Retinal Changes in Astrocytes and Müller Glia in a Mouse Model of Laser-Induced Glaucoma: A Time-Course Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Groups
2.3. Anesthetics
2.4. Laser Treatment and Measurement of IOP
2.5. Immunohistochemistry
2.6. Quantitative Retinal Analysis
2.6.1. GFAP-RA
2.6.2. GFAP-IRI and MHC-II-IRI
2.7. Statistical Analysis
3. Results
3.1. Intraocular Pressure (IOP)
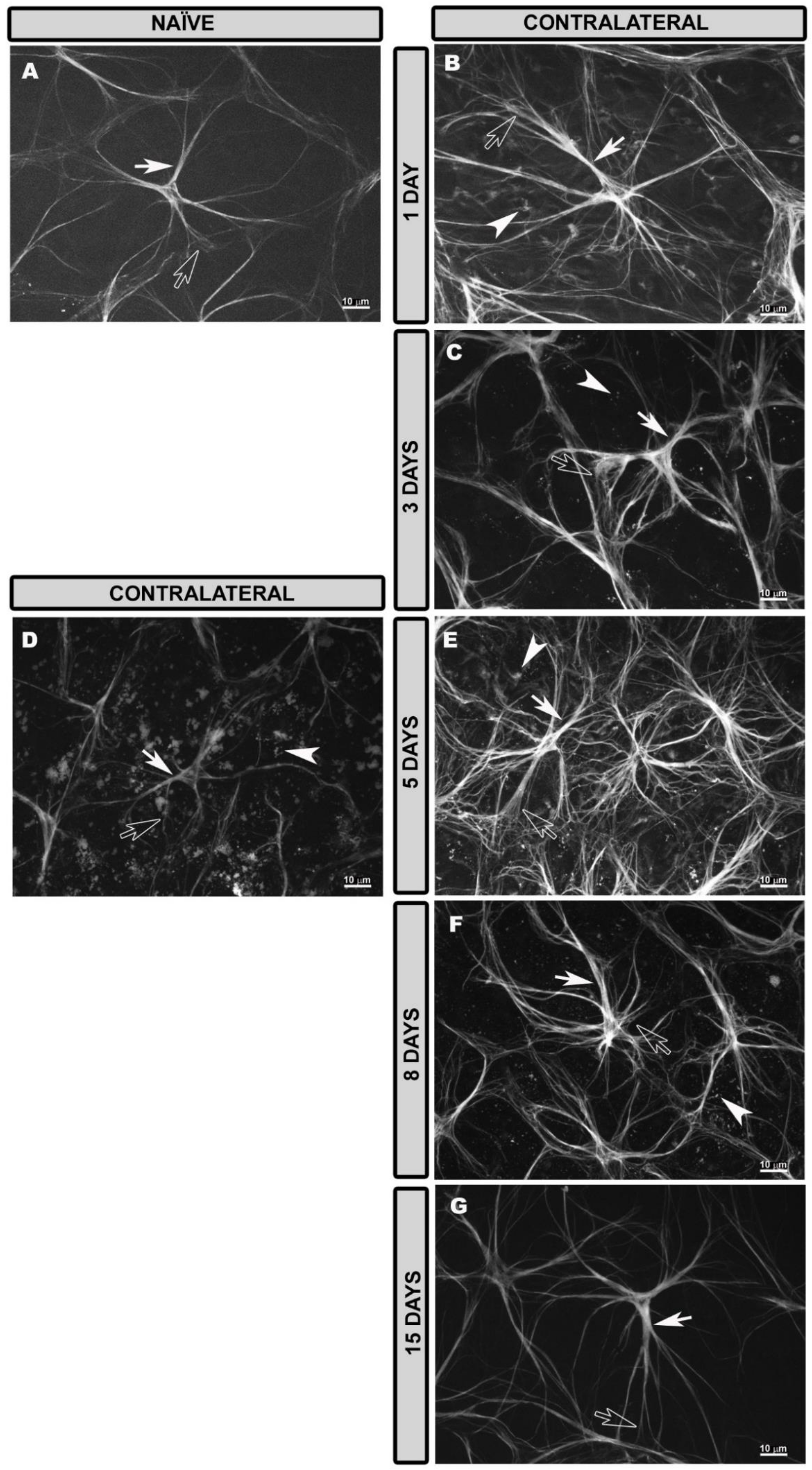
3.2. General Characteristics of Astrocytes and Müller Glia in the Naïve Retina
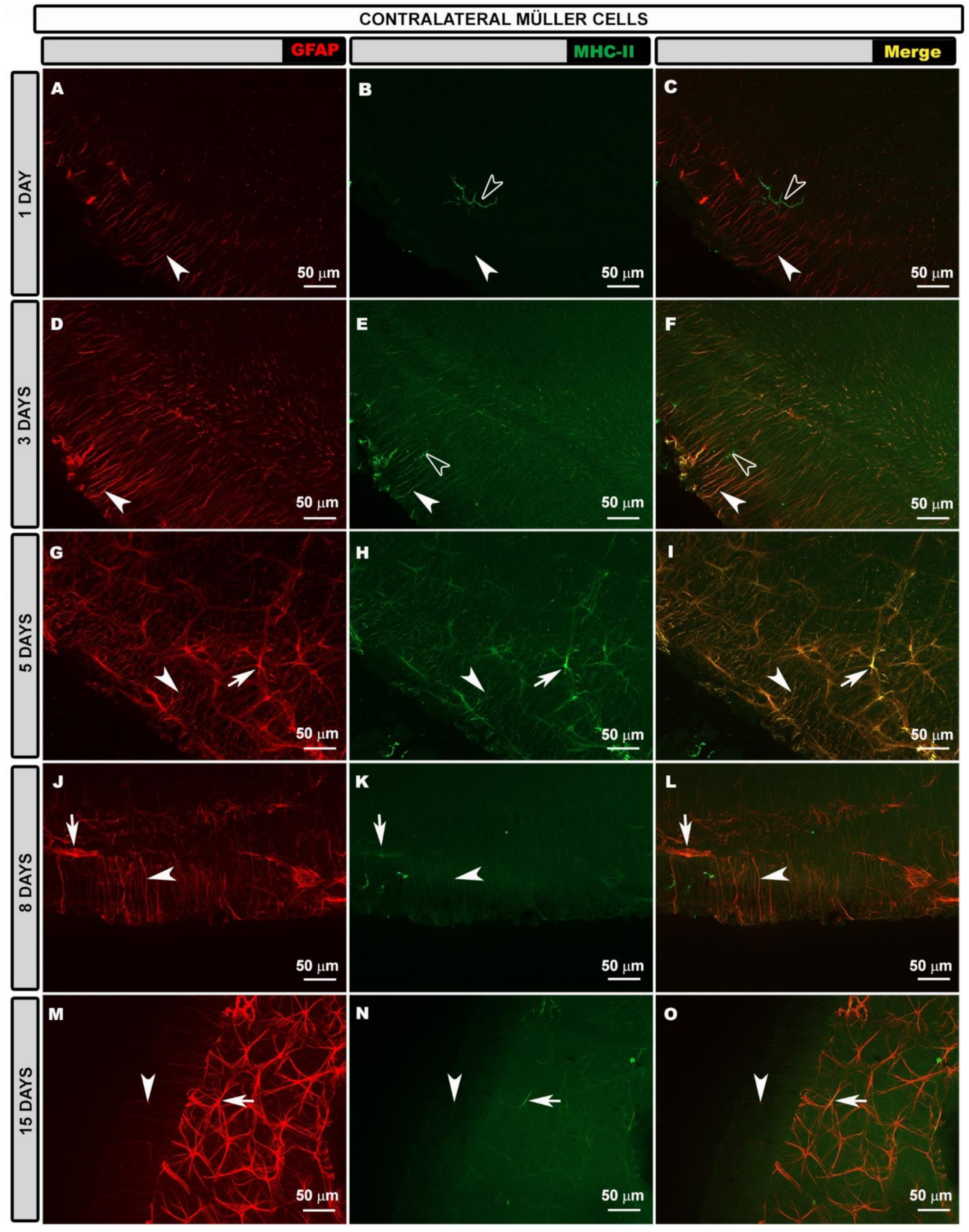
3.3. Morphological Changes in Retinal Macroglia of Contralateral Eyes at Different Time Points after OHT Induction
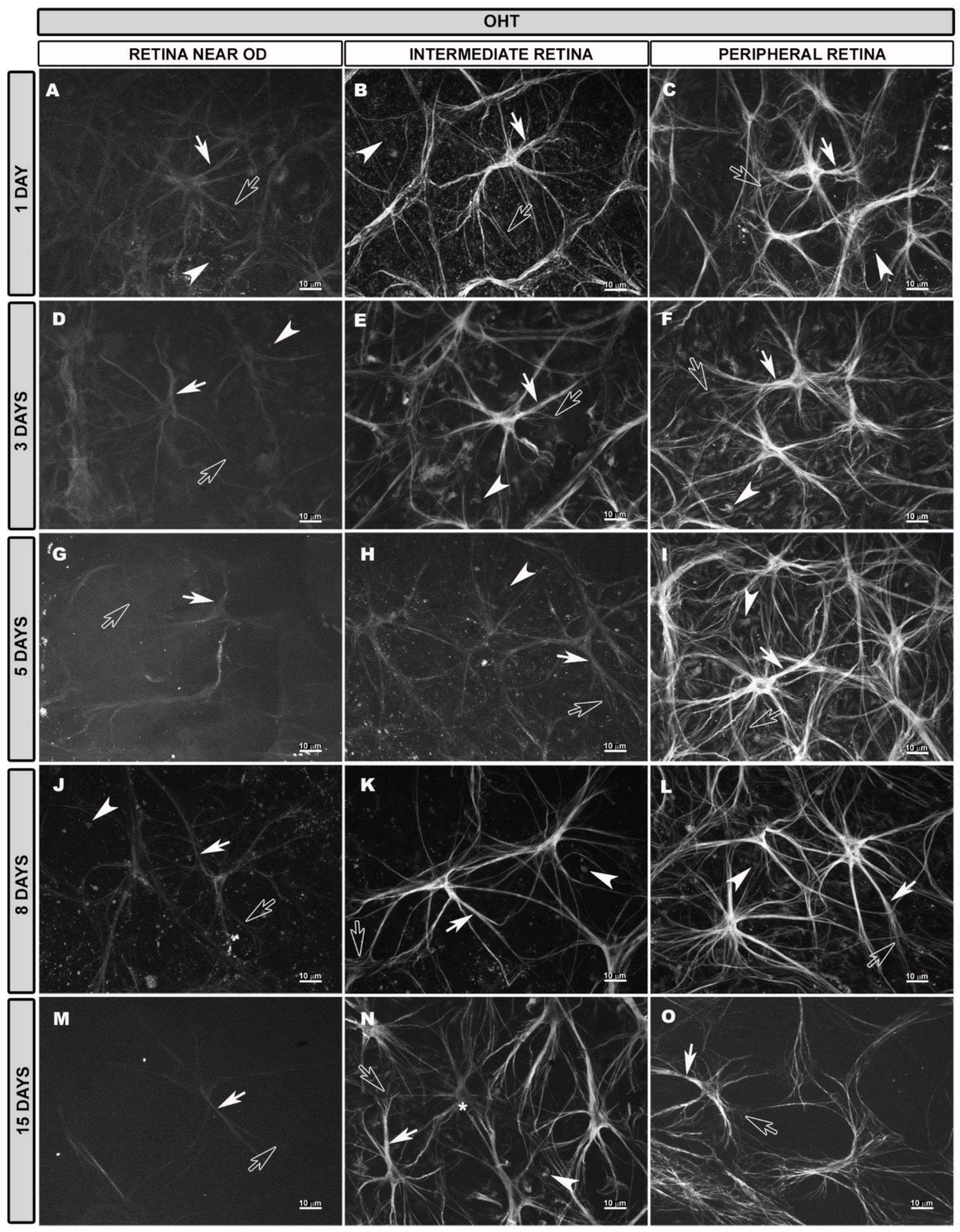
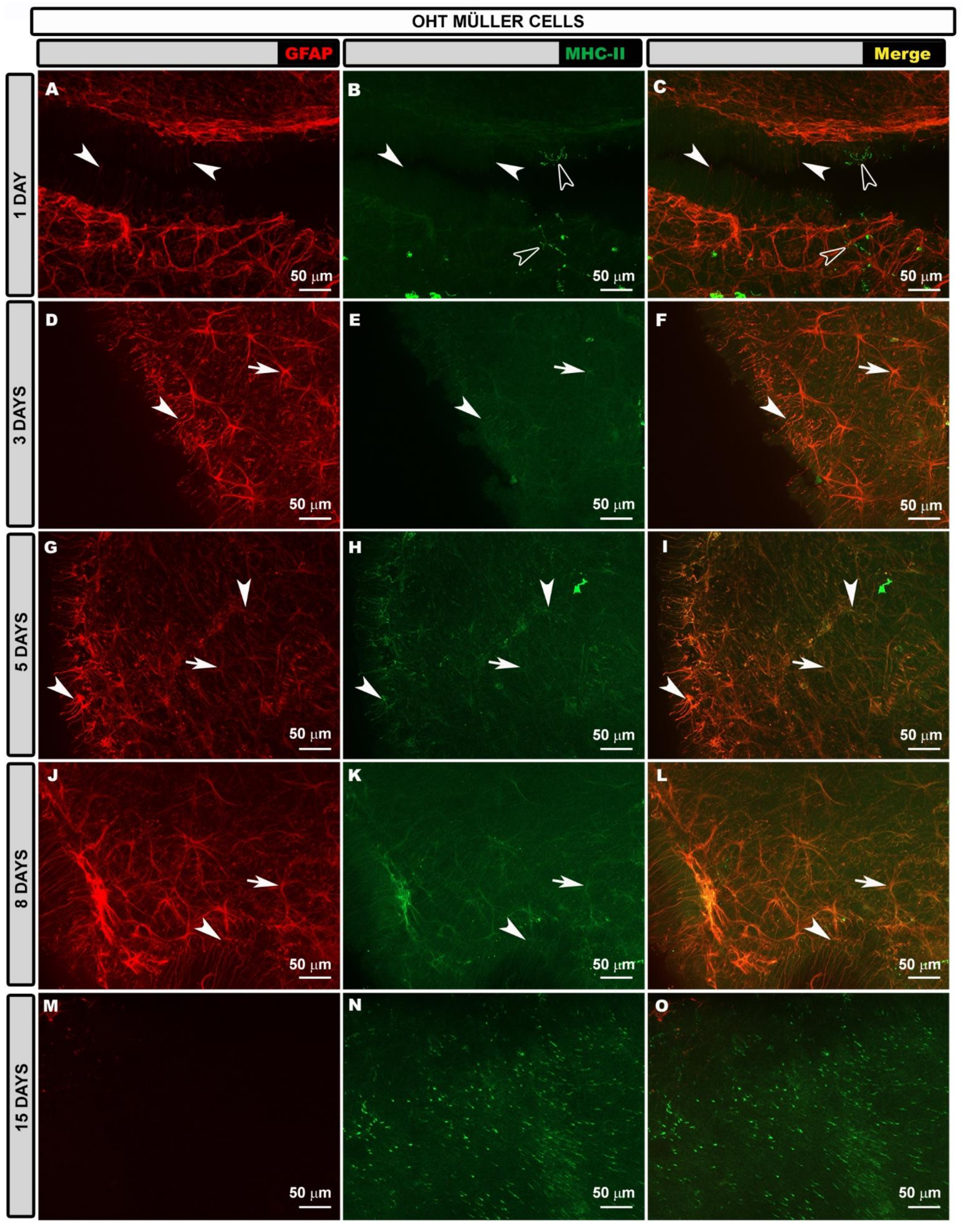
3.4. Morphological Changes in Retinal Macroglia of OHT Eyes at Different Time Points after OHT Induction
3.5. GFAP-Labeled Retinal Area at Different Time Points after OHT Induction
3.5.1. GFAP-RA Total Value
3.5.2. GFAP-RA Values by Retinal Sectors
- -
- Superior retina: (i) the S-OD at 1, 3, 5 and 8 (all p < 0.01) days; (ii) the S-IR at 1, 3, 5, 8 (all p < 0.01) and 15 (p < 0.05) days; (iii) the S-PR at all the time points analyzed (p < 0.01).
- -
- Inferior retina: (i) the I-OD at 1 (p < 0.05), 5 (p < 0.01) and 8 (p < 0.05) days; (ii) the I-IR at 1, 3 (p < 0.05), 5 (p < 0.01) and 8 (p < 0.05) days; (iii) the I-PR at 1, 3, 5 (p < 0.05) and 8 (p < 0.01) days.
- -
- Nasal retina: (i) the N-OD at 1 (p < 0.01), 3 (p < 0.05), 5 (p < 0.01) and 8 (p < 0.05) days; (ii) the N-IR at 1, 3, 5, 8 (p < 0.01) and 15 (p < 0.05) days; (iii) the N-PR at 1, 3, 5 (p < 0.01) and 8 (p < 0.05) days.
- -
- Temporal retina: (i) the T-OD only at 5 days (p < 0.05); (ii) the T-IR at 1, 3, 5 and 8 (p < 0.01) days; (iii) the T-PR at 1, 3, 5 and 8 (p < 0.01) days.
- -
- Superior retina: (i) the S-OD at 1, 3, 5 (all p < 0.01) and 8 (p < 0.05) days; (ii) the S-IR at 1, 3, 5 and 8 days (all p < 0.01); (iii) the S-PR at 1, 3, 5 and 8 (p < 0.01) days.
- -
- Inferior retina: (i) the I-OD at 3 days (p < 0.01); (ii) the I-IR at 1 and 3 days (both p < 0.01) and at 5 and 8 days (p < 0.05); (iii) the I-PR at 3 (p < 0.01), 5 and 8 (both p < 0.05) days.
- -
- Nasal retina: (i) the N-OD at 1 (p < 0.05), 3 (p < 0.01) and 5 (p < 0.05) days; (ii) the N-IR at 1, 3, 5 and 8 days (all p < 0.01); (iii) the N-PR at 1, 3 (both p < 0.01), 5 (p < 0.05) and 8 (p < 0.01) days. At 15 days, in the N-IR, we found a significant decrease in GFAP-RA (p < 0.05).
- -
- Temporal retina: (i) the T-OD only at Day 3 (p < 0.01); (ii) the T-IR at 1, 3, 5 and 8 days (all p < 0.01); (iii) the T-PR at 1 (p < 0.05), 3, 5 and 8 (all p < 0.01) days.
3.6. Intensity of GFAP Immunoreactivity at Different Time Points after OHT Induction
- -
- Superior retina: (i) the S-IR at 3, 5 and 8 days (all p < 0.05); (ii) the S-PR at 1 (p < 0.05), 3, 5, 8 (all p < 0.01) and 15 (p < 0.05) days.
- -
- Inferior retina: (i) the I-OD at 15 days (p < 0.05); (ii) the I-IR at 1, 3 and 8 days (all p < 0.05); (iii) the I-PR at all the time points analyzed (p < 0.05).
- -
- Nasal retina: (i) the N-IR at 1, 3, and 8 days (all p < 0.05); (ii) the N-PR at all the time points analyzed (p < 0.05).
- -
- Temporal retina: (i) the T-OD at 15 days (p < 0.05); (ii) the T-PR at 3, 5, (p < 0.01), 8 (p < 0.05) and 15 (p < 0.01) days.
- -
- Superior retina: (i) a decrease in the S-OD at 1 day (p < 0.05); (ii) an increase in the S-IR at 1 and 3 (p < 0.05) days; (iii) an increase in the S-PR at 1, 3, 5 (p < 0.05) and 8 (p < 0.01) days.
- -
- Inferior retina: (i) a decrease in the I-OD at 5 days (p < 0.05); (ii) an increase in the I-IR at 8 (p < 0.05) days; (iii) an increase in the I-PR at 1, 3, 8 and 15 (p < 0.05) days.
- -
- Nasal retina: (i) an increase in the N-IR at 5 and 8 (p < 0.05) days; (ii) an increase in the N-PR at 1, 3, 5 and 8 (p < 0.05) days.
- -
- Temporal retina: (i) a decrease in the T-OD at 5 days (p < 0.05); (ii) an increase in the T-IR at 1 day (p < 0.05); (iii) an increase in the T-PR at 1 day (p < 0.01), 3 days (p < 0.05) and 8 days (p < 0.01).
3.7. Intensity of MHC-II Immunoreactivity at Different Time Points after OHT Induction
- -
- Superior retina: (i) the S-OD only at 3 days (p < 0.05); (ii) the S-IR at 1, 3, 5 (all p < 0.01) and 8 (p < 0.05) days; (iii) the S-PR at all the time points analyzed (all p < 0.01).
- -
- Inferior retina: (i) I-IR at 1 (p < 0.01), 3 (p < 0.05), 5 and 8 (p < 0.01) days; (ii) the I-PR at all the time points analyzed (all p < 0.01).
- -
- Nasal retina: (i) the N-IR at all the time points analyzed (all p < 0.01); (ii) the N-PR at all the time points analyzed (all p < 0.01).
- -
- Temporal retina: (i) the T-OD only at 8 days (p < 0.05); (ii) the T-IR at all the time points analyzed (all p < 0.01); (iii) the T-PR at all the time points analyzed (all p < 0.01).
- -
- Superior retina: (i) the S-IR at 1 (p < 0.05), 3, 5 (both p < 0.01) and 8 (p < 0.05) days; (ii) the S-PR at all the time points analyzed (all p < 0.01).
- -
- Inferior retina: (i) the I-IR at 3 (p < 0.01) and 8 (p < 0.05) days; (ii) the I-PR at 1 (p < 0.05), 3 (p < 0.01), 5 and 8 (both p < 0.05) days.
- -
- Nasal retina: (i) the N-IR at 3 (p < 0.01), 5 and 8 (both p < 0.05) days; (ii) the N-PR at 1, 3 (both p < 0.01), 5 and 8 (p < 0.05) days.
- -
- Temporal retina: (i) the T-IR at 3, 5 and 8 (all p < 0.05) days; (ii) the T-PR at 1, 3 (both p < 0.01), 5 (p < 0.05) and 8 (p < 0.01) days.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global Prevalence of Glaucoma and Projections of Glaucoma Burden through 2040: A Systematic Review and Meta-Analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Flaxman, S.R.; Bourne, R.R.A.; Resnikoff, S.; Ackland, P.; Braithwaite, T.; Cicinelli, M.V.; Das, A.; Jonas, J.B.; Keeffe, J.; Kempen, J.; et al. Global Causes of Blindness and Distance Vision Impairment 1990–2020: A Systematic Review and Meta-Analysis. Lancet Glob. Health 2017, 5, e1221–e1234. [Google Scholar] [CrossRef] [Green Version]
- Qu, J.; Wang, D.; Grosskreutz, C.L. Mechanisms of Retinal Ganglion Cell Injury and Defense in Glaucoma. Exp. Eye Res. 2010, 91, 48–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meier-Gibbons, F.; Töteberg-Harms, M. Structure/Function/Treatment in Glaucoma: Progress over the Last 10 Years. Ophthalmologe 2021, 118, 1216–1221. [Google Scholar] [CrossRef] [PubMed]
- Gallego, B.I.; Salazar, J.J.; de Hoz, R.; Rojas, B.; Ramírez, A.I.; Salinas-Navarro, M.; Ortín-Martínez, A.; Valiente-Soriano, F.J.; Avilés-Trigueros, M.; Villegas-Perez, M.P.; et al. IOP Induces Upregulation of GFAP and MHC-II and Microglia Reactivity in Mice Retina Contralateral to Experimental Glaucoma. J. Neuroinflamm. 2012, 9, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramírez, J.M.; Rojas, B.; Gallego, B.I.; García-Martín, E.S.; Triviño, A.; Ramírez, A.I.; Salazar, J.J.; de Hoz, R. Glia and Blood Retinal Barrier: Effects of Ocular Hypertension. In Cardiovascular Disease II; iConcept Press Ltd.: Hong Kong, China, 2014; pp. 123–162. [Google Scholar]
- Ramírez, A.I.; de Hoz, R.; Fernández-Albarral, J.A.; Salobrar-García, E.; Rojas, B.; Valiente-Soriano, F.J.; Avilés-Trigueros, M.; Villegas-Pérez, M.P.; Vidal-Sanz, M.; Triviño, A.; et al. Time Course of Bilateral Microglial Activation in a Mouse Model of Laser-Induced Glaucoma. Sci. Rep. 2020, 10, 4890. [Google Scholar] [CrossRef] [Green Version]
- Ramírez, A.I.; Salazar, J.J.; de Hoz, R.; Rojas, B.; Gallego, B.I.; Salinas-Navarro, M.; Alarcón-Martínez, L.; Ortín-Martínez, A.; Avilés-Trigueros, M.; Vidal-Sanz, M.; et al. Quantification of the Effect of Different Levels of IOP in the Astroglia of the Rat Retina Ipsilateral and Contralateral to Experimental Glaucoma. Investig. Opthalmol. Vis. Sci. 2010, 51, 5690–5696. [Google Scholar] [CrossRef] [Green Version]
- De Hoz, R.; Ramírez, A.I.; González-Martín, R.; Ajoy, D.; Rojas, B.; Salobrar-García, E.; Valiente-Soriano, F.J.; Avilés-Trigueros, M.; Villegas-Pérez, M.P.; Vidal-Sanz, M.; et al. Bilateral Early Activation of Retinal Microglial Cells in a Mouse Model of Unilateral Laser-Induced Experimental Ocular Hypertension. Exp. Eye Res. 2018, 171, 12–29. [Google Scholar] [CrossRef]
- Tezel, G.; Ben-Hur, T.; Gibson, G.E.; Stevens, B.; Streit, W.J.; Wekerle, H.; Bhattacharya, S.K.; Borras, T.; Burgoyne, C.F.; Caspi, R.R.; et al. The Role of Glia, Mitochondria, and the Immune System in Glaucoma. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1001–1012. [Google Scholar] [CrossRef]
- Tezel, G. The Immune Response in Glaucoma: A Perspective on the Roles of Oxidative Stress. Exp. Eye Res. 2011, 93, 178–186. [Google Scholar] [CrossRef] [Green Version]
- de Hoz, R.; Rojas, B.; Ramírez, A.I.; Salazar, J.J.; Gallego, B.I.; Triviño, A.; Ramírez, J.M. Retinal Macroglial Responses in Health and Disease. BioMed Res. Int. 2016, 2016, 2954721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pannicke, T.; Biedermann, B.; Uckermann, O.; Weick, M.; Bringmann, A.; Wolf, S.; Wiedemann, P.; Habermann, G.; Buse, E.; Reichenbach, A. Physiological Properties of Retinal Müller Glial Cells from the Cynomolgus Monkey, Macaca Fascicularis—A Comparison to Human Müller Cells. Vis. Res. 2005, 45, 1781–1791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bringmann, A.; Pannicke, T.; Grosche, J.; Francke, M.; Wiedemann, P.; Skatchkov, S.N.; Osborne, N.N.; Reichenbach, A. Müller Cells in the Healthy and Diseased Retina. Prog. Retin. Eye Res. 2006, 25, 397–424. [Google Scholar] [CrossRef]
- Bringmann, A.; Wiedemann, P. Müller Glial Cells in Retinal Disease. Ophthalmologica 2012, 227, 1–19. [Google Scholar] [CrossRef]
- Pekny, M.; Pekna, M.; Messing, A.; Steinhäuser, C.; Lee, J.-M.; Parpura, V.; Hol, E.M.; Sofroniew, M.V.; Verkhratsky, A. Astrocytes: A Central Element in Neurological Diseases. Acta Neuropathol. 2015, 131, 323–345. [Google Scholar] [CrossRef]
- Oberheim, N.A.; Wang, X.; Goldman, S.; Nedergaard, M. Astrocytic Complexity Distinguishes the Human Brain. Trends Neurosci. 2006, 29, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Reichenbach, A.; Bringmann, A. New Functions of Müller Cells. Glia 2013, 61, 651–678. [Google Scholar] [CrossRef]
- Sanderson, J.; Dartt, D.A.; Trinkaus-Randall, V.; Pintor, J.; Civan, M.M.; Delamere, N.A.; Fletcher, E.L.; Salt, T.E.; Grosche, A.; Mitchell, C.H. Purines in the Eye: Recent Evidence for the Physiological and Pathological Role of Purines in the RPE, Retinal Neurons, Astrocytes, Müller Cells, Lens, Trabecular Meshwork, Cornea and Lacrimal Gland. Exp. Eye Res. 2014, 127, 270–279. [Google Scholar] [CrossRef]
- Johnson, E.C.; Morrison, J.C. Friend or Foe? Resolving the Impact of Glial Responses in Glaucoma. J. Glaucoma 2009, 18, 341–353. [Google Scholar] [CrossRef] [Green Version]
- Perea, G.; Navarrete, M.; Araque, A. Tripartite Synapses: Astrocytes Process and Control Synaptic Information. Trends Neurosci. 2009, 32, 421–431. [Google Scholar] [CrossRef]
- Macht, V.A. Neuro-Immune Interactions across Development: A Look at Glutamate in the Prefrontal Cortex. Neurosci. Biobehav. Rev. 2016, 71, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Tsacopoulos, M.; Magistretti, P.J. Metabolic Coupling between Glia and Neurons. J. Neurosci. 1996, 16, 877–885. [Google Scholar] [CrossRef]
- Fernández-Albarral, J.A.; Salazar, J.J.; de Hoz, R.; Marco, E.M.; Martín-Sánchez, B.; Flores-Salguero, E.; Salobrar-García, E.; López-Cuenca, I.; Barrios-Sabador, V.; Avilés-Trigueros, M.; et al. Retinal Molecular Changes Are Associated with Neuroinflammation and Loss of RGCs in an Experimental Model of Glaucoma. Int. J. Mol. Sci. 2021, 22, 2066. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.X. Antioxidant Defense of the Brain: A Role for Astrocytes. Can. J. Physiol. Pharmacol. 1997, 75, 1149–1163. [Google Scholar] [CrossRef]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte-Endothelial Interactions at the Blood-Brain Barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef]
- Tout, S.; Chan-Ling, T.; Holländer, H.; Stone, J. The Role of Müller Cells in the Formation of the Blood-Retinal Barrier. Neuroscience 1993, 55, 291–301. [Google Scholar] [CrossRef]
- Paisley, C.E.; Kay, J.N. Seeing Stars: Development and Function of Retinal Astrocytes. Dev. Biol. 2021, 478, 144–154. [Google Scholar] [CrossRef]
- Koehler, R.C.; Roman, R.J.; Harder, D.R. Astrocytes and the Regulation of Cerebral Blood Flow. Trends Neurosci. 2009, 32, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Hablitz, L.M.; Nedergaard, M. The Glymphatic System: A Novel Component of Fundamental Neurobiology. J. Neurosci. 2021, 41, 7698–7711. [Google Scholar] [CrossRef]
- Dong, Y.; Benveniste, E.N. Immune Function of Astrocytes. Glia 2001, 36, 180–190. [Google Scholar] [CrossRef]
- Peng, L.; Parpura, V.; Verkhratsky, A. Editorial (Thematic Issue: Neuroglia as a Central Element of Neurological Diseases: An Underappreciated Target for Therapeutic Intervention). Curr. Neuropharmacol. 2014, 12, 303–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pekny, M.; Pekna, M. Astrocyte Reactivity and Reactive Astrogliosis: Costs and Benefits. Physiol. Rev. 2014, 94, 1077–1098. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.-B.; Song, L.-J.; Wang, Q.; Kumar, G.; Yan, Y.-Q.; Ma, C.-G. Astrocytes: A Double-Edged Sword in Neurodegenerative Diseases. Neural Regen. Res. 2021, 16, 1702–1710. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.P.; Fisher, S.K. Up-Regulation of Glial Fibrillary Acidic Protein in Response to Retinal Injury: Its Potential Role in Glial Remodeling and a Comparison to Vimentin Expression. Int. Rev. Cytol. 2003, 230, 263–290. [Google Scholar] [CrossRef] [PubMed]
- Pekny, M.; Wilhelmsson, U.; Pekna, M. The Dual Role of Astrocyte Activation and Reactive Gliosis. Neurosci. Lett. 2014, 565, 30–38. [Google Scholar] [CrossRef]
- Bringmann, A.; Iandiev, I.; Pannicke, T.; Wurm, A.; Hollborn, M.; Wiedemann, P.; Osborne, N.N.; Reichenbach, A. Cellular Signaling and Factors Involved in Müller Cell Gliosis: Neuroprotective and Detrimental Effects. Prog. Retin. Eye Res. 2009, 28, 423–451. [Google Scholar] [CrossRef]
- Coorey, N.J.; Shen, W.; Chung, S.H.; Zhu, L.; Gillies, M.C. The Role of Glia in Retinal Vascular Disease. Clin. Exp. Optom. 2012, 95, 266–281. [Google Scholar] [CrossRef]
- Seitz, R.; Ohlmann, A.; Tamm, E.R. The Role of Müller Glia and Microglia in Glaucoma. Cell Tissue Res. 2013, 353, 339–345. [Google Scholar] [CrossRef]
- González, H.; Elgueta, D.; Montoya, A.; Pacheco, R. Neuroimmune Regulation of Microglial Activity Involved in Neuroinflammation and Neurodegenerative Diseases. J. Neuroimmunol. 2014, 274, 1–13. [Google Scholar] [CrossRef]
- Kettenmann, H.; Hanisch, U.K.; Noda, M.; Verkhratsky, A. Physiology of Microglia. Physiol. Rev. 2011, 91, 461–553. [Google Scholar] [CrossRef]
- Barcia, C.; Ros, C.M.; Annese, V.; Gómez, A.; Ros-Bernal, F.; Aguado-Year, C.; Martínez-Paǵn, M.E.; De Pablos, V.; Fernandez-Villalba, E.; Herrero, M.T. IFN-γ Signaling, with the Synergistic Contribution of TNF-α, Mediates Cell Specific Microglial and Astroglial Activation in Experimental Models of Parkinson’s Disease. Cell Death Dis. 2011, 2, e142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bezzi, P.; Domercq, M.; Brambilla, L.; Galli, R.; Schols, D.; De Clercq, E.; Vescovi, A.; Bagetta, G.; Kollias, G.; Meldolesi, J.; et al. CXCR4-Activated Astrocyte Glutamate Release via TNFα: Amplification by Microglia Triggers Neurotoxicity. Nat. Neurosci. 2001, 4, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Stolp, H.B.; Dziegielewska, K.M. Review: Role of Developmental Inflammation and Blood-Brain Barrier Dysfunction in Neurodevelopmental and Neurodegenerative Diseases. Neuropathol. Appl. Neurobiol. 2009, 35, 132–146. [Google Scholar] [CrossRef] [PubMed]
- Bringmann, A.; Wiedemann, P. Involvement of Müller Glial Cells in Epiretinal Membrane Formation. Graefe’s Arch. Clin. Exp. Ophthalmol. 2009, 247, 865–883. [Google Scholar] [CrossRef] [PubMed]
- Inman, D.M.; Horner, P.J. Reactive Nonproliferative Gliosis Predominates in a Chronic Mouse Model of Glaucoma. Glia 2007, 55, 942–953. [Google Scholar] [CrossRef]
- Hernandez, M.R.; Miao, H.; Lukas, T. Astrocytes in Glaucomatous Optic Neuropathy. Prog. Brain Res. 2008, 173, 353–373. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tay, S.S.W.; Ng, Y.K. An Immunohistochemical Study of Neuronal and Glial Cell Reactions in Retinae of Rats with Experimental Glaucoma. Exp. Brain Res. 2000, 132, 476–484. [Google Scholar] [CrossRef]
- Tezel, G.; Chauhan, B.C.; LeBlanc, R.P.; Wax, M.B. Immunohistochemical Assessment of the Glial Mitogen-Activated Protein Kinase Activation in Glaucoma. Investig. Ophthalmol. Vis. Sci. 2003, 44, 3025–3033. [Google Scholar] [CrossRef] [Green Version]
- Bolz, S.; Schuettauf, F.; Fries, J.E.; Thaler, S.; Reichenbach, A.; Pannicke, T. K+ Currents Fail to Change in Reactive Retinal Glial Cells in a Mouse Model of Glaucoma. Graefe’s Arch. Clin. Exp. Ophthalmol. 2008, 246, 1249–1254. [Google Scholar] [CrossRef]
- Salinas-Navarro, M.; Alarcón-Martínez, L.; Valiente-Soriano, F.J.; Jiménez-López, M.; Mayor-Torroglosa, S.; Avilés-Trigueros, M.; Villegas-Pérez, M.P.; Vidal-Sanz, M. Ocular Hypertension Impairs Optic Nerve Axonal Transport Leading to Progressive Retinal Ganglion Cell Degeneration. Exp. Eye Res. 2010, 90, 168–183. [Google Scholar] [CrossRef]
- Cuenca, N.; Pinilla, I.; Fernández-Sánchez, L.; Salinas-Navarro, M.; Alarcón-Martínez, L.; Avilés-Trigueros, M.; de la Villa, P.; Miralles de Imperial, J.; Villegas-Pérez, M.P.; Vidal-Sanz, M. Changes in the Inner and Outer Retinal Layers after Acute Increase of the Intraocular Pressure in Adult Albino Swiss Mice. Exp. Eye Res. 2010, 91, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Salinas-Navarro, M.; Alarcón-Martínez, L.; Valiente-Soriano, F.J.; Ortín-Martínez, A.; Jiménez-López, M.; Avilés-Trigueros, M.; Villegas-Pérez, M.P.; de la Villa, P.; Vidal-Sanz, M. Functional and Morphological Effects of Laser-Induced Ocular Hypertension in Retinas of Adult Albino Swiss Mice. Mol. Vis. 2009, 15, 2578–2598. [Google Scholar] [PubMed]
- de Gracia, P.; Gallego, B.I.; Rojas, B.; Ramírez, A.I.; de Hoz, R.; Salazar, J.J.; Triviño, A.; Ramírez, J.M. Automatic Counting of Microglial Cells in Healthy and Glaucomatous Mouse Retinas. PLoS ONE 2015, 10, e0143278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Vidal-Sanz, M.; Salinas-Navarro, M.; Nadal-Nicolás, F.M.; Alarcón-Martínez, L.; Valiente-Soriano, F.J.; Miralles de Imperial, J.; Avilés-Trigueros, M.; Agudo-Barriuso, M.; Villegas-Pérez, M.P. Understanding Glaucomatous Damage: Anatomical and Functional Data from Ocular Hypertensive Rodent Retinas. Prog. Retin. Eye Res. 2012, 31, 1–27. [Google Scholar] [CrossRef] [Green Version]
- Ramírez, A.I.; Fernández-Albarral, J.A.; de Hoz, R.; López-Cuenca, I.; Salobrar-García, E.; Rojas, P.; Valiente-Soriano, F.J.; Avilés-Trigueros, M.; Villegas-Pérez, M.P.; Vidal-Sanz, M.; et al. Microglial Changes in the Early Aging Stage in a Healthy Retina and an Experimental Glaucoma Model. Prog. Brain Res. 2020, 256, 125–149. [Google Scholar] [CrossRef]
- Yuan, L.; Neufeld, A.H. Activated Microglia in the Human Glaucomatous Optic Nerve Head. J. Neurosci. Res. 2001, 64, 523–532. [Google Scholar] [CrossRef]
- Middeldorp, J.; Hol, E.M. GFAP in Health and Disease. Prog. Neurobiol. 2011, 93, 421–443. [Google Scholar] [CrossRef]
- Varela, H.J.; Hernandez, M.R. Astrocyte Responses in Human Optic Nerve Head with Primary Open-Angle Glaucoma. J. Glaucoma 1997, 6, 303–313. [Google Scholar] [CrossRef]
- Hernandez, M.R. The Optic Nerve Head in Glaucoma: Role of Astrocytes in Tissue Remodeling. Prog. Retin. Eye Res. 2000, 19, 297–321. [Google Scholar] [CrossRef]
- Napper, G.A.; Kalloniatis, M. Neurochemical Changes Following Postmortem Ischemia in the Rat Retina. Vis. Neurosci. 1999, 16, 1169–1180. [Google Scholar] [CrossRef] [PubMed]
- de Raad, S.; Szczesny, P.J.; Munz, K.; Remé, C.E. Light Damage in the Rat Retina: Glial Fibrillary Acidic Protein Accumulates in Müller Cells in Correlation with Photoreceptor Damage. Ophthalmic Res. 1996, 28, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Ostrow, L.W.; Suchyna, T.M.; Sachs, F. Stretch Induced Endothelin-1 Secretion by Adult Rat Astrocytes Involves Calcium Influx via Stretch-Activated Ion Channels (SACs). Biochem. Biophys. Res. Commun. 2011, 410, 81–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rungger-Brändle, E.; Dosso, A.A.; Leuenberger, P.M. Glial Reactivity, an Early Feature of Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1971–1980. [Google Scholar]
- Shinozaki, Y.; Koizumi, S. Potential Roles of Astrocytes and Müller Cells in the Pathogenesis of Glaucoma. J. Pharm. Sci. 2021, 145, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Cheung, H.; King, B.J.; Gast, T.J. Presumed Activated Retinal Astrocytes and Müller Cells in Healthy and Glaucomatous Eyes Detected by Spectral Domain Optical Coherence Tomography. Ophthalmic Physiol. Opt. 2020, 40, 738–751. [Google Scholar] [CrossRef] [PubMed]
- Luna, G.; Lewis, G.P.; Banna, C.D.; Skalli, O.; Fisher, S.K. Expression Profiles of Nestin and Synemin in Reactive Astrocytes and Müller Cells Following Retinal Injury: A Comparison with Glial Fibrillar Acidic Protein and Vimentin. Mol. Vis. 2010, 16, 2511–2523. [Google Scholar]
- Penn, J.S.; Madan, A.; Caldwell, R.B.; Bartoli, M.; Caldwell, R.W.; Hartnett, M.E. Vascular Endothelial Growth Factor in Eye Disease. Prog. Retin. Eye Res. 2008, 27, 331–371. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Álvarez, M.J.; Isiegas, C.; Santano, C.; Salazar, J.J.; Ramírez, A.I.; Triviño, A.; Ramírez, J.M.; Albar, J.P.; De La Rosa, E.J.; Prada, C. Vimentin Isoform Expression in the Human Retina Characterized with the Monoclonal Antibody 3CB2. J. Neurosci. Res. 2008, 86, 1871–1883. [Google Scholar] [CrossRef] [Green Version]
- Hatten, M.E.; Liem, R.K.H.; Shelanski, M.L.; Mason, C.A. Astroglia in CNS Injury. Glia 1991, 4, 233–243. [Google Scholar] [CrossRef]
- Brenner, M.; Messing, A. Regulation of GFAP Expression. ASN Neuro 2021, 13, 1759091420981206. [Google Scholar] [CrossRef] [PubMed]
- Kanamori, A.; Nakamura, M.; Nakanishi, Y.; Yamada, Y.; Negi, A. Long-Term Glial Reactivity in Rat Retinas Ipsilateral and Contralateral to Experimental Glaucoma. Exp. Eye Res. 2005, 81, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Gardner, T.W.; Antonetti, D.A.; Barber, A.J.; LaNoue, K.F.; Levison, S.W. Diabetic Retinopathy: More than Meets the Eye. Surv. Ophthalmol. 2002, 47 (Suppl. 2), S253–S262. [Google Scholar] [CrossRef]
- Cobb, J.A.; O’Neill, K.; Milner, J.; Mahajan, G.J.; Lawrence, T.J.; May, W.L.; Miguel-Hidalgo, J.; Rajkowska, G.; Stockmeier, C.A. Density of GFAP-Immunoreactive Astrocytes Is Decreased in Left Hippocampi in Major Depressive Disorder. Neuroscience 2016, 316, 209–220. [Google Scholar] [CrossRef] [Green Version]
- Dalik, H.; Yardimoglu, M.; Filiz, S.; Gonca, S.; Dalik, C.; Erden, B.F. Chronic Ethanol-Induced Glial Fibrillary Acidic Protein (GFAP) Immunoreactivity: An Immunocytochemical Observation in Various Regions of Adult Rat Brain. Int. J. Neurosci. 2009, 119, 1303–1318. [Google Scholar] [CrossRef]
- Alfonso-Loeches, S.; Ureña-Peralta, J.; Morillo-Bargues, M.J.; Gómez-Pinedo, U.; Guerri, C. Ethanol-Induced TLR4/NLRP3 Neuroinflammatory Response in Microglial Cells Promotes Leukocyte Infiltration Across the BBB. Neurochem. Res. 2016, 41, 193–209. [Google Scholar] [CrossRef]
- Tezel, G. Multifactorial Pathogenic Processes of Retinal Ganglion Cell Degeneration in Glaucoma towards Multi-Target Strategies for Broader Treatment Effects. Cells 2021, 10, 1372. [Google Scholar] [CrossRef]
- Hughes, E.G.; Maguire, J.L.; McMinn, M.T.; Scholz, R.E.; Sutherland, M.L. Loss of Glial Fibrillary Acidic Protein Results in Decreased Glutamate Transport and Inhibition of PKA-Induced EAAT2 Cell Surface Trafficking. Mol. Brain Res. 2004, 124, 114–123. [Google Scholar] [CrossRef]
- Liedtke, W.; Edelmann, W.; Bieri, P.L.; Chiu, F.C.; Cowan, N.J.; Kucherlapati, R.; Raine, C.S. GFAP Is Necessary for the Integrity of CNS White Matter Architecture and Long-Term Maintenance of Myelination. Neuron 1996, 17, 607–615. [Google Scholar] [CrossRef] [Green Version]
- Quaranta, L.; Bruttini, C.; Micheletti, E.; Konstas, A.G.P.; Michelessi, M.; Oddone, F.; Katsanos, A.; Sbardella, D.; De Angelis, G.; Riva, I. Glaucoma and Neuroinflammation: An Overview. Surv. Ophthalmol. 2021, 66, 693–713. [Google Scholar] [CrossRef]
- Blum, J.; Wearsch, P.; Cresswell, P. Pathways of Antigen Processing. Annu. Rev. Immunol. 2013, 31, 443–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carson, M.J.; Reilly, C.R.; Sutcliffe, J.G.; Lo, D. Mature Microglia Resemble Immature Antigen-Presenting Cells. Glia 1998, 22, 72–85. [Google Scholar] [CrossRef]
- Carson, M.J.; Sutcliffe, J.G.; Campbell, I.L. Microglia Stimulate Naive T-Cell Differentiation without Stimulating T-Cell Proliferation. J. Neurosci. Res. 1999, 55, 127–134. [Google Scholar] [CrossRef]
- Nikcevich, K.M.; Gordon, K.B.; Tan, L.; Hurst, S.D.; Kroepfl, J.F.; Gardinier, M.; Barrett, T.A.; Miller, S.D. IFN-Gamma-Activated Primary Murine Astrocytes Express B7 Costimulatory Molecules and Prime Naive Antigen-Specific T Cells. J. Immunol. 1997, 158, 614–621. [Google Scholar] [PubMed]
- Yang, J.; Yang, P.; Tezel, G.; Patil, R.V.; Hernandez, M.R.; Wax, M.B. Induction of HLA-DR Expression in Human Lamina Cribrosa Astrocytes by Cytokines and Simulated Ischemia. Investig. Ophthalmol. Vis. Sci. 2001, 42, 365–371. [Google Scholar]
- Lucas-Ruiz, F.; Galindo-Romero, C.; Albaladejo-García, V.; Vidal-Sanz, M.; Agudo-Barriuso, M. Mechanisms Implicated in the Contralateral Effect in the Central Nervous System after Unilateral Injury: Focus on the Visual System. Neural Regen. Res. 2021, 16, 2125. [Google Scholar] [CrossRef]
- Bozic, I.; Savic, D.; Lavrnja, I. Astrocyte Phenotypes: Emphasis on Potential Markers in Neuroinflammation. Histol. Histopathol. 2020, 36, 18284. [Google Scholar] [CrossRef]
- Neumann, H.; Boucraut, J.; Hahnel, C.; Misgeld, T.; Wekerle, H. Neuronal Control of MHC Class II Inducibility in Rat Astrocytes and Microglia. Eur. J. Neurosci. 1996, 8, 2582–2590. [Google Scholar] [CrossRef]
- Rostami, J.; Fotaki, G.; Sirois, J.; Mzezewa, R.; Bergström, J.; Essand, M.; Healy, L.; Erlandsson, A. Astrocytes Have the Capacity to Act as Antigen-Presenting Cells in the Parkinson’s Disease Brain. J. Neuroinflamm. 2020, 17, 119. [Google Scholar] [CrossRef]
- Green, D.S.; Young, H.A.; Valencia, J.C. Current Prospects of Type II Interferon γ Signaling & Autoimmunity. J. Biol. Chem. 2017, 292, 13925–13933. [Google Scholar] [CrossRef]
- Matejuk, A.; Ransohoff, R.M. Crosstalk Between Astrocytes and Microglia: An Overview. Front. Immunol. 2020, 11, 1416. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, Y.; Shibata, K.; Yoshida, K.; Shigetomi, E.; Gachet, C.; Ikenaka, K.; Tanaka, K.F.; Koizumi, S. Transformation of Astrocytes to a Neuroprotective Phenotype by Microglia via P2Y1 Receptor Downregulation. Cell Rep. 2017, 19, 1151–1164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanisch, U.K.; Kettenmann, H. Microglia: Active Sensor and Versatile Effector Cells in the Normal and Pathologic Brain. Nat. Neurosci. 2007, 10, 1387–1394. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Albarral, J.A.; de Hoz, R.; Matamoros, J.A.; Chen, L.; López-Cuenca, I.; Salobrar-García, E.; Sánchez-Puebla, L.; Ramírez, J.M.; Triviño, A.; Salazar, J.J.; et al. Retinal Changes in Astrocytes and Müller Glia in a Mouse Model of Laser-Induced Glaucoma: A Time-Course Study. Biomedicines 2022, 10, 939. https://doi.org/10.3390/biomedicines10050939
Fernández-Albarral JA, de Hoz R, Matamoros JA, Chen L, López-Cuenca I, Salobrar-García E, Sánchez-Puebla L, Ramírez JM, Triviño A, Salazar JJ, et al. Retinal Changes in Astrocytes and Müller Glia in a Mouse Model of Laser-Induced Glaucoma: A Time-Course Study. Biomedicines. 2022; 10(5):939. https://doi.org/10.3390/biomedicines10050939
Chicago/Turabian StyleFernández-Albarral, Jose A., Rosa de Hoz, José A. Matamoros, Lejing Chen, Inés López-Cuenca, Elena Salobrar-García, Lidia Sánchez-Puebla, José M. Ramírez, Alberto Triviño, Juan J. Salazar, and et al. 2022. "Retinal Changes in Astrocytes and Müller Glia in a Mouse Model of Laser-Induced Glaucoma: A Time-Course Study" Biomedicines 10, no. 5: 939. https://doi.org/10.3390/biomedicines10050939
APA StyleFernández-Albarral, J. A., de Hoz, R., Matamoros, J. A., Chen, L., López-Cuenca, I., Salobrar-García, E., Sánchez-Puebla, L., Ramírez, J. M., Triviño, A., Salazar, J. J., & Ramírez, A. I. (2022). Retinal Changes in Astrocytes and Müller Glia in a Mouse Model of Laser-Induced Glaucoma: A Time-Course Study. Biomedicines, 10(5), 939. https://doi.org/10.3390/biomedicines10050939












