drug Target. 2016, 24, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in Cancer Therapy: Challenges, Opportunities, and Clinical Applications. J. Control. Release 2015, 200, 138. [Google Scholar] [CrossRef] [PubMed]
- Abed, S.N.; Deb, P.K.; Surchi, H.S.; Kokaz, S.F.; Jamal, S.M.; Bandopadhyay, S.; Tekade, R.K. Chapter 17—Nanocarriers in Different Preclinical and Clinical Stages. In Advances in Pharmaceutical Product Development and Research; Tekade, R., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 685–731. ISBN 978-0-12-817909-3. [Google Scholar]
- Chung, I.-M.; Subramanian, U.; Thirupathi, P.; Venkidasamy, B.; Samynathan, R.; Gangadhar, B.H.; Rajakumar, G.; Thiruvengadam, M. Resveratrol Nanoparticles: A Promising Therapeutic Advancement over Native Resveratrol. Processes 2020, 8, 458. [Google Scholar] [CrossRef]
- Trucillo, P. Drug Carriers: Classification, Administration, Release Profiles, and Industrial Approach. Processes 2021, 9, 470. [Google Scholar] [CrossRef]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Huo, P.; Liu, B. Formulation Strategies for Folate-Targeted Liposomes and Their Biomedical Applications. Pharmaceutics 2019, 11, 381. [Google Scholar] [CrossRef]
- Arias, J.L.; Clares, B.; Morales, M.E.; Gallardo, V.; Ruiz, M.A. Lipid-based drug delivery systems for cancer treatment. Curr. Drug Targets 2011, 12, 1151–1165. [Google Scholar] [CrossRef]
- Patil, Y.P.; Jadhav, S. Novel methods for liposome preparation. Chem. Phys. Lipids 2014, 177, 8–18. [Google Scholar] [CrossRef]
- Riaz, M.K.; Riaz, M.A.; Zhang, X.; Lin, C.; Wong, K.H.; Chen, X.; Zhang, G.; Lu, A.; Yang, Z. Surface functionalization and targeting strategies of liposomes in solid tumor therapy: A review. Int. J. Mol. Sci. 2018, 19, 195. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.K.; Tiwari, S.K.; Mohapatra, S.; Thomas, S. Chapter 1—Efficient Nanocarriers for Drug-Delivery Systems: Types and Fabrication. In Micro and Nano Technologies; Mohapatra, S.S., Ranjan, S., Dasgupta, N., Mishra, R.K., Thomas, S.B.T.-N., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–41. ISBN 978-0-12-814033-8. [Google Scholar]
- Lorente, C.; Cabeza, L.; Clares, B.; Ortiz, R.; Halbaut, L.; Delgado, Á.V.; Perazzoli, G.; Prados, J.; Arias, J.L.; Melguizo, C. Formulation and in vitro evaluation of magnetoliposomes as a potential nanotool in colorectal cancer therapy. Colloids Surf. B Biointerfaces 2018, 171, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Clares, B.; Biedma-Ortiz, R.A.; Sáez-Fernández, E.; Prados, J.C.; Melguizo, C.; Cabeza, L.; Ortiz, R.; Arias, J.L. Nano-engineering of 5-fluorouracil-loaded magnetoliposomes for combined hyperthermia and chemotherapy against colon cancer. Eur. J. Pharm. Biopharm. 2013, 85, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, P.P.; Biswas, S.; Torchilin, V.P. Current trends in the use of liposomes for tumor targeting. Nanomedicine 2013, 8, 1509–1528. [Google Scholar] [CrossRef] [PubMed]
- Daraee, H.; Etemadi, A.; Kouhi, M.; Alimirzalu, S.; Akbarzadeh, A. Application of liposomes in medicine and drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Rideau, E.; Dimova, R.; Schwille, P.; Wurm, F.R.; Landfester, K. Liposomes and polymersomes: A comparative review towards cell mimicking. Chem. Soc. Rev. 2018, 47, 8572–8610. [Google Scholar] [CrossRef] [PubMed]
- Kauscher, U.; Holme, M.N.; Björnmalm, M.; Stevens, M.M. Physical stimuli-responsive vesicles in drug delivery: Beyond liposomes and polymersomes. Adv. Drug Deliv. Rev. 2019, 138, 259–275. [Google Scholar] [CrossRef]
- Antimisiaris, S.G.; Marazioti, A.; Kannavou, M.; Natsaridis, E.; Gkartziou, F.; Kogkos, G.; Mourtas, S. Overcoming barriers by local drug delivery with liposomes. Adv. Drug Deliv. Rev. 2021, 174, 53–86. [Google Scholar] [CrossRef]
- Abbasi, E.; Aval, S.F.; Akbarzadeh, A.; Milani, M.; Nasrabadi, H.T.; Joo, S.W.; Hanifehpour, Y.; Nejati-Koshki, K.; Pashaei-Asl, R. Dendrimers: Synthesis, applications, and properties. Nanoscale Res. Lett. 2014, 9, 247. [Google Scholar] [CrossRef]
- Chauhan, A.S. Dendrimers for Drug Delivery. Molecules 2018, 23, 938. [Google Scholar] [CrossRef]
- Santos, A.; Veiga, F.; Figueiras, A. Dendrimers as Pharmaceutical Excipients: Synthesis, Properties, Toxicity and Biomedical Applications. Materials 2020, 13, 65. [Google Scholar] [CrossRef] [PubMed]
- Karatas, O.; Keyikoglu, R.; Atalay Gengec, N.; Vatanpour, V.; Khataee, A. A review on dendrimers in preparation and modification of membranes: Progress, applications, and challenges. Mater. Today Chem. 2022, 23, 100683. [Google Scholar] [CrossRef]
- Arseneault, M.; Wafer, C.; Morin, J.-F. Recent Advances in Click Chemistry Applied to Dendrimer Synthesis. Molecules 2015, 20, 9263. [Google Scholar] [CrossRef]
- Sherje, A.P.; Jadhav, M.; Dravyakar, B.R.; Kadam, D. Dendrimers: A versatile nanocarrier for drug delivery and targeting. Int. J. Pharm. 2018, 548, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.P.; da Silva Santos, S.; da Silva, J.V.; Parise-Filho, R.; Igne Ferreira, E.; El Seoud, O.; Giarolla, J. Dendrimers in the context of nanomedicine. Int. J. Pharm. 2020, 573, 118814. [Google Scholar] [CrossRef] [PubMed]
- Pooresmaeil, M.; Namazi, H. Advances in development of the dendrimers having natural saccharides in their structure for efficient and controlled drug delivery applications. Eur. Polym. J. 2021, 148, 110356. [Google Scholar] [CrossRef]
- Moughton, A.O.; Hillmyer, M.A.; Lodge, T.P. Multicompartment Block Polymer Micelles. Macromolecules 2012, 45, 2–19. [Google Scholar] [CrossRef]
- Nishiyama, N.; Kataoka, K. Current state, achievements, and future prospects of polymeric micelles as nanocarriers for drug and gene delivery. Pharmacol. Ther. 2006, 112, 630–648. [Google Scholar] [CrossRef]
- Di, Y.; Li, T.; Zhu, Z.; Chen, F.; Jia, L.; Liu, W.; Gai, X.; Wang, Y.; Pan, W.; Yang, X. pH-sensitive and folic acid-targeted MPEG-PHIS/FA-PEG-VE mixed micelles for the delivery of PTX-VE and their antitumor activity. Int. J. Nanomed. 2017, 12, 5863–5877. [Google Scholar] [CrossRef][Green Version]
- Hao, W.; Wang, T.; Liu, D.; Shang, Y.; Zhang, J.; Xu, S.; Liu, H. Folate-conjugated pH-controllable fluorescent nanomicelles acting as tumor targetable drug carriers. Mikrochim. Acta 2017, 184, 2881–2891. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Zhai, G.-X. Intelligent polymeric micelles: Development and application as drug delivery for docetaxel. J. Drug Target. 2017, 25, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Pham, D.T.; Chokamonsirikun, A.; Phattaravorakarn, V.; Tiyaboonchai, W. Polymeric micelles for pulmonary drug delivery: A comprehensive review. J. Mater. Sci. 2021, 56, 2016–2036. [Google Scholar] [CrossRef]
- Allen, C.; Maysinger, D.; Eisenberg, A. Nano-engineering block copolymer aggregates for drug delivery. Colloids Surf. B Biointerfaces 1999, 16, 3–27. [Google Scholar] [CrossRef]
- Wu, X.; El Ghzaoui, A.; Li, S. Anisotropic self-assembling micelles prepared by the direct dissolution of PLA/PEG block copolymers with a high PEG fraction. Langmuir 2011, 27, 8000–8008. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jackson, J.K.; Burt, H.M. Development of Amphiphilic Diblock Copolymers as Micellar Carriers of Taxol. Int. J. Pharm. 1996, 132, 195. [Google Scholar] [CrossRef]
- Burt, H.M.; Zhang, X.; Toleikis, P.; Embree, L.; Hunter, W.L. Development of copolymers of poly(d,l-lactide) and methoxypolyethylene glycol as micellar carriers of paclitaxel. Colloids Surf. B Biointerfaces 1999, 16, 161–171. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Yun, P.; Shen, X.; Su, F.; Chen, Y.; Li, S.; Song, D. Self-assembled filomicelles prepared from polylactide-poly(ethylene glycol) diblock copolymers for sustained delivery of cycloprotoberberine derivatives. Saudi Pharm. J. 2018, 26, 342–348. [Google Scholar] [CrossRef]
- Jelonek, K.; Li, S.; Wu, X.; Kasperczyk, J.; Marcinkowski, A. Self-assembled filomicelles prepared from polylactide/poly(ethylene glycol) block copolymers for anticancer drug delivery. Int. J. Pharm. 2015, 485, 357–364. [Google Scholar] [CrossRef]
- Kataoka, K.; Harada, A.; Nagasaki, Y. Block copolymer micelles for drug delivery: Design, characterization and biological significance. Adv. Drug Deliv. Rev. 2012, 64, 37–48. [Google Scholar] [CrossRef]
- Biswas, S.; Kumari, P.; Lakhani, P.M.; Ghosh, B. Recent advances in polymeric micelles for anti-cancer drug delivery. Eur. J. Pharm. Sci. 2016, 83, 184–202. [Google Scholar] [CrossRef]
- Gothwal, A.; Khan, I.; Gupta, U. Polymeric Micelles: Recent Advancements in the Delivery of Anticancer Drugs. Pharm. Res. 2016, 33, 18–39. [Google Scholar] [CrossRef] [PubMed]
- Cabral, H.; Miyata, K.; Osada, K.; Kataoka, K. Block Copolymer Micelles in Nanomedicine Applications. Chem. Rev. 2018, 118, 6844–6892. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, E.; Yang, J.; Cao, Z. Strategies to improve micelle stability for drug delivery. Nano Res. 2018, 11, 4985–4998. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, M.; Pescina, S.; Padula, C.; Santi, P.; Del Favero, E.; Cantù, L.; Nicoli, S. Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. J. Control. Release 2021, 332, 312–336. [Google Scholar] [CrossRef]
- Wang, Z.; Deng, X.; Ding, J.; Zhou, W.; Zheng, X.; Tang, G. Mechanisms of drug release in pH-sensitive micelles for tumour targeted drug delivery system: A review. Int. J. Pharm. 2018, 535, 253–260. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, L.; Yang, T.; Wu, H. Stimuli-responsive polymeric micelles for drug delivery and cancer therapy. Int. J. Nanomed. 2018, 13, 2921–2942. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, K.; Zhu, W.; Deng, W.; Lam, K.S. Stimuli-responsive cross-linked micelles for on-demand drug delivery against cancers. Adv. Drug Deliv. Rev. 2014, 66, 58–73. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Liu, G.; Luo, Z.; Zhou, L.; Xue, Y.; Liu, M. Recent progress in the applications of gold-based nanoparticles towards tumor-targeted imaging and therapy. RSC Adv. 2022, 12, 7635–7651. [Google Scholar] [CrossRef]
- Rand, D.; Ortiz, V.; Liu, Y.; Derdak, Z.; Wands, J.R.; Tatíček, M.; Rose-Petruck, C. Nanomaterials for X-ray Imaging: Gold Nanoparticle Enhancement of X-ray Scatter Imaging of Hepatocellular Carcinoma. Nano Lett. 2011, 11, 2678–2683. [Google Scholar] [CrossRef]
- Xu, J.-J.; Zhang, W.-C.; Guo, Y.-W.; Chen, X.-Y.; Zhang, Y.-N. Metal nanoparticles as a promising technology in targeted cancer treatment. Drug Deliv. 2022, 29, 664–678. [Google Scholar] [CrossRef]
- Mu, H.; Holm, R. Solid lipid nanocarriers in drug delivery: Characterization and design. Expert Opin. Drug Deliv. 2018, 15, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Uchechi, O. Nanoparticles for Dermal and Transdermal Drug Delivery. In Application of Nanotechnology in Drug Delivery; Ogbonna, J.D.N., Ed.; IntechOpen: Rijeka, Croatia, 2014; Chapter 6. [Google Scholar]
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.-H.; Qoronfleh, M.W. Therapeutic efficacy of nanoparticles and routes of administration. Biomater. Res. 2019, 23, 20. [Google Scholar] [CrossRef] [PubMed]
- Abraxane. Pharm. Times 2014, 80, 44.
- Barenholz, Y. (Chezy) Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Leonard, R.C.F.; Williams, S.; Tulpule, A.; Levine, A.M.; Oliveros, S. Improving the therapeutic index of anthracycline chemotherapy: Focus on liposomal doxorubicin (MyocetTM). Breast 2009, 18, 218–224. [Google Scholar] [CrossRef]
- Guo, X.; Zhao, Z.; Chen, D.; Qiao, M.; Wan, F.; Cun, D.; Sun, Y.; Yang, M. Co-delivery of resveratrol and docetaxel via polymeric micelles to improve the treatment of drug-resistant tumors. Asian J. Pharm. Sci. 2019, 14, 78–85. [Google Scholar] [CrossRef]
- de Weger, V.A.; Beijnen, J.H.; Schellens, J.H.M. Cellular and clinical pharmacology of the taxanes docetaxel and paclitaxel—A review. Anticancer. Drugs 2014, 25, 488–494. [Google Scholar] [CrossRef]
- Lyseng-Williamson, K.A.; Fenton, C. Docetaxel. Drugs 2005, 65, 2513–2531. [Google Scholar] [CrossRef]
- Hitt, R.; Lopez-Martin, A. Docetaxel BT—Encyclopedia of Cancer; Schwab, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1409–1412. ISBN 978-3-662-46875-3. [Google Scholar]
- Baker, S.D.; Sparreboom, A.; Verweij, J. Clinical Pharmacokinetics of Docetaxel. Clin. Pharmacokinet. 2006, 45, 235–252. [Google Scholar] [CrossRef]
- Hua, H.; Zhang, N.; Liu, D.; Song, L.; Liu, T.; Li, S.; Zhao, Y. Multifunctional gold nanorods and docetaxel-encapsulated liposomes for combined thermo-and chemotherapy. Int. J. Nanomed. 2017, 12, 7869. [Google Scholar] [CrossRef]
- Eloy, J.O.; Ruiz, A.; de Lima, F.T.; Petrilli, R.; Raspantini, G.; Nogueira, K.A.B.; Santos, E.; de Oliveira, C.S.; Borges, J.C.; Marchetti, J.M.; et al. EGFR-targeted immunoliposomes efficiently deliver docetaxel to prostate cancer cells. Colloids Surf. B Biointerfaces 2020, 194, 111185. [Google Scholar] [CrossRef] [PubMed]
- Wenbo, Y.; Lan, H.A.O.; Zhigang2, W.; Ronggui, Z. CREKA peptide-modified docetaxel loaded liposomes for targeted treatment of prostate cancer in vitro. Di 3 Jun Yi Da Xue Xue Bao 2020, 42, 2017–2115. [Google Scholar]
- Aires Fernandes, M.O.; Eloy, J.; Tavares Luiz, M.; Ramos Junior, S.L.; Borges, J.C.; Rodríguez de la Fuente, L.; Ortega-de San Luis, C.; Maldonado Marchetti, J.; Santos-Martinez, M.J.; Chorilli, M. Transferrin-functionalized liposomes for docetaxel delivery to prostate cancer cells. Colloids Surf. A Physicochem. Eng. Asp. 2021, 611, 125806. [Google Scholar] [CrossRef]
- Bi, X.; Yuan, Q.; Han, J.; Cong, W.; Ge, Y.; Ma, D.; Dai, Z.; Li, Y. Docetaxel-loaded solid lipid nanoparticles suppress breast cancer cells growth with reduced myelosuppression toxicity. Int. J. Nanomed. 2014, 9, 4829. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.; Maiti, S.; Lee, J.H.; Lee, J.H.; Kim, J.S. Rational design of biotin–disulfide–coumarin conjugates: A cancer targeted thiol probe and bioimaging. Chem. Commun. 2014, 50, 3044–3047. [Google Scholar] [CrossRef]
- da Rocha, M.C.O.; da Silva, P.B.; Radicchi, M.A.; Andrade, B.Y.G.; de Oliveira, J.V.; Venus, T.; Merker, C.; Estrela-Lopis, I.; Longo, J.P.F.; Báo, S.N. Docetaxel-loaded solid lipid nanoparticles prevent tumor growth and lung metastasis of 4T1 murine mammary carcinoma cells. J. Nanobiotechnol. 2020, 18, 43. [Google Scholar] [CrossRef]
- Kim, K.S.; Youn, Y.S.; Bae, Y.H. Immune-triggered cancer treatment by intestinal lymphatic delivery of docetaxel-loaded nanoparticle. J. Control. Release 2019, 311–312, 85–95. [Google Scholar] [CrossRef]
- Wang, Y.; Zuo, A.; Huang, X.; Ying, Y.; Shu, X.; Chen, X.; Yang, Y.; Ma, J.; Lin, G.; Wang, X.; et al. Docetaxel-loaded PAMAM-based poly (γ-benzyl-l-glutamate)-b-d-α-tocopheryl polyethylene glycol 1000 succinate nanoparticles in human breast cancer and human cervical cancer therapy. J. Microencapsul. 2019, 36, 552–565. [Google Scholar] [CrossRef]
- Bai, S.-B.; Cheng, Y.; Liu, D.-Z.; Ji, Q.-F.; Liu, M.; Zhang, B.-L.; Mei, Q.-B.; Zhou, S.-Y. Bone-targeted PAMAM nanoparticle to treat bone metastases of lung cancer. Nanomedicine 2020, 15, 833–849. [Google Scholar] [CrossRef]
- Marcinkowska, M.; Stanczyk, M.; Janaszewska, A.; Sobierajska, E.; Chworos, A.; Klajnert-Maculewicz, B. Multicomponent Conjugates of Anticancer Drugs and Monoclonal Antibody with PAMAM Dendrimers to Increase Efficacy of HER-2 Positive Breast Cancer Therapy. Pharm. Res. 2019, 36, 154. [Google Scholar] [CrossRef]
- Song, S.Y.; Kim, K.-P.; Jeong, S.-Y.; Park, J.; Park, J.; Jung, J.; Chung, H.K.; Lee, S.-W.; Seo, M.H.; Lee, J.-S.; et al. Polymeric nanoparticle-docetaxel for the treatment of advanced solid tumors: Phase I clinical trial and preclinical data from an orthotopic pancreatic cancer model. Oncotarget 2016, 7, 77348–77357. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.I.; Ayub, A.D.; Mat Yusuf, S.N.; Yahaya, N.; Abd Kadir, E.; Lim, V. Docetaxel-Loaded Disulfide Cross-Linked Nanoparticles Derived from Thiolated Sodium Alginate for Colon Cancer Drug Delivery. Pharmaceutics 2020, 12, 38. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Wang, X.; Zhao, S.; Qiu, H.; Han, M.; Li, J.; Zhao, N.; Wang, R.; Guo, Y. The influence of nanocarrier architectures on antitumor efficacy of docetaxel nanoparticles. RSC Adv. 2020, 10, 11074–11078. [Google Scholar] [CrossRef]
- Li, Y.; Chen, X.; Ji, J.; Li, L.; Zhai, G. Redox-responsive nanoparticles based on Chondroitin Sulfate and Docetaxel prodrug for tumor targeted delivery of Docetaxel. Carbohydr. Polym. 2021, 255, 117393. [Google Scholar] [CrossRef]
- Zhang, E.; Xing, R.; Liu, S.; Li, K.; Qin, Y.; Yu, H.; Li, P. Vascular targeted chitosan-derived nanoparticles as docetaxel carriers for gastric cancer therapy. Int. J. Biol. Macromol. 2019, 126, 662–672. [Google Scholar] [CrossRef]
- Kolluru, L.P.; Chandran, T.; Shastri, P.N.; Rizvi, S.A.A.; D’Souza, M.J. Development and evaluation of polycaprolactone based docetaxel nanoparticle formulation for targeted breast cancer therapy. J. Nanopart. Res. 2020, 22, 372. [Google Scholar] [CrossRef]
- Yu, Z.; Li, X.; Duan, J.; Yang, X.-D. Targeted Treatment of Colon Cancer with Aptamer-Guided Albumin Nanoparticles Loaded with Docetaxel. Int. J. Nanomed. 2020, 15, 6737–6748. [Google Scholar] [CrossRef] [PubMed]
- Scheetz, L.M.; Yu, M.; Li, D.; Castro, M.G.; Moon, J.J.; Schwendeman, A. Synthetic HDL Nanoparticles Delivering Docetaxel and CpG for Chemoimmunotherapy of Colon Adenocarcinoma. Int. J. Mol. Sci. 2020, 21, 1777. [Google Scholar] [CrossRef]
- Mahmood, M.A.; Madni, A.; Rehman, M.; Rahim, M.A.; Jabar, A. Ionically Cross-Linked Chitosan Nanoparticles for Sustained Delivery of Docetaxel: Fabrication, Post-Formulation and Acute Oral Toxicity Evaluation. Int. J. Nanomed. 2019, 14, 10035–10046. [Google Scholar] [CrossRef]
- Liu, N.; Ji, J.; Qiu, H.; Shao, Z.; Wen, X.; Chen, A.; Yao, S.; Zhang, X.; Yao, H.; Zhang, L. Improving radio-chemotherapy efficacy of prostate cancer by co-deliverying docetaxel and dbait with biodegradable nanoparticles. Artif. Cells Nanomed. Biotechnol. 2020, 48, 305–314. [Google Scholar] [CrossRef]
- Atrafi, F.; Dumez, H.; Mathijssen, R.H.J.; Menke van der Houven van Oordt, C.W.; Rijcken, C.J.F.; Hanssen, R.; Eskens, F.A.L.M.; Schöffski, P. A phase I dose-escalation and pharmacokinetic study of a micellar nanoparticle with entrapped docetaxel (CPC634) in patients with advanced solid tumours. J. Control. Release 2020, 325, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Thambiraj, S.; Shruthi, S.; Vijayalakshmi, R.; Ravi Shankaran, D. Evaluation of cytotoxic activity of docetaxel loaded gold nanoparticles for lung cancer drug delivery. Cancer Treat. Res. Commun. 2019, 21, 100157. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Kang, N.-W.; Kim, H.; Kim, D.H.; Chae, J.; Lee, W.; Song, G.Y.; Cho, C.-W.; Kim, D.-D.; Lee, J.-Y. Chondroitin sulfate-hybridized zein nanoparticles for tumor-targeted delivery of docetaxel. Carbohydr. Polym. 2021, 253, 117187. [Google Scholar] [CrossRef]
- Karimi, N.; Soleiman-Beigi, M.; Fattahi, A. Co-delivery of all-trans-retinoic acid and docetaxel in drug conjugated polymeric nanoparticles: Improving controlled release and anticancer effect. Mater. Today Commun. 2020, 25, 101280. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, D.; Liu, M.; Ji, Q.; Mei, Q.; Cheng, Y.; Zhou, S. Bone-Targeted Calcium Phosphate-Polymer Hybrid Nanoparticle Co-Deliver Zoledronate and Docetaxel to Treat Bone Metastasis of Prostate Cancer. J. Pharm. Sci. 2021, 110, 876–887. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Diao, L.; Chen, F.; Shen, A.; Wang, S.; Jin, H.; Cai, D.; Hu, Y. pH-Sensitive Nanoparticles Codelivering Docetaxel and Dihydroartemisinin Effectively Treat Breast Cancer by Enhancing Reactive Oxidative Species-Mediated Mitochondrial Apoptosis. Mol. Pharm. 2021, 18, 74–86. [Google Scholar] [CrossRef]
- Afsharzadeh, M.; Hashemi, M.; Babaei, M.; Abnous, K.; Ramezani, M. PEG-PLA nanoparticles decorated with small-molecule PSMA ligand for targeted delivery of galbanic acid and docetaxel to prostate cancer cells. J. Cell. Physiol. 2020, 235, 4618–4630. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.; Li, F.; Liu, H.; Feng, S.; Zhang, Y.; Zhou, D.; Zhang, R. Docetaxel-loaded biomimetic nanoparticles for targeted lung cancer therapy in vivo. J. Nanopart. Res. 2019, 21, 144. [Google Scholar] [CrossRef]
- Rodallec, A.; Brunel, J.-M.; Giacometti, S.; Maccario, H.; Correard, F.; Mas, E.; Orneto, C.; Savina, A.; Bouquet, F.; Lacarelle, B.; et al. Docetaxel-trastuzumab stealth immunoliposome: Development and in vitro proof of concept studies in breast cancer. Int. J. Nanomed. 2018, 13, 3451–3465. [Google Scholar] [CrossRef]
- Deeken, J.F.; Slack, R.; Weiss, G.J.; Ramanathan, R.K.; Pishvaian, M.J.; Hwang, J.; Lewandowski, K.; Subramaniam, D.; He, A.R.; Cotarla, I.; et al. A phase I study of liposomal-encapsulated docetaxel (LE-DT) in patients with advanced solid tumor malignancies. Cancer Chemother. Pharmacol. 2013, 71, 627–633. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; Mita, M.M.; Ramanathan, R.K.; Weiss, G.J.; Mita, A.C.; LoRusso, P.M.; Burris, H.A.; Hart, L.L.; Low, S.C.; Parsons, D.M.; et al. Phase I Study of PSMA-Targeted Docetaxel-Containing Nanoparticle BIND-014 in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2016, 22, 3157–3163. [Google Scholar] [CrossRef] [PubMed]
- Rezvantalab, S.; Drude, N.I.; Moraveji, M.K.; Güvener, N.; Koons, E.K.; Shi, Y.; Lammers, T.; Kiessling, F. PLGA-Based Nanoparticles in Cancer Treatment. Front. Pharmacol. 2018, 9, 1260. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Rijcken, C.J.; Bansal, R.; Hennink, W.E.; Storm, G.; Prakash, J. Complete regression of breast tumour with a single dose of docetaxel-entrapped core-cross-linked polymeric micelles. Biomaterials 2015, 53, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Braal, C.L.; de Bruijn, P.; Atrafi, F.; van Geijn, M.; Rijcken, C.J.F.; Mathijssen, R.H.J.; Koolen, S.L.W. A new method for the determination of total and released docetaxel from docetaxel-entrapped core-crosslinked polymeric micelles (CriPec®) by LC–MS/MS and its clinical application in plasma and tissues in patients with various tumours. J. Pharm. Biomed. Anal. 2018, 161, 168–174. [Google Scholar] [CrossRef]
- Ahmadi, R.; Ebrahimzadeh, M.A. Resveratrol—A comprehensive review of recent advances in anticancer drug design and development. Eur. J. Med. Chem. 2020, 200, 112356. [Google Scholar] [CrossRef]
- Kiskova, T.; Kubatka, P.; Büsselberg, D.; Kassayova, M. The Plant-Derived Compound Resveratrol in Brain Cancer: A Review. Biomolecules 2020, 10, 161. [Google Scholar] [CrossRef]
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Potential adverse effects of resveratrol: A literature review. Int. J. Mol. Sci. 2020, 6, 2084. [Google Scholar] [CrossRef]
- Elshaer, M.; Chen, Y.; Wang, X.J.; Tang, X. Resveratrol: An overview of its anti-cancer mechanisms. Life Sci. 2018, 207, 340–349. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhu, W.; Feng, W.; Lee, S.S.; Leung, A.W.; Shen, J.; Gao, L.; Xu, C. A Review of Resveratrol as a Potent Chemoprotective and Synergistic Agent in Cancer Chemotherapy. Front. Pharmacol. 2019, 9, 1534. [Google Scholar] [CrossRef]
- Patra, S.; Pradhan, B.; Nayak, R.; Behera, C.; Rout, L.; Jena, M.; Efferth, T.; Bhutia, S.K. Chemotherapeutic efficacy of curcumin and resveratrol against cancer: Chemoprevention, chemoprotection, drug synergism and clinical pharmacokinetics. Semin. Cancer Biol. 2021, 73, 310–320. [Google Scholar] [CrossRef]
- Shukla, Y.; Singh, R. Resveratrol and cellular mechanisms of cancer prevention. Ann. N. Y. Acad. Sci. 2011, 1215, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.K.; Das, D.K. Resveratrol and chemoprevention. Cancer Lett. 2009, 284, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, I.A.; Sanna, V.; Ahmad, N.; Sechi, M.; Mukhtar, H. Resveratrol nanoformulation for cancer prevention and therapy. Ann. N. Y. Acad. Sci. 2015, 1348, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Abba, Y.; Hassim, H.; Hamzah, H.; Noordin, M.M. Antiviral Activity of Resveratrol against Human and Animal Viruses. Adv. Virol. 2015, 2015, 184241. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, M.; Ingmer, H. Antibacterial and antifungal properties of resveratrol. Int. J. Antimicrob. Agents 2019, 53, 716–723. [Google Scholar] [CrossRef]
- Santos, M.A.; Franco, F.N.; Caldeira, C.A.; de Araújo, G.R.; Vieira, A.; Chaves, M.M.; Lara, R.C. Antioxidant effect of Resveratrol: Change in MAPK cell signaling pathway during the aging process. Arch. Gerontol. Geriatr. 2021, 92, 104266. [Google Scholar] [CrossRef]
- Lu, Y.; Xu, H.-m; Han, Y.; Zhang, Y. li Analgesic effect of resveratrol on colitis-induced visceral pain via inhibition of TRAF6/NF-κB signaling pathway in the spinal cord. Brain Res. 2019, 1724, 146464. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Milajerdi, A.; Sheikhi, A.; Kord-Varkaneh, H.; Feinle-Bisset, C.; Larijani, B.; Esmaillzadeh, A. Resveratrol supplementation significantly influences obesity measures: A systematic review and dose–response meta-analysis of randomized controlled trials. Obes. Rev. 2019, 20, 487–498. [Google Scholar] [CrossRef]
- Yan, Y.; Yang, H.; Xie, Y.; Ding, Y.; Kong, D.; Yu, H. Research Progress on Alzheimer’s Disease and Resveratrol. Neurochem. Res. 2020, 45, 989–1006. [Google Scholar] [CrossRef]
- Xia, D.; Sui, R.; Zhang, Z. Administration of resveratrol improved Parkinson’s disease-like phenotype by suppressing apoptosis of neurons via modulating the MALAT1/miR-129/SNCA signaling pathway. J. Cell. Biochem. 2019, 120, 4942–4951. [Google Scholar] [CrossRef]
- Repossi, G.; Das, U.N.; Eynard, A.R. Molecular Basis of the Beneficial Actions of Resveratrol. Arch. Med. Res. 2020, 51, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Al-Abd, A.M.; Mahmoud, A.M.; El-Sherbiny, G.A.; El-Moselhy, M.A.; Nofal, S.M.; El-Latif, H.A.; El-Eraky, W.I.; El-Shemy, H.A. Resveratrol enhances the cytotoxic profile of docetaxel and doxorubicin in solid tumour cell lines in vitro. Cell Prolif. 2011, 44, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.W.; Fong, H.H.S.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G.; et al. Cancer Chemopreventive Activity of Resveratrol, a Natural Product Derived from Grapes. Science 1997, 275, 218–220. [Google Scholar] [CrossRef]
- Davoodvandi, A.; Darvish, M.; Borran, S.; Nejati, M.; Mazaheri, S.; Reza Tamtaji, O.; Hamblin, M.R.; Masoudian, N.; Mirzaei, H. The therapeutic potential of resveratrol in a mouse model of melanoma lung metastasis. Int. Immunopharmacol. 2020, 88, 106905. [Google Scholar] [CrossRef]
- Wu, H.; Chen, L.; Zhu, F.; Han, X.; Sun, L.; Chen, K. The Cytotoxicity Effect of Resveratrol: Cell Cycle Arrest and Induced Apoptosis of Breast Cancer 4T1 Cells. Toxins 2019, 11, 731. [Google Scholar] [CrossRef] [PubMed]
- Kuo, I.-M.; Lee, J.-J.; Wang, Y.-S.; Chiang, H.-C.; Huang, C.-C.; Hsieh, P.-J.; Han, W.; Ke, C.-H.; Liao, A.T.C.; Lin, C.-S. Potential enhancement of host immunity and anti-tumor efficacy of nanoscale curcumin and resveratrol in colorectal cancers by modulated electro- hyperthermia. BMC Cancer 2020, 20, 603. [Google Scholar] [CrossRef] [PubMed]
- Park Young, S.; Chae Yeong, S.; Park Oh, J.; Lee Jin, K.; Park, G. Gold-conjugated resveratrol nanoparticles attenuate the invasion and MMP-9 and COX-2 expression in breast cancer cells. Oncol. Rep. 2016, 35, 3248–3256. [Google Scholar] [CrossRef] [PubMed]
- Carletto, B.; Berton, J.; Ferreira, T.N.; Dalmolin, L.F.; Paludo, K.S.; Mainardes, R.M.; Farago, P.V.; Favero, G.M. Resveratrol-loaded nanocapsules inhibit murine melanoma tumor growth. Colloids Surf. B Biointerfaces 2016, 144, 65–72. [Google Scholar] [CrossRef]
- Sanna, V.; Siddiqui, I.A.; Sechi, M.; Mukhtar, H. Resveratrol-Loaded Nanoparticles Based on Poly(epsilon-caprolactone) and Poly(d,l-lactic-co-glycolic acid)–Poly(ethylene glycol) Blend for Prostate Cancer Treatment. Mol. Pharm. 2013, 10, 3871–3881. [Google Scholar] [CrossRef]
- Nassir, A.M.; Shahzad, N.; Ibrahim, I.A.A.; Ahmad, I.; Md, S.; Ain, M.R. Resveratrol-loaded PLGA nanoparticles mediated programmed cell death in prostate cancer cells. Saudi Pharm. J. 2018, 26, 876–885. [Google Scholar] [CrossRef]
- Hsu, H.-T.; Tseng, Y.-T.; Wong, W.-J.; Liu, C.-M.; Lo, Y.-C. Resveratrol prevents nanoparticles-induced inflammation and oxidative stress via downregulation of PKC-α and NADPH oxidase in lung epithelial A549 cells. BMC Complement. Altern. Med. 2018, 18, 211. [Google Scholar] [CrossRef] [PubMed]
- Lian, B.; Wu, M.; Feng, Z.; Deng, Y.; Zhong, C.; Zhao, X. Folate-conjugated human serum albumin-encapsulated resveratrol nanoparticles: Preparation, characterization, bioavailability and targeting of liver tumors. Artif. Cells Nanomed. Biotechnol. 2019, 47, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Gregoriou, Y.; Gregoriou, G.; Yilmaz, V.; Kapnisis, K.; Prokopi, M.; Anayiotos, A.; Strati, K.; Dietis, N.; Constantinou, A.I.; Andreou, C. Resveratrol loaded polymeric micelles for theranostic targeting of breast cancer cells. Nanotheranostics 2021, 5, 113–124. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, L.; Chen, T.; Guo, W.; Bao, X.; Wang, D.; Ren, B.; Wang, H.; Li, Y.; Wang, Y.; et al. Anticancer Effects of Resveratrol-Loaded Solid Lipid Nanoparticles on Human Breast Cancer Cells. Molecules 2017, 22, 1814. [Google Scholar] [CrossRef] [PubMed]
- Neves, A.R.; Queiroz, J.F.; Reis, S. Brain-targeted delivery of resveratrol using solid lipid nanoparticles functionalized with apolipoprotein E. J. Nanobiotechnol. 2016, 14, 27. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, Y.; Gao, L.; Zhang, Y.; Yi, J. Improved chemical stability and cellular antioxidant activity of resveratrol in zein nanoparticle with bovine serum albumin-caffeic acid conjugate. Food Chem. 2018, 261, 283–291. [Google Scholar] [CrossRef]
- Jeon, Y.O.; Lee, J.-S.; Lee, H.G. Improving solubility, stability, and cellular uptake of resveratrol by nanoencapsulation with chitosan and γ-poly (glutamic acid). Colloids Surf. B Biointerfaces 2016, 147, 224–233. [Google Scholar] [CrossRef]
- Nguyen, A.V.; Martinez, M.; Stamos, M.J.; Moyer, M.P.; Planutis, K.; Hope, C.; Holcombe, R.F. Results of a phase I pilot clinical trial examining the effect of plant-derived resveratrol and grape powder on Wnt pathway target gene expression in colonic mucosa and colon cancer. Cancer Manag. Res. 2009, 1, 25–37. [Google Scholar]
- Bunaciu, R.P.; Yen, A. Resveratrol and Malignancies. Curr. Pharmacol. Rep. 2015, 1, 266–271. [Google Scholar] [CrossRef][Green Version]
- Popat, R.; Plesner, T.; Davies, F.; Cook, G.; Cook, M.; Elliott, P.; Jacobson, E.; Gumbleton, T.; Oakervee, H.; Cavenagh, J. A phase 2 study of SRT501 (resveratrol) with bortezomib for patients with relapsed and or refractory multiple myeloma. Br. J. Haematol. 2013, 160, 714–717. [Google Scholar] [CrossRef]
- Chow, H.-H.S.; Garland, L.L.; Heckman-Stoddard, B.M.; Hsu, C.-H.; Butler, V.D.; Cordova, C.A.; Chew, W.M.; Cornelison, T.L. A pilot clinical study of resveratrol in postmenopausal women with high body mass index: Effects on systemic sex steroid hormones. J. Transl. Med. 2014, 12, 223. [Google Scholar] [CrossRef] [PubMed]
- Rosso, F.; Grimaldi, A.; Barbarisi, A.; Avvisati, V.; De Chiaro, M.; Di Lazzaro, A.; Arra, C.; Barbieri, A.; Palma, G.; Iaffaioli, R. V 1219 Trans-resveratrol reverse drug resistance to docetaxel: A preliminary in vivo study. Eur. J. Cancer Suppl. 2009, 7, 126. [Google Scholar] [CrossRef]
- Vinod, B.S.; Nair, H.H.; Vijayakurup, V.; Shabna, A.; Shah, S.; Krishna, A.; Pillai, K.S.; Thankachan, S.; Anto, R.J. Resveratrol chemosensitizes HER-2-overexpressing breast cancer cells to docetaxel chemoresistance by inhibiting docetaxel-mediated activation of HER-2–Akt axis. Cell Death Discov. 2015, 1, 15061. [Google Scholar] [CrossRef] [PubMed]
- Kumar Singh, S.; Banerjee, S.; Acosta, E.P.; Lillard, J.W.; Singh, R. Resveratrol induces cell cycle arrest and apoptosis with docetaxel in prostate cancer cells via a p53/ p21 WAF1/CIP1 and p27 KIP1 pathway. Oncotarget 2017, 8, 17216. [Google Scholar]
- Singh, S.K.; Lillard, J.W.; Singh, R. Reversal of drug resistance by planetary ball milled (PBM) nanoparticle loaded with resveratrol and docetaxel in prostate cancer. Cancer Lett. 2018, 427, 49–62. [Google Scholar] [CrossRef]
- Lee, S.-H.; Lee, Y.-J. Synergistic anticancer activity of resveratrol in combination with docetaxel in prostate carcinoma cells. Nutr. Res. Pract. 2021, 15, 12–25. [Google Scholar] [CrossRef]
- Aw, M.S.; Kurian, M.; Losic, D. Polymeric micelles for multidrug delivery and combination therapy. Chemistry 2013, 19, 12586–12601. [Google Scholar] [CrossRef]
- Cho, H.; Lai, T.; Tomoda, K.; Kwon, G. Polymeric Micelles for Multi-Drug Delivery in Cancer. AAPS PharmSciTech 2014, 16, 10–20. [Google Scholar] [CrossRef]
- Anwar, D.M.; El-Sayed, M.; Reda, A.; Fang, J.-Y.; Khattab, S.N.; Elzoghby, A.O. Recent advances in herbal combination nanomedicine for cancer: Delivery technology and therapeutic outcomes. Expert Opin. Drug Deliv. 2021, 18, 1609–1625. [Google Scholar] [CrossRef]
- Song, Z.; Shi, Y.; Han, Q.; Dai, G. Endothelial growth factor receptor-targeted and reactive oxygen species-responsive lung cancer therapy by docetaxel and resveratrol encapsulated lipid-polymer hybrid nanoparticles. Biomed. Pharmacother. 2018, 105, 18–26. [Google Scholar] [CrossRef]
- Singh, S.K.; Gordetsky, J.B.; Bae, S.; Acosta, E.P.; Lillard, J.W.; Singh, R. Selective Targeting of the Hedgehog Signaling Pathway by PBM Nanoparticles in Docetaxel-Resistant Prostate Cancer. Cells 2020, 9, 1976. [Google Scholar] [CrossRef] [PubMed]
- Fredenberg, S.; Wahlgren, M.; Reslow, M.; Axelsson, A. The mechanisms of drug release in poly(lactic-co-glycolic acid)-based drug delivery systems—A review. Int. J. Pharm. 2011, 415, 34–52. [Google Scholar] [CrossRef] [PubMed]
- Jelonek, K.; Li, S.; Kaczmarczyk, B.; Marcinkowski, A.; Orchel, A.; Musiał-Kulik, M.; Kasperczyk, J. Multidrug PLA-PEG filomicelles for concurrent delivery of anticancer drugs—The influence of drug-drug and drug-polymer interactions on drug loading and release properties. Int. J. Pharm. 2016, 510, 365–374. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).