Osseointegration of a New, Ultrahydrophilic and Nanostructured Dental Implant Surface: A Comparative In Vivo Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Implants
2.2. Animals
2.3. Surgery
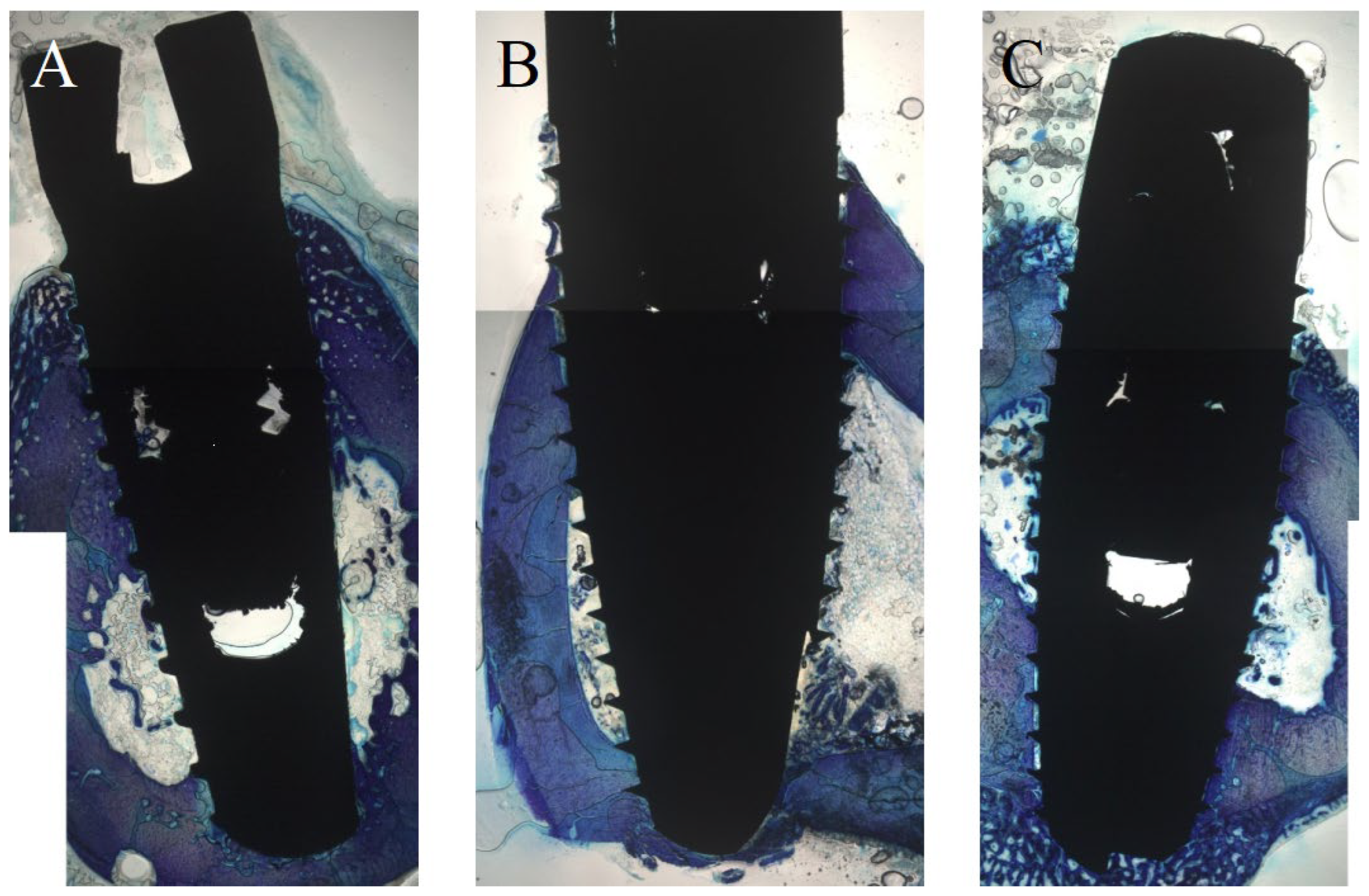
2.4. Histology
2.5. Histomorphometry
2.6. Statistics
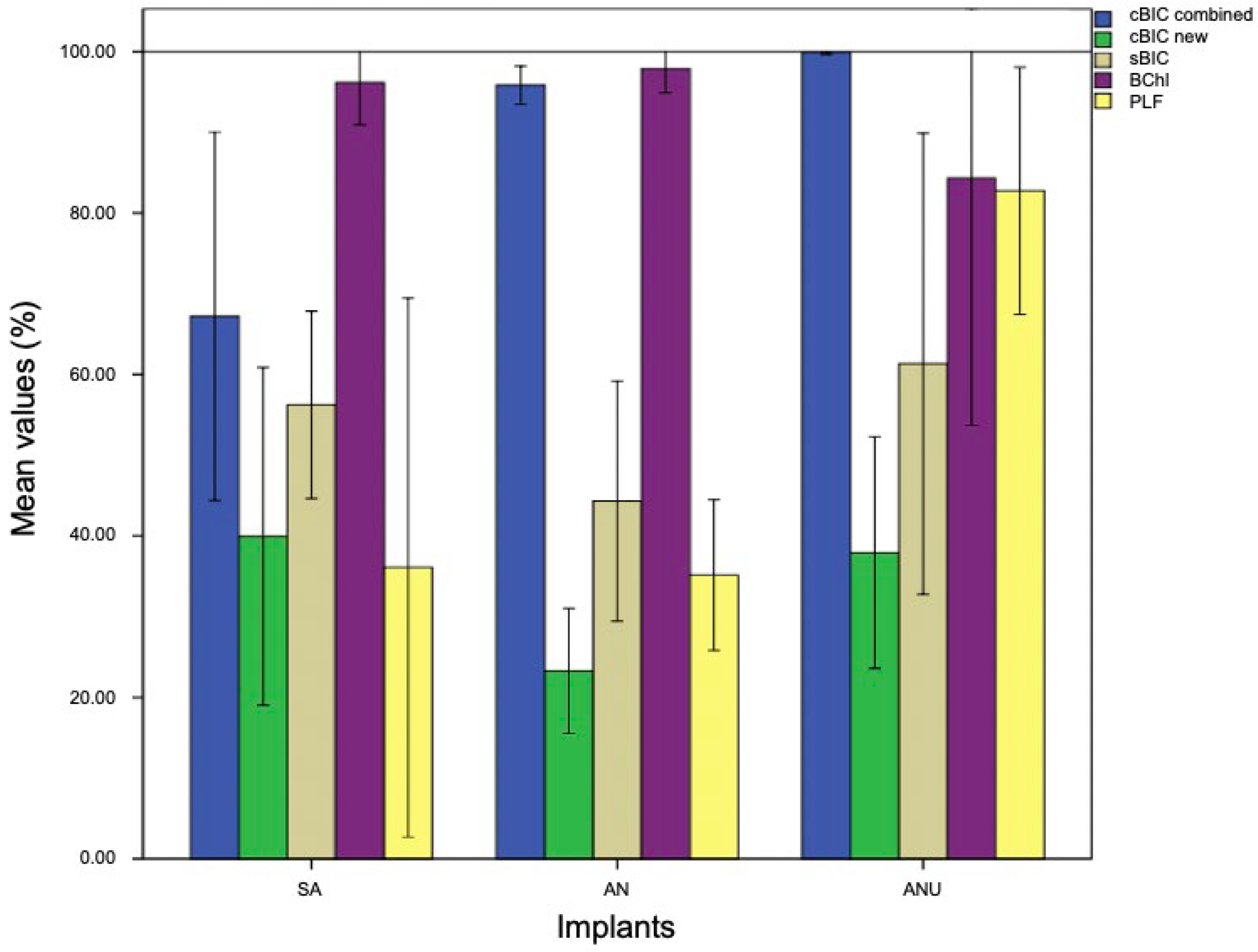
3. Results
3.1. Histological Evaluation after One Week
3.2. Histological Evaluation after Two Weeks
3.3. Histological Evaluation after Four Weeks
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ae | acid-etched |
| uh | ultrahydrophilic |
| nuh | non-ultrahydrophilic |
| ms | microstructured |
| ns | nanostructured |
| sb | sandblasted |
| nsb | non-sandblasted |
| SA | sandblasted, acid-etched |
| AN | acid-etched, nanostructured |
| ANU | acid-etched, nanostructured, ultrahydrophilic |
| BIC | bone-implant contact |
| BChI | bone chamber ingrowth |
| PLF | percentage of linear bone fill |
| BMP-2 | bone morphogenic protein |
References
- Minervini, G.; Romano, A.; Petruzzi, M.; Maio, C.; Serpico, R.; Lucchese, A.; Candotto, V.; Di Stasio, D. Telescopic overdenture on natural teeth: Prosthetic rehabilitation on (OFD) syndromic patient and a review on available literature. J. Biol. Regul. Homeost. Agents 2018, 32, 131–134. [Google Scholar] [PubMed]
- Duong, H.; Roccuzzo, A.; Stähli, A.; Salvi, G.E.; Lang, N.P.; Sculean, A. Oral health-related quality of life of patients rehabilitated with fixed and removable implant-supported dental prostheses. Periodontol. 2000 2022, 88, 201–237. [Google Scholar] [CrossRef] [PubMed]
- Schiegnitz, E.; Müller, L.K.; Sagheb, K.; Theis, L.; Cagiran, V.; Kämmerer, P.W.; Wegener, J.; Wagner, W.; Al-Nawas, B. Clinical long-term and patient-reported outcomes of dental implants in oral cancer patients. Int. J. Implant Dent. 2021, 7, 93. [Google Scholar] [CrossRef] [PubMed]
- Schiegnitz, E.; Al-Nawas, B.; Tegner, A.; Sagheb, K.; Berres, M.; Kämmerer, P.W.; Wagner, W. Clinical and Radiological Long-Term Outcome of a Tapered Implant System with Special Emphasis on the Influence of Augmentation Procedures. Clin. Implant Dent. Relat. Res. 2015, 18, 810–820. [Google Scholar] [CrossRef]
- Francetti, L.; Cavalli, N.; Taschieri, S.; Corbella, S. Ten years follow-up retrospective study on implant survival rates and prevalence of peri-implantitis in implant-supported full-arch rehabilitations. Clin. Oral. Implants Res. 2019, 30, 252–260. [Google Scholar] [CrossRef]
- Malchiodi, L.; Ricciardi, G.; Salandini, A.; Caricasulo, R.; Cucchi, A.; Ghensi, P. Influence of crown–implant ratio on implant success rate of ultra-short dental implants: Results of a 8- to 10-year retrospective study. Clin. Oral Investig. 2020, 24, 3213–3222. [Google Scholar] [CrossRef]
- Al-Nawas, B.; Kämmerer, P.W.; Morbach, T.; Ladwein, C.; Wegener, J.; Wagner, W. Ten-Year Retrospective Follow-Up Study of the TiOblast™ Dental Implant. Clin. Implant Dent. Relat. Res. 2010, 14, 127–134. [Google Scholar] [CrossRef]
- Cheung, M.C.; Hopcraft, M.S.; Darby, I.B. Patient-reported oral hygiene and implant outcomes in general dental practice. Aust. Dent. J. 2020, 66, 49–60. [Google Scholar] [CrossRef]
- Sakka, S.; Baroudi, K.; Nassani, M.Z. Factors associated with early and late failure of dental implants. J. Investig. Clin. Dent. 2012, 3, 258–261. [Google Scholar] [CrossRef]
- Cattoni, F.; Tetè, G.; D’orto, B.; Bergamaschi, A.; Polizzi, E.; Gastaldi, G. Comparison of hygiene levels in metal-ceramic and stratified zirconia in prosthetic rehabilitation on teeth and implants: A retrospective clinical study of a three-year follow-up. J. Biol. Regul. Homeost. Agents 2021, 35, 41–49. [Google Scholar]
- Ye, Z.; Sang, T.; Li, K.; Fischer, N.G.; Mutreja, I.; Echeverría, C.; Kumar, D.; Tang, Z.; Aparicio, C. Hybrid nanocoatings of self-assembled organic-inorganic amphiphiles for prevention of implant infections. Acta Biomater. 2021, 140, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Contaldo, M.; Della Vella, F.; Raimondo, E.; Minervini, G.; Buljubasic, M.; Ogodescu, A.; Sinescu, C.; Serpico, R. Early Childhood Oral Health Impact Scale (ECOHIS): Literature review and Italian validation. Int. J. Dent. Hyg. 2020, 18, 396–402. [Google Scholar] [CrossRef]
- Al-Sawai, A.-A.; Labib, H. Success of immediate loading implants compared to conventionally-loaded implants: A literature review. J. Investig. Clin. Dent. 2015, 7, 217–224. [Google Scholar] [CrossRef]
- Buser, D.; Chappuis, V.; Belser, U.C.; Chen, S. Implant placement post extraction in esthetic single tooth sites: When immediate, when early, when late? Periodontol. 2000 2017, 73, 84–102. [Google Scholar] [CrossRef] [PubMed]
- Gallucci, G.O.; Hamilton, A.; Zhou, W.; Buser, D.; Chen, S. Implant placement and loading protocols in partially edentulous patients: A systematic review. Clin. Oral Implant. Res. 2018, 29, 106–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staedt, H.; Heimes, D.; Lehmann, K.; Ottl, P.; Bjelopavlovic, M.; Wagner, W.; Al-Nawas, B.; Kämmerer, P. Does the Modification of the Apical Geometry of a Dental Implant Affect Its Primary Stability? A Comparative Ex Vivo Study. Materials 2021, 14, 1728. [Google Scholar] [CrossRef]
- Heller, M.; Kumar, V.V.; Pabst, A.; Brieger, J.; Al-Nawas, B.; Kämmerer, P.W. Osseous response on linear and cyclic RGD-peptides immobilized on titanium surfaces in vitro and in vivo. J. Biomed. Mater. Res. Part A 2017, 106, 419–427. [Google Scholar] [CrossRef]
- Schimmel, M.; Srinivasan, M.; McKenna, G.; Müller, F. Effect of advanced age and/or systemic medical conditions on dental implant survival: A systematic review and meta-analysis. Clin. Oral Implant. Res. 2018, 29, 311–330. [Google Scholar] [CrossRef] [Green Version]
- Schmitt, C.M.; Buchbender, M.; Lutz, R.; Neukam, F.W. Oral implant survival in patients with bisphosphonate (BP)/antiresorptive and radiation therapy and their impact on osteonecrosis of the jaws. A systematic review. Eur. J. Oral Implantol. 2018, 11, S93–S111. [Google Scholar]
- Schiegnitz, E.; Al-Nawas, B.; Kämmerer, P.W.; Grötz, K.A. Dental implants in irradiated patients: Which factors influence implant survival? Clin. Oral Investig. 2015, 19, 1691–1692. [Google Scholar] [CrossRef]
- Smeets, R.; Stadlinger, B.; Schwarz, F.; Beck-Broichsitter, B.; Jung, O.; Precht, C.; Kloss, F.; Gröbe, A.; Heiland, M.; Ebker, T. Impact of Dental Implant Surface Modifications on Osseointegration. BioMed Res. Int. 2016, 2016, 6285620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guglielmotti, M.B.; Olmedo, D.G.; Cabrini, R.L. Research on implants and osseointegration. Periodontol. 2000 2019, 79, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Al-Nawas, B.; Kämmerer, P.W.; Morbach, T.; Ophoven, F.; Wagner, W. Retrospective clinical evaluation of an internal tube-in-tube dental implant after 4 years, with special emphasis on peri-implant bone resorption. Int. J. Oral Maxillofac. Implant. 2011, 26, 1309–1316. [Google Scholar]
- Kämmerer, T.A.; Palarie, V.; Schiegnitz, E.; Topalo, V.; Schröter, A.; Al-Nawas, B.; Kämmerer, P.W. A biphasic calcium phosphate coating for potential drug delivery affects early osseointegration of titanium implants. J. Oral Pathol. Med. 2017, 46, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Ettelt, V.; Ekat, K.; Kämmerer, P.W.; Kreikemeyer, B.; Epple, M.; Veith, M. Streptavidin-coated surfaces suppress bacterial colonization by inhibiting non-specific protein adsorption. J. Biomed. Mater. Res. Part A 2017, 106, 758–768. [Google Scholar] [CrossRef] [PubMed]
- Toffoli, A.; Parisi, L.; Tatti, R.; Lorenzi, A.; Verucchi, R.; Manfredi, E.; Lumetti, S.; Macaluso, G.M. Thermal-induced hydrophilicity enhancement of titanium dental implant surfaces. J. Oral Sci. 2020, 62, 217–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.-W.; Kwon, T.-G.; Suh, J.-Y. The relative effect of surface strontium chemistry and super-hydrophilicity on the early osseointegration of moderately rough titanium surface in the rabbit femur. Clin. Oral Implant. Res. 2012, 24, 706–709. [Google Scholar] [CrossRef]
- Albrektsson, T.; Wennerberg, A. On osseointegration in relation to implant surfaces. Clin. Implant. Dent. Relat. Res. 2019, 21, 4–7. [Google Scholar] [CrossRef] [Green Version]
- Ríos-Santos, J.V.; Menjívar-Galán, A.M.; Herrero-Climent, M.; Rios-Carrasco, B.; Fernández-Palacín, A.; Perez, R.A.; Gil, F. Unravelling the effect of macro and microscopic design of dental implants on osseointegration: A randomised clinical study in minipigs. J. Mater. Sci. Mater. Med. 2018, 29, 99. [Google Scholar] [CrossRef]
- Assaf, A.; Saad, M.; Hijawi, S. Use of narrow-diameter implants in the posterior segments of the jaws: A retrospective observational study of 2 to 11 years. J. Prosthet. Dent. 2022. Epub ahead of print. [Google Scholar] [CrossRef]
- Moccia, S.; Nucci, L.; Spagnuolo, C.; D’Apuzzo, F.; Piancino, M.G.; Minervini, G. Polyphenols as Potential Agents in the Management of Temporomandibular Disorders. Appl. Sci. 2020, 10, 5305. [Google Scholar] [CrossRef]
- Bennardo, F.; Barone, S.; Vocaturo, C.; Nucci, L.; Antonelli, A.; Giudice, A. Usefulness of Magnetic Mallet in Oral Surgery and Implantology: A Systematic Review. J. Pers. Med. 2022, 12, 108. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, A.; Inchingolo, A.; Bordea, I.; Xhajanka, E.; Romeo, D.; Romeo, M.; Zappone, C.; Malcangi, G.; Scarano, A.; Lorusso, F.; et al. The Effectiveness of Osseodensification Drilling Protocol for Implant Site Osteotomy: A Systematic Review of the Literature and Meta-Analysis. Materials 2021, 14, 1147. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, A.; Meijer, G.J.; Cuijpers, V.M.; Walboomers, X.F. Combined effect of undersized surgical technique and axial compression on the primary implant stability and host bone architecture. Saudi Dent. J. 2021, 33, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Elias, C.N.; Rocha, F.A.; Nascimento, A.L.; Coelho, P.G. Influence of implant shape, surface morphology, surgical technique and bone quality on the primary stability of dental implants. J. Mech. Behav. Biomed. Mater. 2012, 16, 169–180. [Google Scholar] [CrossRef]
- Cochran, D.L.; Jones, A.A.; Sugita, R.; Brown, M.C.; Prasad, H.; Kay, G.W. Twelve-month evaluation of a novel mineral–organic adhesive material used to stabilize dental implants placed in oversized osteotomies in vivo in an animal model. Clin. Oral Implant. Res. 2022, 33, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Chen, X.; Mu, H.; Xu, Y.; Chen, R.; Xia, R.; Xia, L.; Zhang, S. Titanium Nanobowl-Based Nest-Like Nanofiber Structure Prepared at Room Temperature and Pressure Promotes Osseointegration of Beagle Implants. Front. Bioeng. Biotechnol. 2022, 10, 841591. [Google Scholar] [CrossRef]
- Yan, R.; Li, J.; Wu, Q.; Zhang, X.; Hu, L.; Deng, Y.; Jiang, R.; Wen, J.; Jiang, X. Trace Element-Augmented Titanium Implant With Targeted Angiogenesis and Enhanced Osseointegration in Osteoporotic Rats. Front. Chem. 2022, 10, 839062. [Google Scholar] [CrossRef]
- Cho, W.-T.; Kim, S.-Y.; Jung, S.-I.; Kang, S.-S.; Kim, S.-E.; Hwang, S.-H.; Jeong, C.-M.; Huh, J.-B. Effects of Gamma Radiation-Induced Crosslinking of Collagen Type I Coated Dental Titanium Implants on Osseointegration and Bone Regeneration. Materials 2021, 14, 3268. [Google Scholar] [CrossRef]
- Rupp, F.; Liang, L.; Geis-Gerstorfer, J.; Scheideler, L.; Hüttig, F. Surface characteristics of dental implants: A review. Dent. Mater. 2018, 34, 40–57. [Google Scholar] [CrossRef]
- Hotchkiss, K.M.; Reddy, G.B.; Hyzy, S.L.; Schwartz, Z.; Boyan, B.D.; Olivares-Navarrete, R. Titanium surface characteristics, including topography and wettability, alter macrophage activation. Acta Biomater. 2015, 31, 425–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alfarsi, M.A.; Hamlet, S.M.; Ivanovski, S. Titanium surface hydrophilicity modulates the human macrophage inflammatory cytokine response. J. Biomed. Mater. Res. Part A 2013, 102, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Asensio, G.; Vázquez-Lasa, B.; Rojo, L. Achievements in the Topographic Design of Commercial Titanium Dental Implants: Towards Anti-Peri-Implantitis Surfaces. J. Clin. Med. 2019, 8, 1982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alfarsi, M.A.; Hamlet, S.M.; Ivanovski, S. Titanium surface hydrophilicity enhances platelet activation. Dent. Mater. J. 2014, 33, 749–756. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Hernández, M.; Hannig, M.; García-Pérez, V.I.; Olivares-Navarrete, R.; Fecher-Trost, C.; Almaguer-Flores, A. Roughness and wettability of titanium implant surfaces modify the salivary pellicle composition. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 109, 1017–1028. [Google Scholar] [CrossRef]
- Kämmerer, T.; Lesmeister, T.; Palarie, V.; Schiegnitz, E.; Schröter, A.; Al-Nawas, B.; Kämmerer, P.W. Calcium Phosphate-Coated Titanium Implants in the Mandible: Limitations of the in vivo Minipig Model. Eur. Surg. Res. 2020, 61, 177–187. [Google Scholar] [CrossRef]
- Kämmerer, P.; Palarie, V.; Schiegnitz, E.; Nacu, V.; Draenert, F.; Al-Nawas, B. Influence of a collagen membrane and recombinant platelet-derived growth factor on vertical bone augmentation in implant-fixed deproteinized bovine bone—Animal pilot study. Clin. Oral Implant. Res. 2012, 24, 1222–1230. [Google Scholar] [CrossRef]
- Schiegnitz, E.; Palarie, V.; Nacu, V.; Al-Nawas, B.; Kämmerer, P.W. Vertical Osteoconductive Characteristics of Titanium Implants with Calcium-Phosphate-Coated Surfaces—A Pilot Study in Rabbits. Clin. Implant Dent. Relat. Res. 2014, 16, 194–201. [Google Scholar] [CrossRef]
- Kämmerer, P.W.; Palarie, V.; Schiegnitz, E.; Hagmann, S.; Alshihri, A.; Al-Nawas, B. Vertical osteoconductivity and early bone formation of titanium-zirconium and titanium implants in a subperiosteal rabbit animal model. Clin. Oral Implant. Res. 2014, 25, 774–780. [Google Scholar] [CrossRef]
- Hankenson, K.; Zimmerman, G.; Marcucio, R. Biological perspectives of delayed fracture healing. Injury 2014, 45, S8–S15. [Google Scholar] [CrossRef] [Green Version]
- Solheim, E. Osteoinduction by demineralised bone. Int. Orthop. 1998, 22, 335–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001, 10 (Suppl. S2), S96–S101. [Google Scholar] [PubMed] [Green Version]
- Lang, N.P.; Salvi, G.E.; Huynh-Ba, G.; Ivanovski, S.; Donos, N.; Bosshardt, D.D. Early osseointegration to hydrophilic and hydrophobic implant surfaces in humans. Clin. Oral Implant. Res. 2011, 22, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Schwartz, Z.; Wieland, M.; Rupp, F.; Geis-Gerstorfer, J.; Cochran, D.L.; Boyan, B.D. High surface energy enhances cell response to titanium substrate microstructure. J. Biomed. Mater. Res. Part A 2005, 74A, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Wennerberg, A.; Albrektsson, T.; Galli, S. Current knowledge about the hydrophilic and nanostructured SLActive surface. Clin. Cosmet. Investig. Dent. 2011, 3, 59–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, W.; Smith, R.K.; Zilberman, Y.; Mozsary, P.G.; Smith, R.S. Osseous adaptation to continuous loading of rigid endosseous implants. Am. J. Orthod. 1984, 86, 95–111. [Google Scholar] [CrossRef]
- Kämmerer, P.W.; Lehnert, M.; Kumar, V.V.; Veith, M.; Al-Nawas, B.; Hagmann, S.; Alshihri, A.; Frerich, B. Osseoconductivity of a Specific Streptavidin-Biotin-Fibronectin Surface Coating of Biotinylated Titanium Implants—A Rabbit Animal Study. Clin. Implant Dent. Relat. Res. 2015, 17, e601–e612. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhang, F.; He, F.; Zhang, L.; Guo, C.; Zhao, S.; Yang, G. Osseointegration of titanium implants with a roughened surface containing hydride ion in a rabbit model. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2010, 110, e5–e12. [Google Scholar] [CrossRef]
- De Marco, T.J.; Paine, S. Mandibular dimensional change. J. Prosthet. Dent. 1974, 31, 482–485. [Google Scholar] [CrossRef]
- Sartoretto, S.C.; Alves, A.T.N.N.; Zarranz, L.; Jorge, M.Z.; Granjeiro, J.; Calasans-Maia, M. Hydrophilic surface of Ti6Al4V-ELI alloy improves the early bone apposition of sheep tibia. Clin. Oral Implant. Res. 2016, 28, 893–901. [Google Scholar] [CrossRef]
- Sartoretto, S.C.; Calasans-Maia, J.D.A.; da Costa, Y.O.; Louro, R.S.; Granjeiro, J.M.; Calasans-Maia, M.D. Accelerated Healing Period with Hydrophilic Implant Placed in Sheep Tibia. Braz. Dent. J. 2017, 28, 559–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, G.-L.; He, F.-M.; Yang, X.-F.; Wang, X.-X.; Zhao, S.-F. Bone responses to titanium implants surface-roughened by sandblasted and double etched treatments in a rabbit model. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2008, 106, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.S.; Kim, M.J.; Han, J.S.; Yeo, I.S. Effects of hydrophilicity and fluoride surface modifications to titanium dental implants on early osseointegration: An in vivo study. Implant Dent. 2014, 23, 529–533. [Google Scholar] [CrossRef]
- Burgos, P.M.; Rasmusson, L.; Meirelles, L.; Sennerby, L. Early Bone Tissue Responses to Turned and Oxidized Implants in the Rabbit Tibia. Clin. Implant. Dent. Relat. Res. 2008, 10, 181–190. [Google Scholar] [CrossRef] [PubMed]
- French, D.; Grandin, H.M.; Ofec, R. Retrospective cohort study of 4591 dental implants: Analysis of risk indicators for bone loss and prevalence of peri-implant mucositis and peri-implantitis. J. Periodontol. 2019, 90, 691–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capulli, M.; Paone, R.; Rucci, N. Osteoblast and osteocyte: Games without frontiers. Arch. Biochem. Biophys. 2014, 561, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, M.J.; Schemitsch, E.H.; Kregor, P.J.; Senft, D.; Swiontkowski, M.F. Effect of periosteal stripping on cortical bone perfusion: A laser doppler study in sheep. Calcif. Tissue Res. 1996, 59, 24–26. [Google Scholar] [CrossRef]
- Jennissen, H.P. A macrophage model of osseointegration. Curr. Dir. Biomed. Eng. 2016, 2, 53–56. [Google Scholar] [CrossRef]
- Vercellino, M.; Ceccarelli, G.; Cristofaro, F.; Balli, M.; Bertoglio, F.; Bruni, G.; Benedetti, L.; Avanzini, M.A.; Imbriani, M.; Visai, L. Nanostructured TiO2 Surfaces Promote Human Bone Marrow Mesenchymal Stem Cells Differentiation to Osteoblasts. Nanomaterials 2016, 6, 124. [Google Scholar] [CrossRef] [Green Version]
- Zumstein, T.; Schütz, S.; Sahlin, H.; Sennerby, L. Factors influencing marginal bone loss at a hydrophilic implant design placed with or without GBR procedures: A 5-year retrospective study. Clin. Implant Dent. Relat. Res. 2019, 21, 817–826. [Google Scholar] [CrossRef]
- Degasperi, W.; Andersson, P.; Verrocchi, D.; Sennerby, L. One-Year Clinical and Radiographic Results with a Novel Hydrophilic Titanium Dental Implant. Clin. Implant Dent. Relat. Res. 2012, 16, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.A.F.; Faot, F.; da Silva, W.J.; Cury, A.A.D.B. Does implant surface hydrophilicity influence the maintenance of surface integrity after insertion into low-density artificial bone? Dent. Mater. 2021, 37, e69–e84. [Google Scholar] [CrossRef] [PubMed]
- Marconi, G.D.; Fonticoli, L.; Della Rocca, Y.; Oliva, S.; Rajan, T.S.; Trubiani, O.; Murmura, G.; Diomede, F.; Pizzicannella, J. Enhanced Extracellular Matrix Deposition on Titanium Implant Surfaces: Cellular and Molecular Evidences. Biomedicines 2021, 9, 1710. [Google Scholar] [CrossRef] [PubMed]
- Aresti, A.; Aragoneses, J.; López-Valverde, N.; Suárez, A.; Aragoneses, J.M. Effectiveness of Biofunctionalization of Titanium Surfaces with Phosphonic Acid. Biomedicines 2021, 9, 1663. [Google Scholar] [CrossRef] [PubMed]
- Smargiassi, A.; Bertacchini, J.; Checchi, M.; Cavani, F.; Ferretti, M.; Palumbo, C. Biocompatibility Analyses of Al2O3-Treated Titanium Plates Tested with Osteocyte and Fibroblast Cell Lines. Biomedicines 2017, 5, 32. [Google Scholar] [CrossRef]
- Blanc-Sylvestre, N.; Bouchard, P.; Chaussain, C.; Bardet, C. Pre-Clinical Models in Implant Dentistry: Past, Present, Future. Biomedicines 2021, 9, 1538. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pabst, A.; Asran, A.; Lüers, S.; Laub, M.; Holfeld, C.; Palarie, V.; Thiem, D.G.E.; Becker, P.; Hartmann, A.; Heimes, D.; et al. Osseointegration of a New, Ultrahydrophilic and Nanostructured Dental Implant Surface: A Comparative In Vivo Study. Biomedicines 2022, 10, 943. https://doi.org/10.3390/biomedicines10050943
Pabst A, Asran A, Lüers S, Laub M, Holfeld C, Palarie V, Thiem DGE, Becker P, Hartmann A, Heimes D, et al. Osseointegration of a New, Ultrahydrophilic and Nanostructured Dental Implant Surface: A Comparative In Vivo Study. Biomedicines. 2022; 10(5):943. https://doi.org/10.3390/biomedicines10050943
Chicago/Turabian StylePabst, Andreas, Ashraf Asran, Steffen Lüers, Markus Laub, Christopher Holfeld, Victor Palarie, Daniel G. E. Thiem, Philipp Becker, Amely Hartmann, Diana Heimes, and et al. 2022. "Osseointegration of a New, Ultrahydrophilic and Nanostructured Dental Implant Surface: A Comparative In Vivo Study" Biomedicines 10, no. 5: 943. https://doi.org/10.3390/biomedicines10050943






