Intra-Articular Injection of Platelet-Rich Plasma Is More Effective than Hyaluronic Acid or Steroid Injection in the Treatment of Mild to Moderate Knee Osteoarthritis: A Prospective, Randomized, Triple-Parallel Clinical Trial
Abstract
:1. Introduction
2. Methods
2.1. Patient Selection and Screening
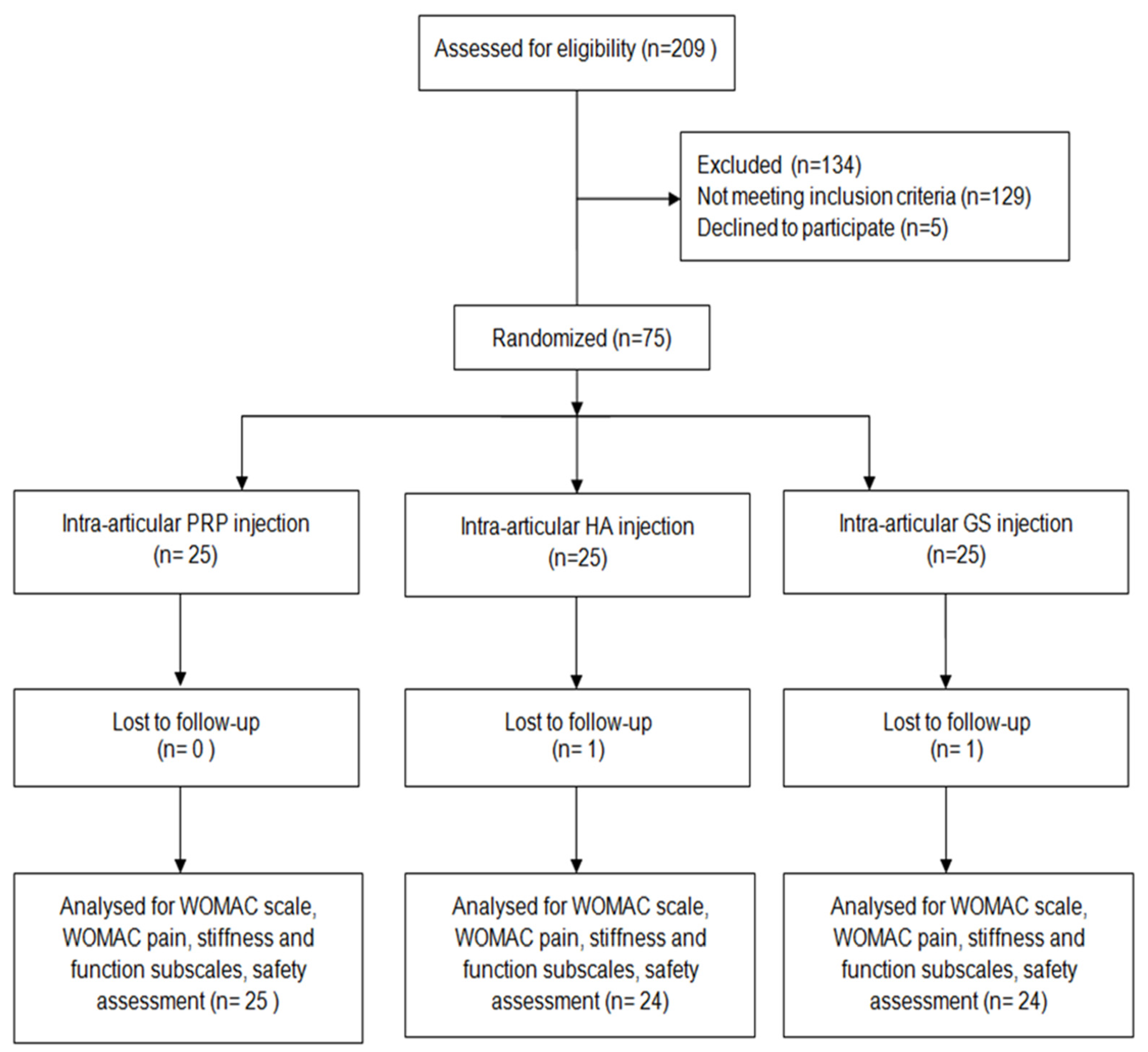
Randomization
2.2. Intervention Protocol
- History of ailments and medications taken.
- Completing the WOMAC questionnaire.
- Physical examination.
- Information on further proceedings.
- Preparation of PRP.
2.3. Statistical Methods
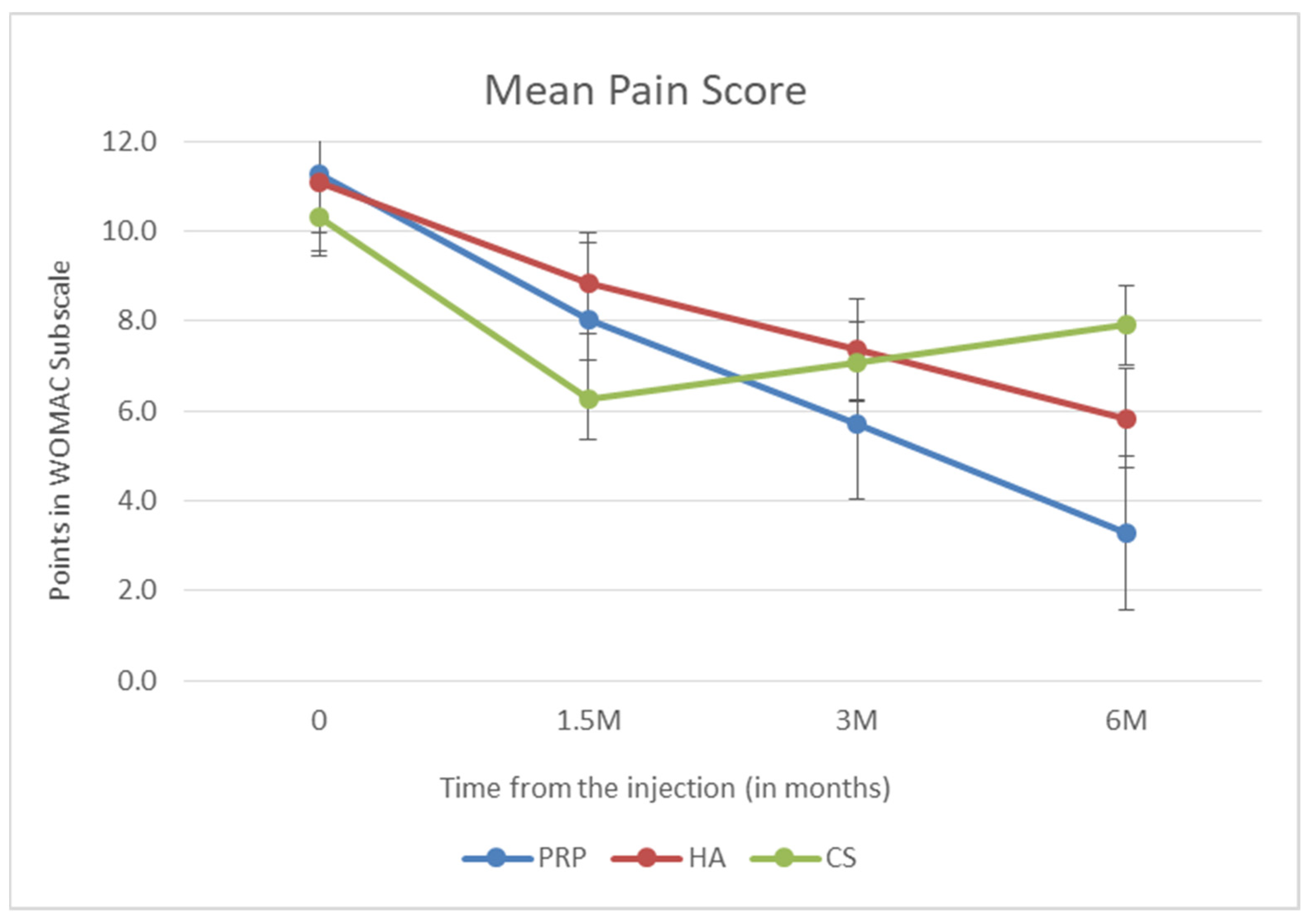
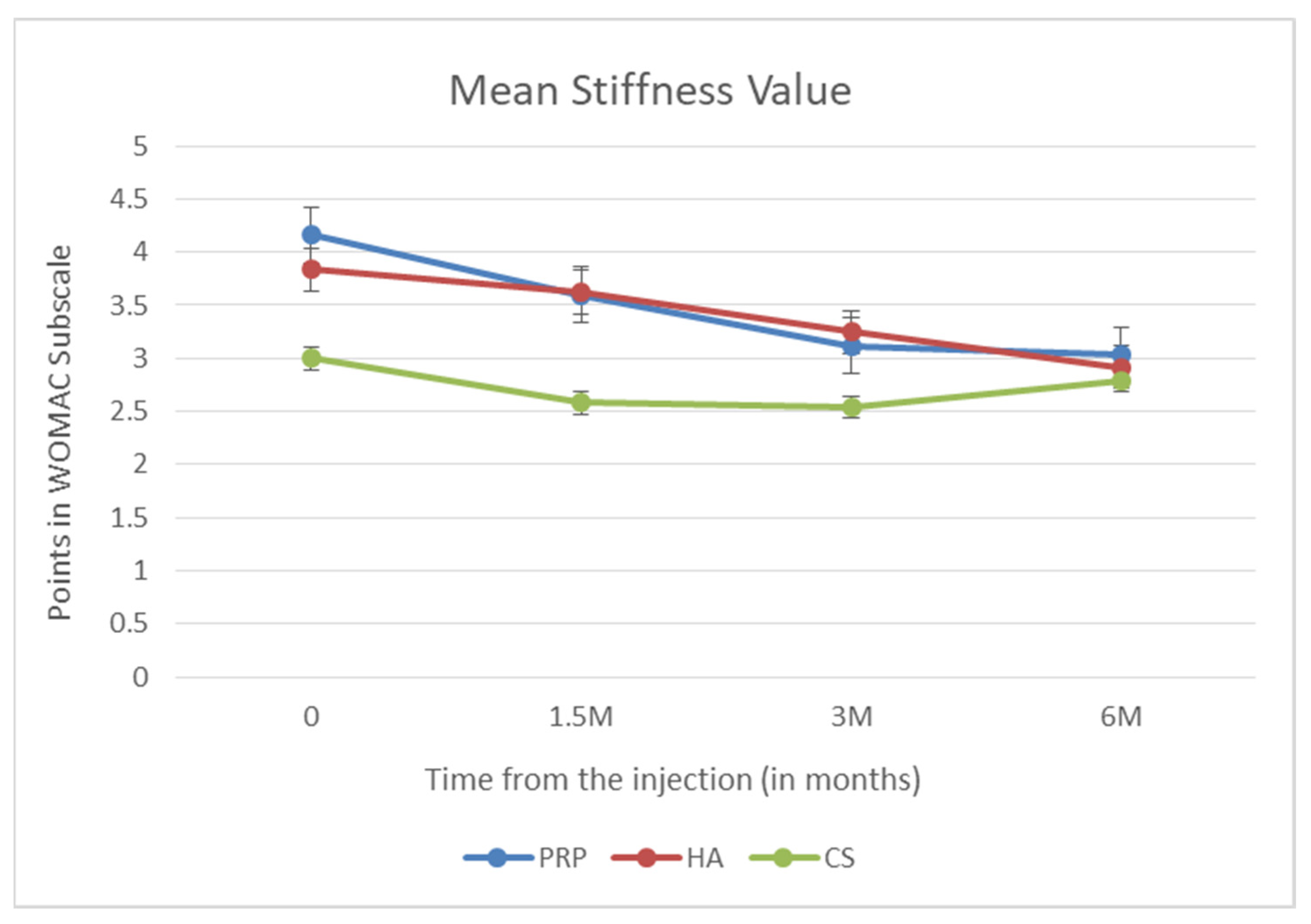
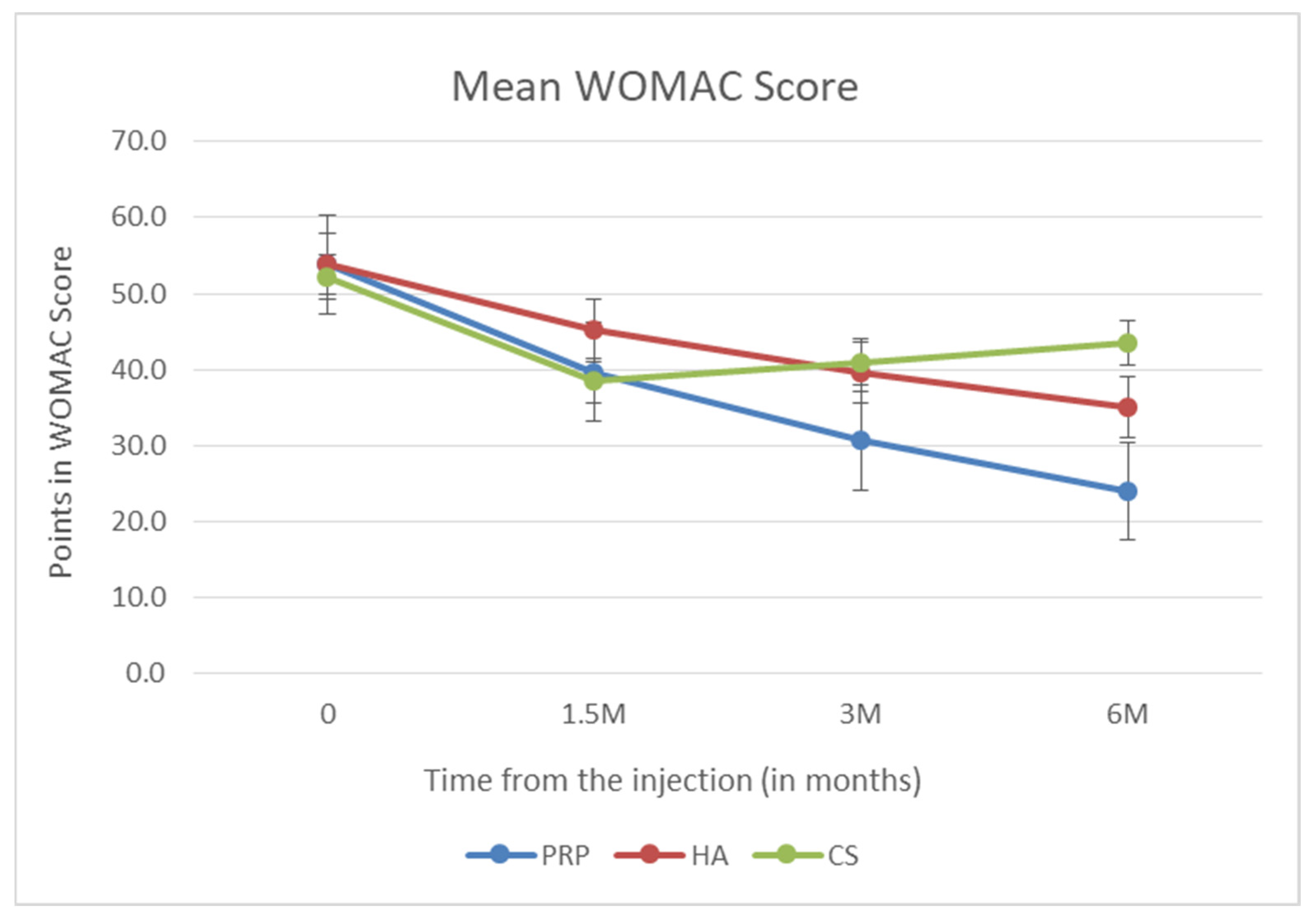
3. Results
Analysis of the Safety Profile of Individual Methods
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pereira, D.; Peleteiro, B.; Araújo, J.; Branco, J.; Santos, R.A.; Ramos, E. The effect of osteoarthritis definition on prevalence and incidence estimates: A systematic review. Osteoarthr. Cartil. 2011, 19, 1270–1285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mobasheri, A.; Rayman, M.P.; Gualillo, O.; Sellam, J.; Van Der Kraan, P.; Fearon, U. The role of metabolism in the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2017, 13, 302–311. [Google Scholar] [CrossRef]
- Szwedowski, D.; Szczepanek, J.; Paczesny, Ł.; Pękała, P.; Zabrzyński, J.; Kruczyński, J. Genetics in cartilage lesions: Basic science and therapy approaches. Int. J. Mol. Sci. 2020, 21, 5430. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Hua, S.H.A.; Yang, T.; Ma, J.; Yu, W.; Chen, X. Platelet-rich plasma shows beneficial effects for patients with knee osteoarthritis by suppressing inflammatory factors. Exp. Ther. Med. 2018, 15, 3096–3102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Buul, G.M.; Koevoet, W.L.M.; Kops, N.; Bos, P.K.; Verhaar, J.A.N.; Weinans, H.; Bernsen, M.R.; Van Osch, G.J.V.M. Platelet-rich plasma releasate inhibits inflammatory processes in osteoarthritic chondrocytes. Am. J. Sports Med. 2011, 39, 2362–2370. [Google Scholar] [CrossRef]
- Szwedowski, D.; Szczepanek, J.; Paczesny, Ł.; Zabrzyński, J.; Gagat, M.; Mobasheri, A.; Jeka, S. The effect of platelet-rich plasma on the intra-articular microenvironment in knee osteoarthritis. Int. J. Mol. Sci. 2021, 22, 5492. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Doherty, M.; Peat, G.; Bierma-Zeinstra, S.M.A.; Arden, N.K.; Bresnihan, B.; Herrero-Beaumont, G.; Kirschner, S.; Leeb, B.F.; Lohmander, L.S.; et al. EULAR evidence-based recommendations for the diagnosis of knee osteoarthritis. Ann. Rheum. Dis. 2010, 69, 483–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cole, B.J.; Karas, V.; Hussey, K.; Merkow, D.B.; Pilz, K.; Fortier, L.A. Hyaluronic Acid Versus Platelet-Rich Plasma: A Prospective. Double-Blind Randomized Controlled Trial Comparing Clinical Outcomes and Effects on Intra-articular Biology for the Treatment of Knee Osteoarthritis. Am. J. Sports Med. 2017, 45, 339–346. [Google Scholar] [CrossRef]
- Annaniemi, J.A.; Pere, J.; Giordano, S. Platelet-rich plasma versus hyaluronic acid injections for knee osteoarthritis: A propensity-score analysis. Scand. J. Surg. 2019, 108, 329–337. [Google Scholar] [CrossRef]
- Di Martino, A.; Di Matteo, B.; Papio, T.; Tentoni, F.; Selleri, F.; Cenacchi, A.; Kon, E.; Filardo, G. Platelet-Rich Plasma Versus Hyaluronic Acid Injections for the Treatment of Knee Osteoarthritis: Results at 5 Years of a Double-Blind. Randomized Controlled Trial. Am. J. Sports Med. 2019, 47, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Belk, J.W.; Kraeutler, M.J.; Houck, D.A.; Goodrich, J.A.; Dragoo, J.L.; McCarty, E.C. Platelet-Rich Plasma Versus Hyaluronic Acid for Knee Osteoarthritis: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Am. J. Sports Med. 2020, 49, 249–260. [Google Scholar] [CrossRef]
- Filardo, G.; Di Matteo, B.; Di Martino, A.; Merli, M.L.; Cenacchi, A.; Fornasari, P.; Marcacci, M.; Kon, E. Platelet-rich plasma intra-articular knee injections show no superiority versus viscosupplementation: A randomized controlled trial. Am. J. Sports Med. 2015, 43, 1575–1582. [Google Scholar] [CrossRef] [PubMed]
- Görmeli, G.; Görmeli, C.A.; Ataoglu, B.; Çolak, C.; Aslantürk, O.; Ertem, K. Multiple PRP injections are more effective than single injections and hyaluronic acid in knees with early osteoarthritis: A randomized. double-blind. placebo-controlled trial. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 958–965. [Google Scholar] [CrossRef]
- Duymus, T.M.; Mutlu, S.; Dernek, B.; Komur, B.; Aydogmus, S.; Kesiktas, F.N. Choice of intra-articular injection in treatment of knee osteoarthritis: Platelet-rich plasma. hyaluronic acid or ozone options. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 485–492. [Google Scholar] [CrossRef]
- Lana, J.F.S.D.; Weglein, A.; Sampson, S.E.; Vicente, E.F.; Huber, S.C.; Souza, C.V.; Ambach, M.A.; Vincent, H.; Urban-Paffaro, A.; Onodera, C.M.K.; et al. Randomized controlled trial comparing hyaluronic acid. platelet-rich plasma and the combination of both in the treatment of mild and moderate osteoarthritis of the knee. J. Stem Cells Regen. Med. 2016, 12, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Matzkin, E.G.; Curry, E.J.; Kong, Q.; Rogers, M.J.; Henry, M.; Smith, E.L. Efficacy and Treatment Response of Intra-articular Corticosteroid Injections in Patients with Symptomatic Knee Osteoarthritis. J. Am. Acad. Orthop. Surg. 2017, 25, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Bliddal, H.; Leeds, A.R.; Christensen, R. Osteoarthritis. obesity and weight loss: Evidence. hypotheses and horizons—A scoping review. Obes. Rev. 2014, 15, 578–586. [Google Scholar] [CrossRef]
- Smith, P.A. Intra-articular Autologous Conditioned Plasma Injections Provide Safe and Efficacious Treatment for Knee Osteoarthritis. Am. J. Sports Med. 2015, 44, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Dhillon, M.S.; Aggarwal, S.; Marwaha, N.; Jain, A. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: A prospective. double-blind. randomized trial. Am. J. Sports Med. 2013, 41, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, A.; Mather, R.C.; Karas, V.; Ellman, M.B.; Young, B.B.; Bach, B.R.; Cole, B.J. An epidemiologic analysis of clinical practice guidelines for non-arthroplasty treatment of osteoarthritis of the knee. Arthrosc.-J. Arthrosc. Relat. Surg. 2014, 30, 65–71. [Google Scholar] [CrossRef]
- Bedard, N.A.; DeMik, D.E.; Glass, N.A.; Burnett, R.A.; Bozic, K.J.; Callaghan, J.J. Impact of clinical practice guidelines on use of intra-articular hyaluronic acid and corticosteroid injections for knee osteoarthritis. J. Bone Jt. Surg.-Am. Vol. 2018, 100, 827–834. [Google Scholar] [CrossRef]
- Kompel, A.J.; Roemer, F.W.; Murakami, A.M.; Diaz, L.E.; Crema, M.D.; Guermazi, A. Intra-articular Corticosteroid Injections in the Hip and Knee: Perhaps not as safe as we thought? Radiology 2019, 293, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Kolasinski, S.L.; Neogi, T.; Hochberg, M.C.; Oatis, C.; Guyatt, G.; Block, J.; Callahan, L.; Copenhaver, C.; Dodge, C.; Felson, D.; et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand. Hip. and Knee. Arthritis Rheumatol. 2020, 72, 220–233. [Google Scholar] [CrossRef]
- Nakazawa, F.; Matsuno, H.; Yudoh, K.; Watanabe, Y.; Katayama, R.; Kimura, T. Corticosteroid treatment induces chondrocyte apoptosis in an experimental arthritis model in chondrocyte cultures. Clin. Exp. Rheumatol. 2002, 20, 773–782. [Google Scholar] [PubMed]
- Han, S.-B.; Seo, I.-W.; Shin, Y.-S. Intra-articular injections of hyaluronic acid or steroid associated with better outcomes than platelet-rich plasma. adipose mesenchymal stromal cell. or placebo in knee osteoarthritis: A network meta-analysis. Arthrosc. J. Arthrosc. Relat. Surg. 2020, 37, 292–306. [Google Scholar] [CrossRef]


| Inclusion Criteria |
|
| Exclusion Criteria |
|
| Age | BMI | WOMAC Pain | WOMAC Stiffness | WOMAC Function of the Knee Joint | Total WOMAC | ||
|---|---|---|---|---|---|---|---|
| PRP (n = 25) | Minimum | 40.00 | 20.30 | 4.00 | 0.00 | 22.00 | 31.00 |
| Maximum | 70.00 | 38.10 | 18.00 | 8.00 | 60.00 | 85.00 | |
| Mean | 57.92 | 27.48 | 11.28 | 4.16 | 38.40 | 53.84 | |
| Standard Deviation | 9.67 | 4.99 | 3.34 | 2.32 | 10.71 | 14.96 | |
| HA (n = 24) | Minimum | 40.00 | 20.80 | 4.00 | 0.00 | 24.00 | 32.00 |
| Maximum | 66.00 | 32.20 | 17.00 | 8.00 | 60.00 | 84.00 | |
| Mean | 52.58 | 26.82 | 11.88 | 3.83 | 39.00 | 53.92 | |
| Standard Deviation | 7.40 | 3.81 | 3.62 | 2.10 | 10.40 | 15.19 | |
| CS (n = 24) | Minimum | 46.00 | 18.21 | 5.00 | 0.00 | 22.00 | 32.00 |
| Maximum | 69.00 | 29.75 | 17.00 | 8.00 | 60.00 | 84.00 | |
| Mean | 57.29 | 25.12 | 10.33 | 3.00 | 38.83 | 52.17 | |
| Standard Deviation | 7.56 | 3.30 | 3.32 | 2.00 | 9.14 | 12.89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szwedowski, D.; Mobasheri, A.; Moniuszko, A.; Zabrzyński, J.; Jeka, S. Intra-Articular Injection of Platelet-Rich Plasma Is More Effective than Hyaluronic Acid or Steroid Injection in the Treatment of Mild to Moderate Knee Osteoarthritis: A Prospective, Randomized, Triple-Parallel Clinical Trial. Biomedicines 2022, 10, 991. https://doi.org/10.3390/biomedicines10050991
Szwedowski D, Mobasheri A, Moniuszko A, Zabrzyński J, Jeka S. Intra-Articular Injection of Platelet-Rich Plasma Is More Effective than Hyaluronic Acid or Steroid Injection in the Treatment of Mild to Moderate Knee Osteoarthritis: A Prospective, Randomized, Triple-Parallel Clinical Trial. Biomedicines. 2022; 10(5):991. https://doi.org/10.3390/biomedicines10050991
Chicago/Turabian StyleSzwedowski, Dawid, Ali Mobasheri, Andrzej Moniuszko, Jan Zabrzyński, and Sławomir Jeka. 2022. "Intra-Articular Injection of Platelet-Rich Plasma Is More Effective than Hyaluronic Acid or Steroid Injection in the Treatment of Mild to Moderate Knee Osteoarthritis: A Prospective, Randomized, Triple-Parallel Clinical Trial" Biomedicines 10, no. 5: 991. https://doi.org/10.3390/biomedicines10050991
APA StyleSzwedowski, D., Mobasheri, A., Moniuszko, A., Zabrzyński, J., & Jeka, S. (2022). Intra-Articular Injection of Platelet-Rich Plasma Is More Effective than Hyaluronic Acid or Steroid Injection in the Treatment of Mild to Moderate Knee Osteoarthritis: A Prospective, Randomized, Triple-Parallel Clinical Trial. Biomedicines, 10(5), 991. https://doi.org/10.3390/biomedicines10050991








