Total Tumor Volume on 18F-PSMA-1007 PET as Additional Imaging Biomarker in mCRPC Patients Undergoing PSMA-Targeted Alpha Therapy with 225Ac-PSMA-I&T
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
2.2. 225Ac-PSMA-I&T Radiopharmaceutical and Treatment Protocol
2.3. 18F-PSMA-1007 Radiopharmaceutical and Imaging Protocol
2.4. Blood Values and Pain Score
2.5. Image-Based Therapy Response Assessment
2.5.1. Modified PERCIST on 18F-PSMA-1007 PET
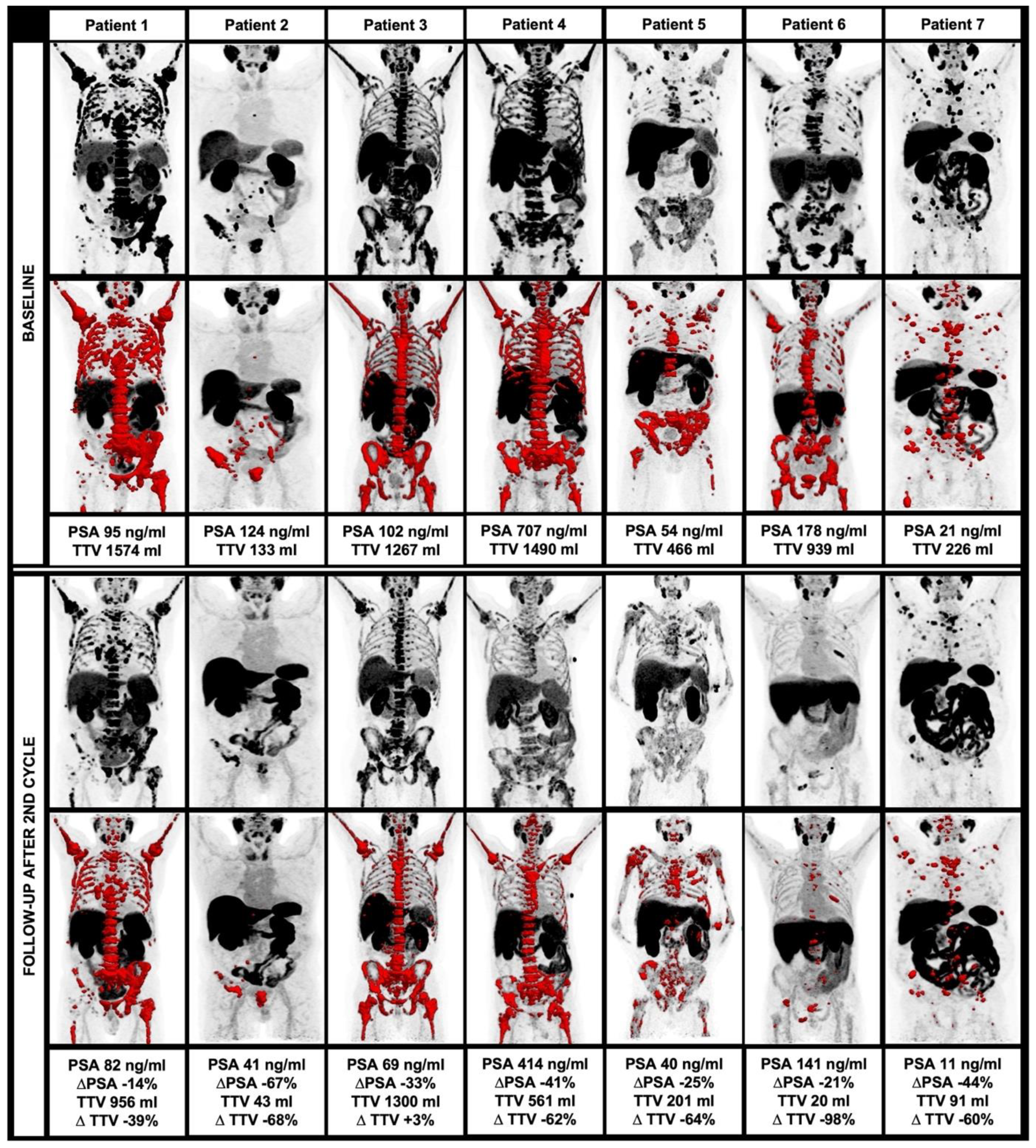
2.5.2. Evaluation of Total Tumor Volume (TTV) on 18F-PSMA-1007 PET
2.5.3. CT (RECIST 1.1)
2.6. Follow-Up
2.7. Statistical Analysis
3. Results
3.1. Patient Characteristics at Baseline Time Point
3.2. Correlation of TTV and Further Pretherapeutic Parameters
3.3. Correlation of TTV and Pretherapeutic Parameters with Clinical Outcome
3.4. Changes of Clinical and Image-Derived Parameters during 225Ac-PSMA-I&T Treatment
3.5. Intercorrelation of Clinical and Imaged Derived Changes after 225Ac-PSMA-I&T Treatment
3.6. Changes of TTV in Comparison to Further Response Criteria
3.7. Occurrence of New Lesions in Correlation to TTV and Further Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Zacherl, M.J.; Gildehaus, F.J.; Mittlmeier, L.; Böning, G.; Gosewisch, A.; Wenter, V.; Unterrainer, M.; Schmidt-Hegemann, N.; Belka, C.; Kretschmer, A.; et al. First clinical results for PSMA-targeted α-therapy using 225Ac-PSMA-I&T in advanced-mCRPC patients. J. Nucl. Med. 2021, 62, 669–674. [Google Scholar] [PubMed]
- Marshall, C.H.; Antonarakis, E.S. Emerging treatments for metastatic castration-resistant prostate cancer: Immunotherapy, PARP inhibitors, and PSMA-targeted approaches. Cancer Treat. Res. Commun. 2020, 23, 100164. [Google Scholar] [CrossRef] [PubMed]
- Angelergues, A.; Maillet, D.; Flechon, A.; Ozguroglu, M.; Mercier, F.; Guillot, A.; Le Moulec, S.; Gravis, G.; Beuzeboc, P.; Massard, C.; et al. Duration of response to androgen-deprivation therapy (ADT) and efficacy of secondary hormone therapy, docetaxel (D), and cabazitaxel (C) in metastatic castration-resistant prostate cancer (mCRPC). Am. Soc. Clin. Oncol. 2014, 32, 282. [Google Scholar] [CrossRef]
- Bauckneht, M.; Capitanio, S.; Donegani, M.I.; Zanardi, E.; Miceli, A.; Murialdo, R.; Raffa, S.; Tomasello, L.; Vitti, M.; Cavo, A.; et al. Role of baseline and post-therapy 18F-FDG PET in the prognostic stratification of metastatic castration-resistant prostate cancer (mCRPC) patients treated with radium-223. Cancers 2020, 12, 31. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, A.J.; Lin, P.; Tombal, B.; Saad, F.; Higano, C.S.; Joshua, A.M.; Parli, T.; Rosbrook, B.; van Os, S.; Beer, T.M. Five-year survival prediction and safety outcomes with enzalutamide in men with chemotherapy-naive metastatic castration-resistant prostate cancer from the PREVAIL trial. Eur. Urol. 2020, 78, 347–357. [Google Scholar] [CrossRef]
- Czernin, J.; Calais, J. 177Lu-PSMA617 and the VISION trial: One of the greatest success stories in the history of nuclear medicine. J. Nucl. Med. 2021, 62, 1025–1026. [Google Scholar] [CrossRef] [PubMed]
- Sartor, A.O.; Morris, M.J.; Krause, B.J. VISION: An international, prospective, open-label, multicenter, randomized phase 3 study of 177Lu-PSMA-617 in the treatment of patients with progressive PSMA-positive metastatic castration-resistant prostate cancer (mCRPC). Am. Soc. Clin. Oncol. 2019, 37, TPS259. [Google Scholar] [CrossRef]
- Morris, M.J.; De Bono, J.S.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Phase III study of lutetium-177-PSMA-617 in patients with metastatic castration-resistant prostate cancer (VISION). Am. Soc. Clin. Oncol. 2021, 39, LBA4. [Google Scholar] [CrossRef]
- Seitzer, K.E.; Seifert, R.; Kessel, K.; Roll, W.; Schlack, K.; Boegemann, M.; Rahbar, K. Lutetium-177 Labelled PSMA Targeted Therapy in Advanced Prostate Cancer: Current Status and Future Perspectives. Cancers 2021, 13, 3715. [Google Scholar] [CrossRef]
- Kratochwil, C.; Bruchertseifer, F.; Rathke, H.; Bronzel, M.; Apostolidis, C.; Weichert, W.; Haberkorn, U.; Giesel, F.L.; Morgenstern, A. Targeted α-therapy of metastatic castration-resistant prostate cancer with 225Ac-PSMA-617: Dosimetry estimate and empiric dose finding. J. Nucl. Med. 2017, 58, 1624–1631. [Google Scholar] [CrossRef] [Green Version]
- Sathekge, M.; Bruchertseifer, F.; Vorster, M.; Lawal, I.; Knoesen, O.; Mahapane, J.; Davis, C.; Mdlophane, A.; Maes, A.; Mokoala, K.; et al. mCRPC patients receiving 225Ac-PSMA-617 therapy in post androgen deprivation therapy setting: Response to treatment and survival analysis. J. Nucl. Med. 2022. [Google Scholar] [CrossRef] [PubMed]
- Hooijman, E.L.; Chalashkan, Y.; Ling, S.W.; Kahyargil, F.F.; Segbers, M.; Bruchertseifer, F.; Morgenstern, A.; Seimbille, Y.; Koolen, S.L.; Brabander, T.; et al. Development of [225Ac] Ac-PSMA-I&T for Targeted Alpha Therapy According to GMP Guidelines for Treatment of mCRPC. Pharmaceutics 2021, 13, 715. [Google Scholar] [PubMed]
- Ma, J.; Li, L.; Liao, T.; Gong, W.; Zhang, C. Efficacy and Safety of 225Ac-PSMA-617-Targeted Alpha Therapy in Metastatic Castration-Resistant Prostate Cancer: A Systematic Review and Meta-Analysis. Front. Oncol. 2022, 12, 796657. [Google Scholar] [CrossRef] [PubMed]
- Feuerecker, B.; Tauber, R.; Knorr, K.; Heck, M.; Beheshti, A.; Seidl, C.; Bruchertseifer, F.; Pickhard, A.; Gafita, A.; Kratochwil, C.; et al. Activity and adverse events of actinium-225-PSMA-617 in advanced metastatic castration-resistant prostate cancer after failure of lutetium-177-PSMA. Eur. Urol. 2021, 79, 343–350. [Google Scholar] [CrossRef]
- Juzeniene, A.; Stenberg, V.Y.; Bruland, Ø.S.; Larsen, R.H. Preclinical and Clinical Status of PSMA-Targeted Alpha Therapy for Metastatic Castration-Resistant Prostate Cancer. Cancers 2021, 13, 779. [Google Scholar] [CrossRef]
- Schuchardt, C.; Zhang, J.; Kulkarni, H.R.; Chen, X.; Mueller, D.; Baum, R.P. Prostate-specific membrane antigen radioligand therapy using 177Lu-PSMA I&T and 177Lu-PSMA-617 in patients with metastatic castration-resistant prostate cancer: Comparison of safety, biodistribution and dosimetry. J. Nucl. Med. 2021. [Google Scholar] [CrossRef]
- Schmidkonz, C.; Cordes, M.; Schmidt, D.; Bäuerle, T.; Goetz, T.I.; Beck, M.; Prante, O.; Cavallaro, A.; Uder, M.; Wullich, B.; et al. 68Ga-PSMA-11 PET/CT-derived metabolic parameters for determination of whole-body tumor burden and treatment response in prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1862–1872. [Google Scholar] [CrossRef]
- Fanti, S.; Goffin, K.; Hadaschik, B.A.; Herrmann, K.; Maurer, T.; MacLennan, S.; Oprea-Lager, D.E.; Oyen, W.J.; Rouvière, O.; Mottet, N.; et al. Consensus statements on PSMA PET/CT response assessment criteria in prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 469–476. [Google Scholar] [CrossRef]
- Cardinale, J.; Schäfer, M.; Benešová, M.; Bauder-Wüst, U.; Leotta, K.; Eder, M.; Neels, O.C.; Haberkorn, U.; Giesel, F.L.; Kopka, K. Preclinical evaluation of 18F-PSMA-1007, a new prostate-specific membrane antigen ligand for prostate cancer imaging. J. Nucl. Med. 2017, 58, 425–431. [Google Scholar] [CrossRef] [Green Version]
- d’Amico, A.; Gorczewska, I.; Gorczewski, K.; Turska-d’Amico, M.; Di Pietro, M. Effect of furosemide administration before F-18 fluorodeoxyglucose positron emission tomography/computed tomography on urine radioactivity and detection of uterine cervical cancer. Nucl. Med. Rev. 2014, 17, 83–86. [Google Scholar] [CrossRef] [Green Version]
- Seitz, A.K.; Rauscher, I.; Haller, B.; Krönke, M.; Luther, S.; Heck, M.M.; Horn, T.; Gschwend, J.E.; Schwaiger, M.; Eiber, M.; et al. Preliminary results on response assessment using 68 Ga-HBED-CC-PSMA PET/CT in patients with metastatic prostate cancer undergoing docetaxel chemotherapy. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Mittlmeier, L.M.; Brendel, M.; Beyer, L.; Albert, N.L.; Todica, A.; Zacherl, M.J.; Wenter, V.; Herlemann, A.; Kretschmer, A.; Ledderose, S.T.; et al. Feasibility of different tumor delineation approaches for 18F-PSMA-1007 PET/CT imaging in prostate cancer patients. Front. Oncol. 2021, 11, 1612. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Fanti, S.; Hadaschik, B.; Herrmann, K. Proposal for systemic-therapy response-assessment criteria at the time of PSMA PET/CT imaging: The PSMA PET progression criteria. Soc. Nucl. Med. 2020, 61, 678–682. [Google Scholar] [CrossRef]
- Seifert, R.; Kessel, K.; Schlack, K.; Weckesser, M.; Kersting, D.; Seitzer, K.E.; Weber, M.; Bögemann, M.; Rahbar, K. Total tumor volume reduction and low PSMA expression in patients receiving Lu-PSMA therapy. Theranostics 2021, 11, 8143. [Google Scholar] [CrossRef]
- Satapathy, S.; Sood, A.; Das, C.K.; Mittal, B.R. Evolving role of 225Ac-PSMA radioligand therapy in metastatic castration-resistant prostate cancer—A systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2021, 24, 880–890. [Google Scholar] [CrossRef]
- Heinrich, D.; Bruland, Ø.; Guise, T.A.; Suzuki, H.; Sartor, O. Alkaline phosphatase in metastatic castration-resistant prostate cancer: Reassessment of an older biomarker. Future Oncol. 2018, 14, 2543–2556. [Google Scholar] [CrossRef] [Green Version]
- Maruzzo, M.; Basso, U.; Borsatti, E.; Evangelista, L.; Alongi, F.; Caffo, O.; Maines, F.; Galuppo, S.; De Vivo, R.; Zustovich, F.; et al. Results from a large, multicenter, retrospective analysis on radium223 use in metastatic castration-resistant prostate cancer (mCRPC) in the Triveneto Italian Region. Clin. Genitourin. Cancer 2019, 17, e187–e194. [Google Scholar] [CrossRef]
- Rahbar, K.; Ahmadzadehfar, H.; Kratochwil, C.; Haberkorn, U.; Schäfers, M.; Essler, M.; Baum, R.P.; Kulkarni, H.R.; Schmidt, M.; Drzezga, A.; et al. German multicenter study investigating 177Lu-PSMA-617 radioligand therapy in advanced prostate cancer patients. J. Nucl. Med. 2017, 58, 85–90. [Google Scholar] [CrossRef] [Green Version]
- Ferdinandus, J.; Violet, J.; Sandhu, S.; Hicks, R.J.; Ravi Kumar, A.S.; Iravani, A.; Kong, G.; Akhurst, T.; Thang, S.P.; Murphy, D.G.; et al. Prognostic biomarkers in men with metastatic castration-resistant prostate cancer receiving [177Lu]-PSMA-617. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2322–2327. [Google Scholar] [CrossRef]
- Seifert, R.; Kessel, K.; Schlack, K.; Weber, M.; Herrmann, K.; Spanke, M.; Fendler, W.P.; Hadaschik, B.; Kleesiek, J.; Schäfers, M.; et al. PSMA PET total tumor volume predicts outcome of patients with advanced prostate cancer receiving [177Lu] Lu-PSMA-617 radioligand therapy in a bicentric analysis. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1200–1210. [Google Scholar] [CrossRef] [PubMed]
- Seifert, R.; Sandach, P.; Kersting, D.; Fendler, W.P.; Hadaschik, B.; Herrmann, K.; Sunderland, J.; Pollard, J. Repeatability of 68Ga-PSMA-HBED-CC PET/CT-derived total molecular tumor volume. J. Nucl. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Castello, A.; Lopci, E. Response assessment of bone metastatic disease: Seeing the forest for the trees RECIST, PERCIST, iRECIST, and PCWG-2. Q. J. Nucl. Med. Mol. Imaging 2019, 63, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Costelloe, C.M.; Chuang, H.H.; Madewell, J.E.; Ueno, N.T. Cancer response criteria and bone metastases: RECIST 1.1, MDA and PERCIST. J. Cancer 2010, 1, 80. [Google Scholar] [CrossRef] [PubMed]
- Scher, H.I.; Morris, M.J.; Kelly, W.K.; Schwartz, L.H.; Heller, G. Prostate cancer clinical trial end points:“RECIST” ing a step backwards. Clin. Cancer Res. 2005, 11, 5223–5232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jadvar, H. The VISION Forward: Recognition and Implication of PSMA-/FDG+ mCRPC. J. Nucl. Med. 2021. [Google Scholar] [CrossRef] [PubMed]



| Parameters | Value [Median, Range] |
|---|---|
| Age [years] | 74.8 (65.8–80.9) |
| PSA [ng/mL] | 178.0 (13.4–750.0) |
| AP [U/L] | 228.0 (68.0–695.0) (normal range 40.0–130.0) |
| LDH [U/L] | 344.0 (150.0–1457.0) (normal range <249.0) |
| Pain Score | 5.0 (0.0–8.0) |
| TTV [mL] | 835.0 (133.0–1776.0) |
| SUVmean | 9.5 (6.6–15.8) |
| SUVmax | 42.8 (17.5–175.9) |
| Sites of tumor spread | |
| Bone | 13/13 (100.0%) |
| Lymph node | 8/13 (61.5%) |
| Visceral metastases | 4/13 (30.8%) |
| Local tumor | 7/13 (53.8%) |
| Prior therapies | |
| Prostatectomy | 4/13 (30.7%) |
| Radiotherapy | 7/13 (53.8%) |
| ADT | 13/13 (100.0%) |
| Docetaxel | 9/13 (69.2%) |
| 177Lu-PSMA-RLT | 10/13 (76.9%) |
| 223Ra-dichlorode | 1/13 (7.7%) |
| PSA | AP | TTV | SUVmean | SUVmax | LDH | |
|---|---|---|---|---|---|---|
| AP | r = 0.509 (p = 0.076) | - | - | - | - | - |
| TTV | r = −0.022 (p = 0.943) | r = 0.036 (p = 0.908) | - | - | - | - |
| SUVmean | r = −0.324 (p = 0.280) | r = −0.281 (p = 0.353) | r = 0.181 (p = 0.553) | - | - | - |
| SUVmax | r = −0.341 (p = 0.255) | r = −0.426 (p = 0.146) | r = 0.143 (p = 0.642) | r = 0.725 (p = 0.005) | - | - |
| LDH | r = 0.148 (p = 0.629) | r = 0.536 (p = 0.059) | r = 0.346 (p = 0.247) | r = −0.429 (p = 0.144) | r = −0.319 (p = 0.289) | - |
| Pain Score | r = −0.031 (p = 0.920) | r = 0.070 (p = 0.820) | r = 0.020 (p = 0.949) | r = −0.205 (p = 0.503) | r = −0.437 (p = 0.135) | r = 0.014 (p = 0.964) |
| Parameters | STS (n = 6) [Median (Range)] | LTS (n = 7) [Median (Range)] | Significance |
|---|---|---|---|
| PSA [ng/dL] | 224.5 (13.4–750.0) | 124.0 (20.5–707.0) | p = 0.286 |
| AP [U/L] | 412.5 (155.0–695.0) | 98.0 (68.0–326.0) | p = 0.029 |
| LDH [U/L] | 585.0 (264.0–1457.0) | 274.0 (150.0–616.0) | p = 0.286 |
| Pain score | 5.0 (0.0–8.0) | 4.0 (0.0–7.0) | p = 0.559 |
| TTV [mL] | 815.0 (566.0–1776.0) | 939.0 (133.0–1574.0) | p = 0.592 |
| SUVmean | 9.5 (6.9–15.15) | 9.2 (6.6–15.6) | p = 1.000 |
| SUVmax | 36.8 (17.5–113.6) | 55.0 (38.5–175.9) | p = 0.592 |
| Parameters | Baseline [Median (Range)] | Follow-up [Median (Range)] | Δ% [Median (Range)] | Significance |
|---|---|---|---|---|
| PSA [ng/dL] | 178.0 (13.4–750.0) |
68.6 (11.4–414.0) |
−32.8 (−67.3–−14.2) | p = 0.018 |
| AP [U/L] | 228.0 (68.0–695.0) |
148.0 (61.0–487.0) |
−20.5 (−30.6–165.3) | p = 0.176 |
| LDH [U/L] | 344.0 (150.0–1457.0) |
304.0 (174.0–1124.0) |
−3.5 (−29.6–126.6) | p = 0.866 |
| TTV [mL] | 835.0 (133.0–1776.0) |
201.0 (20.3–1300.0) |
−62.3 (−97.8–2.6) | p = 0.028 |
| SUVmean | 9.5 (6.6–15.8) | 7.1 (6.3–9.7) | −24.1 (−42.3–−8.1) | p = 0.018 |
| SUVmax | 42.8 (17.5–175.9) | 32.6 (16.2–54.3) |
−46.0 (−81.6–74.2) | p = 0.063 |
| Δ% PSA | Δ% AP | Δ% TTV | Δ% SUVmean | Δ% SUVmax | |
|---|---|---|---|---|---|
| Δ% AP | r = 0.071 (p = 0.879) | - | - | - | - |
| Δ% TTV | r = 0.107 (p = 0.819) | r = 0.000 (p = 1.000) | - | - | - |
| Δ% SUVmean | r = 0.214 (p = 0.645) | r = 0.571 (p = 0.180) | r = −0.250 (p = 0.589) | - | - |
| Δ% SUVmax | r = −0.286 (p = 0.535) | r = −0.321 (p = 0.482) | r = −0.250 (p = 0.589) | r = −0.071 (p = 0.879) | - |
| Δ% LDH | r = −0.286 (p = 0.535) | r = −0.536 (p = 0.215) | r = 0.107 (p = 0.819) | r = 0.000 (p = 1.000) | r = 0.571 (p = 0.180) |
| Patient | Response TTV | Δ% TTV | Response SUVmean | Δ% SUVmean | Response SUVmax | Δ% SUVmax | Response mPERCIST | Δ% mPERCIST | Response RECIST | Δ% RECIST | Response RECIST | Δ% PSA | Δ% LDH | Δ% AP | OS [mo] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | PR | −39.3% | PR | −37.5% | PR | −54.9% | PR | −36.8% | SD | +2.6% | SD | −14.2% | −3.5% | −30.6% | 10 |
| 2 | PR | −67.9% | SD | −24.1% | PR | −46.0% | PR | −65.3% | SD | −13.9% | SD | −67.3% | +16.0% | −10.3% | 26 |
| 3 | SD | +2.6% | SD | −21.6% | PR | −81.6% | PD * | n.e. | Non-CR/Non-PD | n.a. | Non-CR/Non-PD | −32.8% | −11.6% | +165.3% | 18 |
| 4 | PR | −62.4% | SD | −24.1% | PD | +33.6% | PR | −60.5% | Non-CR/Non-PD | n.a. | Non-CR/Non-PD | −41.4% | −23.8% | +42.9% | 11 ° |
| 5 | PR | −64.5% | SD | −8.1% | PD | +74.2% | PD * | n.e. | PD | n.e. | PD | −25.2% | +126.6% | +20.5% | 5 |
| 6 | PR | −97.8% | SD | −23.3% | PR | −62.2% | PR | −66.4% | SD | +8.9% | SD | −20.8% | −29.6% | +55.8% | 11 |
| 7 | PR | −59.6% | PR | −42.3% | PR | −45.2% | PR | −78.5% | Non-CR/Non-PD | n.a. | Non-CR/Non-PD | −44.4% | +22.0% | −9.2% | 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Unterrainer, L.M.; Beyer, L.; Zacherl, M.J.; Gildehaus, F.J.; Todica, A.; Kunte, S.C.; Holzgreve, A.; Sheikh, G.T.; Herlemann, A.; Casuscelli, J.; et al. Total Tumor Volume on 18F-PSMA-1007 PET as Additional Imaging Biomarker in mCRPC Patients Undergoing PSMA-Targeted Alpha Therapy with 225Ac-PSMA-I&T. Biomedicines 2022, 10, 946. https://doi.org/10.3390/biomedicines10050946
Unterrainer LM, Beyer L, Zacherl MJ, Gildehaus FJ, Todica A, Kunte SC, Holzgreve A, Sheikh GT, Herlemann A, Casuscelli J, et al. Total Tumor Volume on 18F-PSMA-1007 PET as Additional Imaging Biomarker in mCRPC Patients Undergoing PSMA-Targeted Alpha Therapy with 225Ac-PSMA-I&T. Biomedicines. 2022; 10(5):946. https://doi.org/10.3390/biomedicines10050946
Chicago/Turabian StyleUnterrainer, Lena M., Leonie Beyer, Mathias J. Zacherl, Franz J. Gildehaus, Andrei Todica, Sophie C. Kunte, Adrien Holzgreve, Gabriel T. Sheikh, Annika Herlemann, Jozefina Casuscelli, and et al. 2022. "Total Tumor Volume on 18F-PSMA-1007 PET as Additional Imaging Biomarker in mCRPC Patients Undergoing PSMA-Targeted Alpha Therapy with 225Ac-PSMA-I&T" Biomedicines 10, no. 5: 946. https://doi.org/10.3390/biomedicines10050946
APA StyleUnterrainer, L. M., Beyer, L., Zacherl, M. J., Gildehaus, F. J., Todica, A., Kunte, S. C., Holzgreve, A., Sheikh, G. T., Herlemann, A., Casuscelli, J., Brendel, M., Albert, N. L., Wenter, V., Schmidt-Hegemann, N.-S., Kunz, W. G., Cyran, C. C., Ricke, J., Stief, C. G., Bartenstein, P., ... Unterrainer, M. (2022). Total Tumor Volume on 18F-PSMA-1007 PET as Additional Imaging Biomarker in mCRPC Patients Undergoing PSMA-Targeted Alpha Therapy with 225Ac-PSMA-I&T. Biomedicines, 10(5), 946. https://doi.org/10.3390/biomedicines10050946








