Comparison of CD3e Antibody and CD3e-sZAP Immunotoxin Treatment in Mice Identifies sZAP as the Main Driver of Vascular Leakage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice
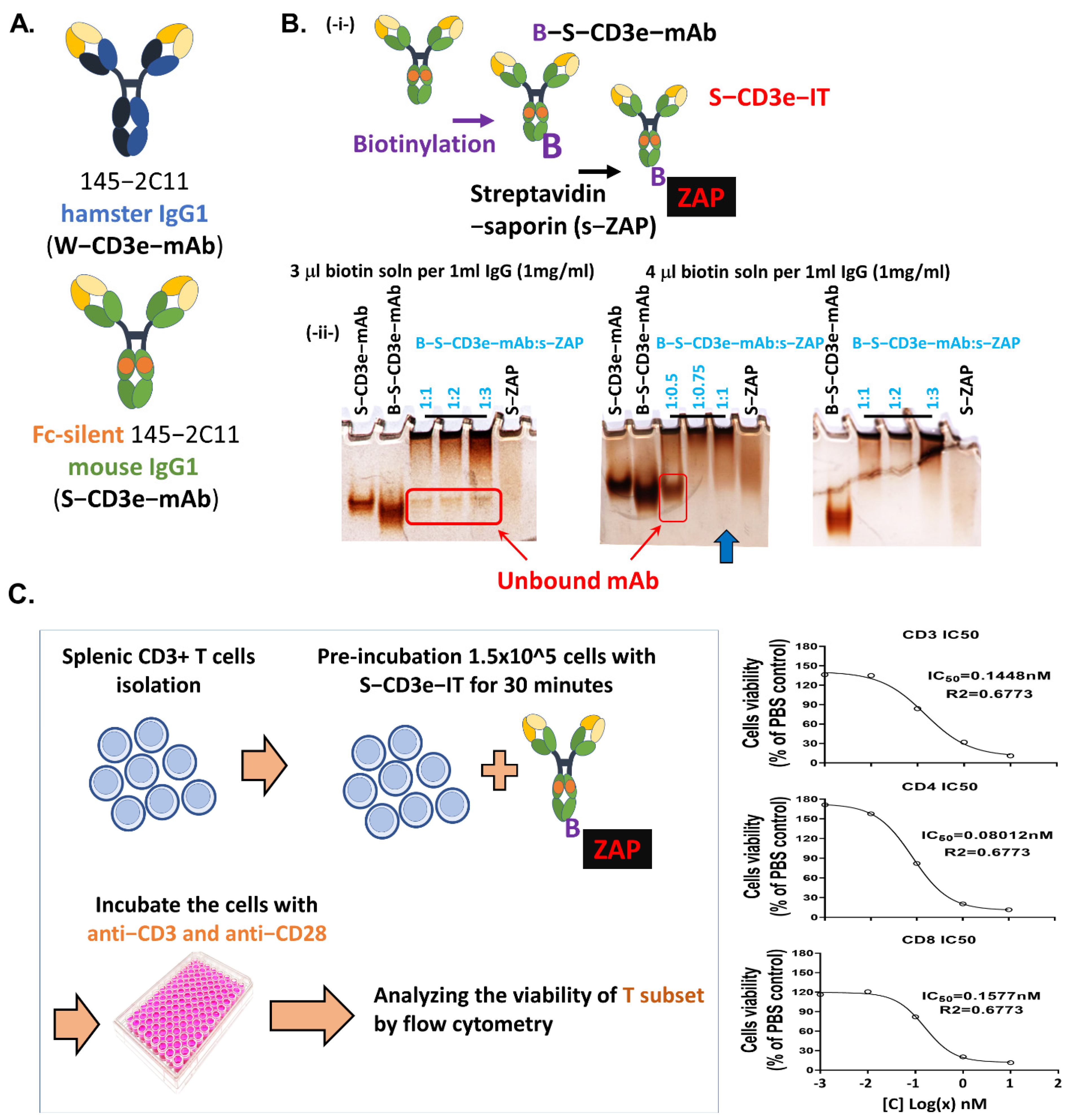
2.2. Biotinylation of Antibodies and S-CD3e-IT Preparation
2.3. S-CD3e-IT In Vitro Cell Killing Assay
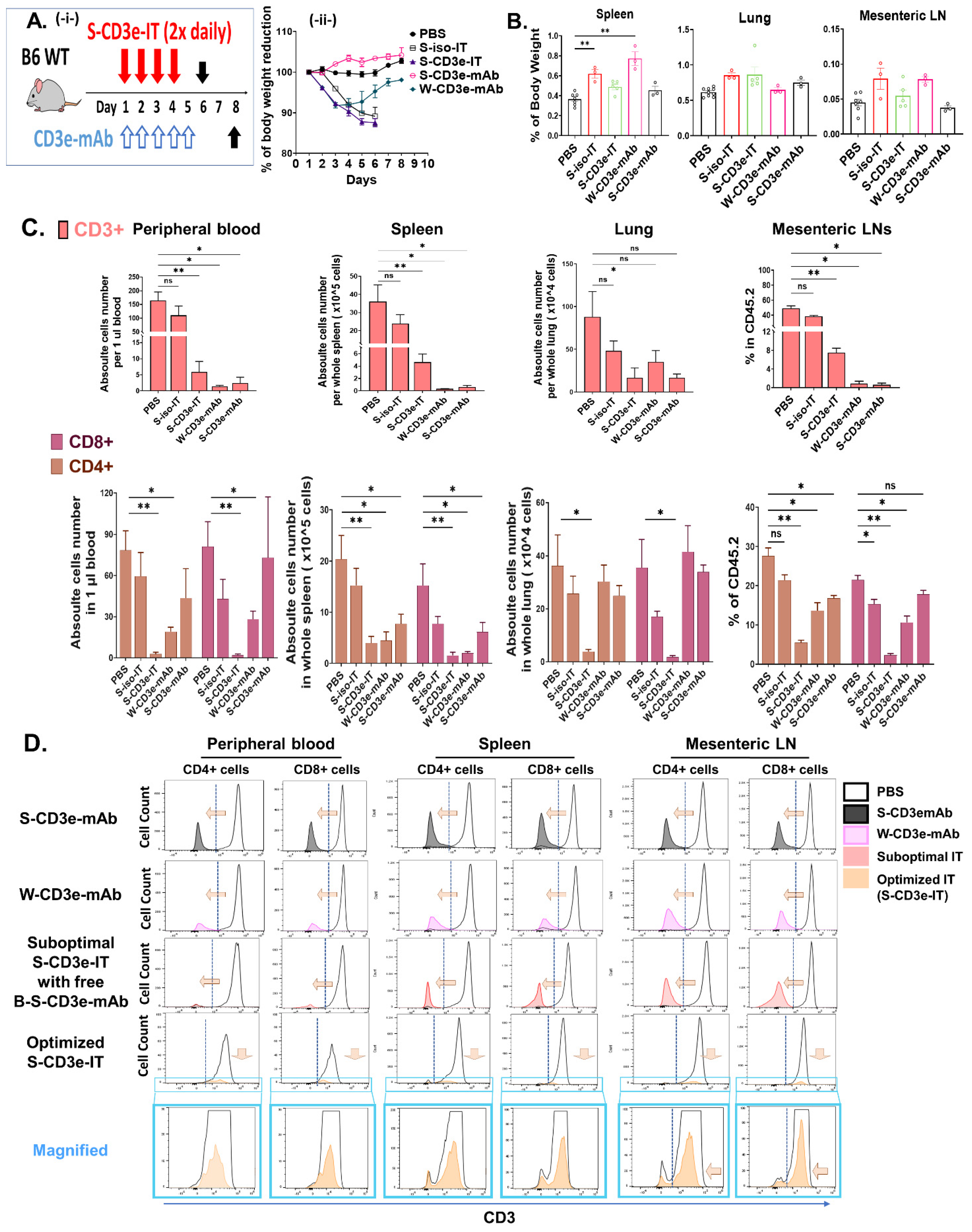
2.4. In Vivo Treatment with CD3e Monoclonal Antibody and S-CD3e-IT
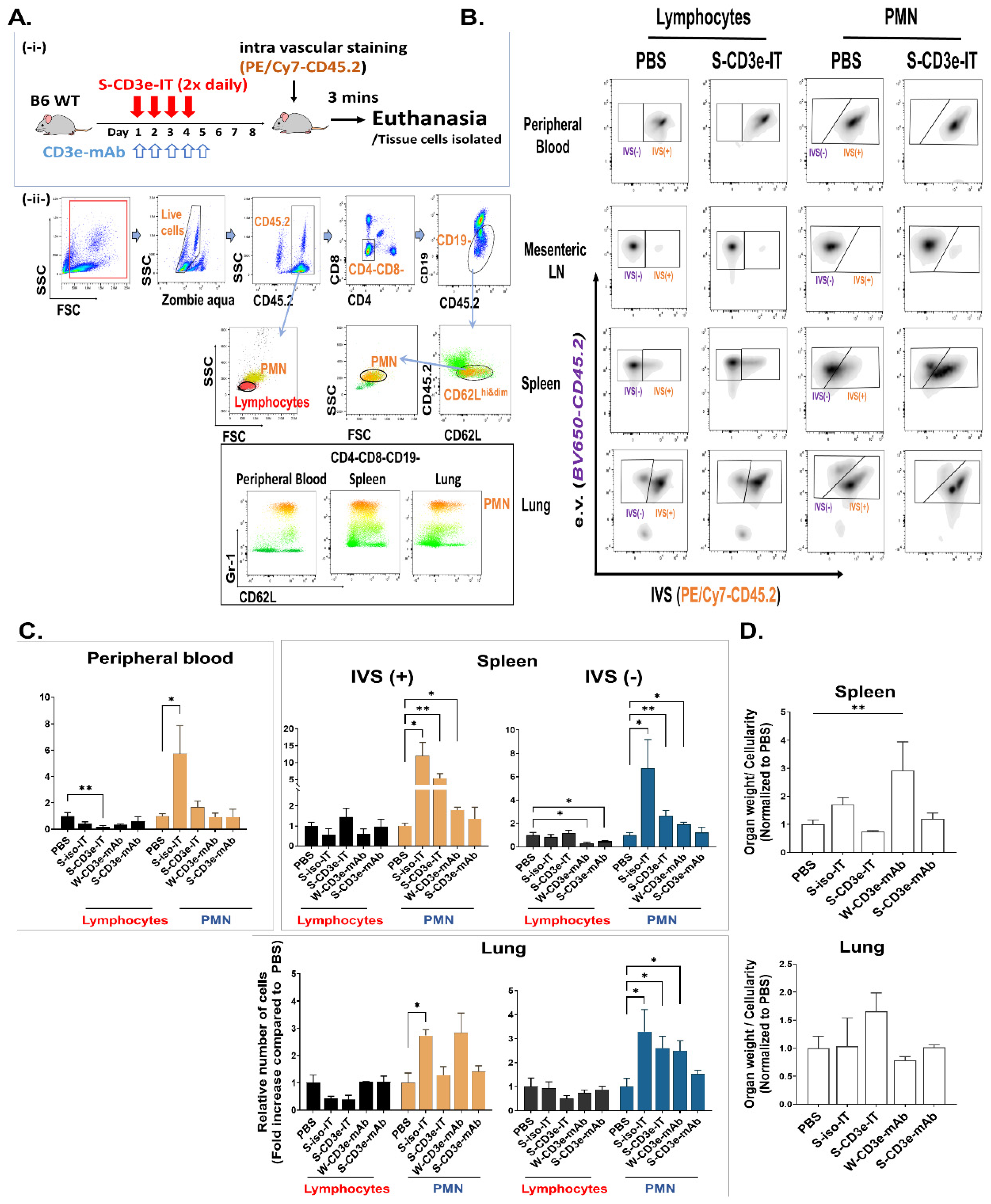
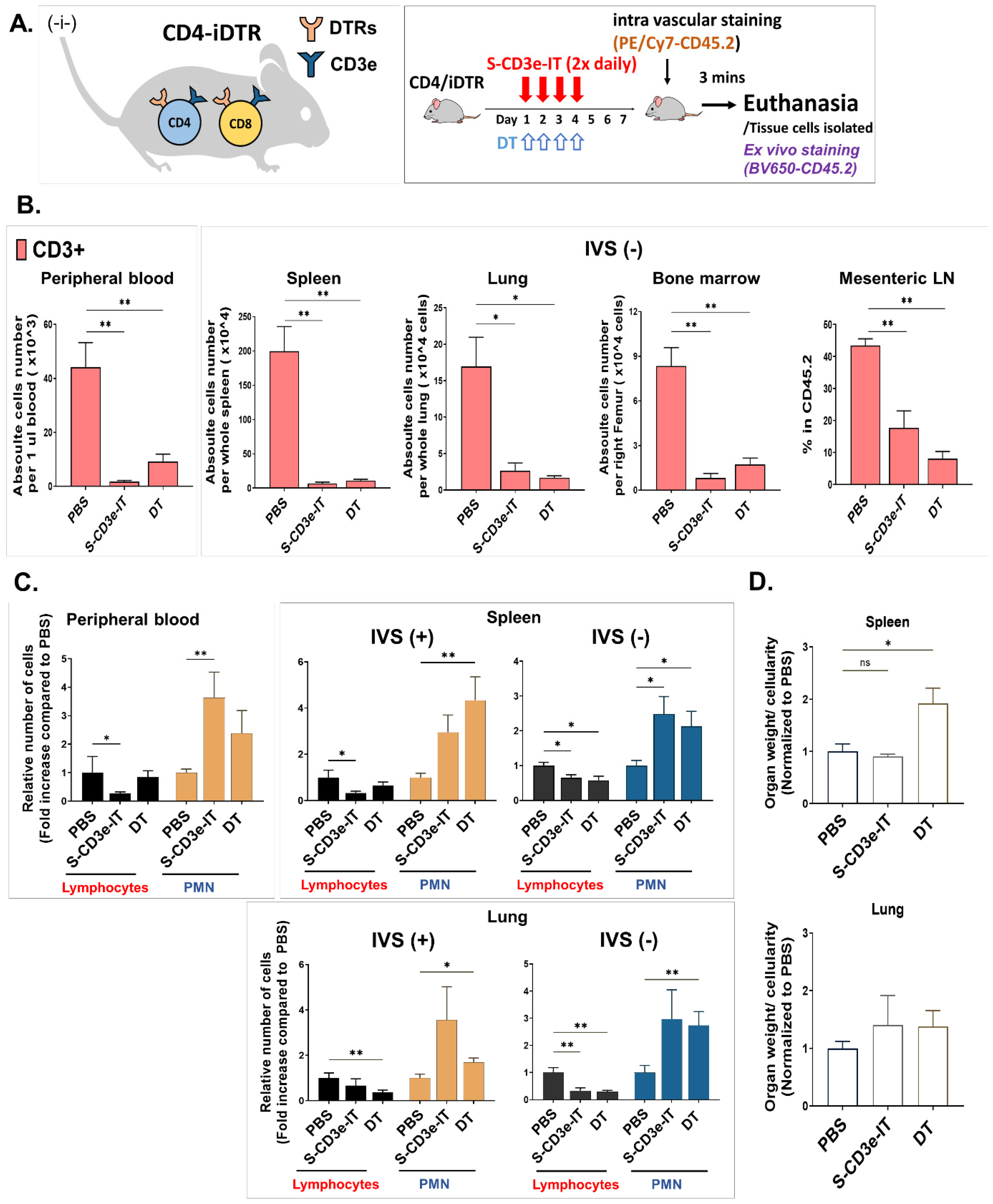
2.5. Intravascular Staining and Cell Isolations
2.6. Flow Cytometry
2.7. PMN Mobilization and W/N Ratios
2.8. In Vivo Short-Term Cell-Binding Assay
2.9. Statistics
3. Results
3.1. Generation of Saporin-Based Anti-Murine CD3epsilon Immunotoxin
3.2. Non-Mitogenic 145-2C11 (S-CD3e-mAb) and S-CD3e-IT Were Well Tolerated in Mice
3.3. Distinct Modes of Action between S-CD3e-mAb and S-CD3e-IT
3.4. S-CD3e-IT Treatment Induces Capillary Leakage
3.5. No Notable Sign of Capillary Leakage Following Administration of Non-Mitogenic S-CD3e-mAb
3.6. Evaluating the Depletion of Local T Cells on IT-Induced Capillary Leakage
3.7. Direct Comparison of S-CD3e-IT and Diphtheria Toxin Treatment in Mice
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Havaei, S.M.; Aucoin, M.G.; Jahanian-Najafabadi, A. Pseudomonas Exotoxin-Based Immunotoxins: Over Three Decades of Efforts on Targeting Cancer Cells with the Toxin. Front. Oncol. 2021, 11, 781800. [Google Scholar] [CrossRef] [PubMed]
- Frankel, A.E.; Woo, J.H.; Ahn, C.; Foss, F.M.; Duvic, M.; Neville, P.H.; Neville, D.M. Resimmune, an Anti-CD3 Recombinant Immunotoxin, Induces Durable Remissions in Patients with Cutaneous T-Cell Lymphoma. Haematologica 2015, 100, 794–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Citores, L.; Iglesias, R.; Ferreras, J.M. Antiviral Activity of Ribosome-Inactivating Proteins. Toxins 2021, 13, 80. [Google Scholar] [CrossRef] [PubMed]
- Yasunaga, M. Antibody Therapeutics and Immunoregulation in Cancer and Autoimmune Disease. Semin. Cancer Biol. 2020, 64, 1–12. [Google Scholar] [CrossRef]
- Hu, H.; Stavrou, S.; Baker, B.C.; Tornatore, C.; Scharff, J.; Okunieff, P.; Neville, D.M. Depletion of T Lymphocytes with Immunotoxin Retards the Progress of Experimental Allergic Encephalomyelitis in Rhesus Monkeys. Cell. Immunol. 1997, 177, 26–34. [Google Scholar] [CrossRef]
- Nishimura, H.; Scalea, J.; Wang, Z.; Shimizu, A.; Moran, S.; Gillon, B.; Sachs, D.H.; Yamada, K. First Experience With the Use of a Recombinant CD3 Immunotoxin as Induction Therapy in Pig-to-Primate Xenotransplantation: The Effect of T-Cell Depletion on Outcome. Transplantation 2011, 92, 641–647. [Google Scholar] [CrossRef] [Green Version]
- Schwarze, M.L.; Menard, M.T.; Fuchimoto, Y.; Huang, C.A.; Houser, S.; Mawulawde, K.; Allison, K.S.; Sachs, D.H.; Madsen, J.C. Mixed Hematopoietic Chimerism Induces Long-Term Tolerance to Cardiac Allografts in Miniature Swine. Ann. Thorac. Surg. 2000, 70, 131–138. [Google Scholar] [CrossRef]
- Zaza, G.; Tomei, P.; Granata, S.; Boschiero, L.; Lupo, A. Monoclonal Antibody Therapy and Renal Transplantation: Focus on Adverse Effects. Toxins 2014, 6, 869–891. [Google Scholar] [CrossRef]
- Casadevall, A.; Pirofski, L.; Joyner, M.J. The Principles of Antibody Therapy for Infectious Diseases with Relevance for COVID-19. mBio 2021, 12, e03372-20. [Google Scholar] [CrossRef]
- Shafiee, F.; Aucoin, M.G.; Jahanian-Najafabadi, A. Targeted Diphtheria Toxin-Based Therapy: A Review Article. Front. Microbiol. 2019, 10, 2340. [Google Scholar] [CrossRef]
- Darvishi, B.; Farahmand, L.; Jalili, N.; Majidzadeh, A.K. Probable Mechanisms Involved in Immunotoxin Mediated Capillary Leak Syndrome (CLS) and Recently Developed Countering Strategies. Curr. Mol. Med. 2018, 18, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Thillainathan, V.; Loh-Trivedi, M.; Rajagopal, A. Pulmonary Capillary Leak Syndrome as a Result of OKT-3 Therapy. Int. Anesthesiol. Clin. 2011, 49, 68–70. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, Y. The Unexpected Side Effects and Safety of Therapeutic Monoclonal Antibodies. Drugs Today 2014, 50, 33. [Google Scholar] [CrossRef] [PubMed]
- Park-Windhol, C.; D’Amore, P.A. Disorders of Vascular Permeability. Annu. Rev. Pathol. 2016, 11, 251–281. [Google Scholar] [CrossRef] [PubMed]
- Baluna, R.; Vitetta, E.S. Vascular Leak Syndrome: A Side Effect of Immunotherapy. Immunopharmacology 1997, 37, 117–132. [Google Scholar] [CrossRef]
- Claesson-Welsh, L.; Dejana, E.; McDonald, D.M. Permeability of the Endothelial Barrier: Identifying and Reconciling Controversies. Trends Mol. Med. 2021, 27, 314–331. [Google Scholar] [CrossRef]
- Xie, Z.; Ghosh, C.C.; Patel, R.; Iwaki, S.; Gaskins, D.; Nelson, C.; Jones, N.; Greipp, P.R.; Parikh, S.M.; Druey, K.M. Vascular Endothelial Hyperpermeability Induces the Clinical Symptoms of Clarkson Disease (the Systemic Capillary Leak Syndrome). Blood 2012, 119, 4321–4332. [Google Scholar] [CrossRef]
- Finsterbusch, M.; Voisin, M.-B.; Beyrau, M.; Williams, T.J.; Nourshargh, S. Neutrophils Recruited by Chemoattractants In Vivo Induce Microvascular Plasma Protein Leakage through Secretion of TNF. J. Exp. Med. 2014, 211, 1307–1314. [Google Scholar] [CrossRef]
- Goldblum, S.E.; Hennig, B.; Jay, M.; Yoneda, K.; McClain, C.J. Tumor Necrosis Factor Alpha-Induced Pulmonary Vascular Endothelial Injury. Infect. Immun. 1989, 57, 1218–1226. [Google Scholar] [CrossRef] [Green Version]
- Chatenoud, L.; Ferran, C.; Legendre, C.; Thouard, I.; Merite, S.; Reuter, A.; Gevaert, Y.; Kreis, H.; Franchimont, P.; Bach, J.-F. In Vivo Cell Activation Following OKT3 Administration. Systemic Cytokine Release and Modulation by Corticosteroids. Transplantation 1990, 49, 697–702. [Google Scholar] [CrossRef]
- Parizel, P.M.; Snoeck, H.W.; Van den Hauwe, L.; Boven, K.; Bosmans, J.L.; Goethem, J.W.V.; Marck, E.A.V.; Cras, P.; Schepper, A.M.D.; Broe, M.E.D. Cerebral Complications of Murine Monoclonal CD3 Antibody (OKT3): CT and MR Findings. Am. J. Neuroradiol. 1997, 18, 1935–1938. [Google Scholar] [PubMed]
- Nouveau, L.; Buatois, V.; Cons, L.; Chatel, L.; Pontini, G.; Pleche, N.; Ferlin, W.G. Immunological Analysis of the Murine Anti-CD3-Induced Cytokine Release Syndrome Model and Therapeutic Efficacy of Anti-Cytokine Antibodies. Eur. J. Immunol. 2021, 51, 2074–2085. [Google Scholar] [CrossRef] [PubMed]
- Becattini, S.; Sorbara, M.T.; Kim, S.G.; Littmann, E.L.; Dong, Q.; Walsh, G.; Wright, R.; Amoretti, L.; Fontana, E.; Hohl, T.M.; et al. Rapid Transcriptional and Metabolic Adaptation of Intestinal Microbes to Host Immune Activation. Cell Host Microbe 2021, 29, 378–393.e5. [Google Scholar] [CrossRef] [PubMed]
- Winkler, U.; Jensen, M.; Manzke, O.; Schulz, H.; Diehl, V.; Engert, A. Cytokine-Release Syndrome in Patients with B-Cell Chronic Lymphocytic Leukemia and High Lymphocyte Counts After Treatment With an Anti-CD20 Monoclonal Antibody (Rituximab, IDEC-C2B8). Blood 1999, 94, 2217–2224. [Google Scholar] [CrossRef] [PubMed]
- Suntharalingam, G.; Perry, M.R.; Ward, S.; Brett, S.J.; Castello-Cortes, A.; Brunner, M.D.; Panoskaltsis, N. Cytokine Storm in a Phase 1 Trial of the Anti-CD28 Monoclonal Antibody TGN1412. N. Engl. J. Med. 2006, 355, 1018–1028. [Google Scholar] [CrossRef]
- Buysmann, S.; Bemelman, F.J.; Schellekens, P.T.A.; Van Kooyk, Y.; Figdor, C.G.; Berge, I.J.M. Activation and Increased Expression of Adhesion Molecules on Peripheral Blood Lymphocytes Is a Mechanism for the Immediate Lymphocytopenia After Administration of OKT3. Blood 1996, 87, 404–411. [Google Scholar] [CrossRef]
- Armstrong, N.; Buckley, P.; Oberley, T.; Fechner, J.J.; Dong, Y.; Hong, X.; Kirk, A.; Neville, D.J.; Knechtle, S. Analysis of Primate Renal Allografts after T-Cell Depletion with Anti-CD3-CRM9. Transplantation 1998, 66, 5–13. [Google Scholar] [CrossRef]
- Fehr, T.; Sykes, M. Tolerance Induction in Clinical Transplantation. Transpl. Immunol. 2004, 13, 117–130. [Google Scholar] [CrossRef]
- Pastan, I.; Hassan, R.; FitzGerald, D.J.; Kreitman, R.J. Immunotoxin Treatment of Cancer. Annu. Rev. Med. 2007, 58, 221–237. [Google Scholar] [CrossRef] [Green Version]
- Alegre, M.L.; Tso, J.Y.; Sattar, H.A.; Smith, J.; Desalle, F.; Cole, M.; Bluestone, J.A. An Anti-Murine CD3 Monoclonal Antibody with a Low Affinity for Fc Gamma Receptors Suppresses Transplantation Responses While Minimizing Acute Toxicity and Immunogenicity. J. Immunol. 1995, 155, 1544–1555. [Google Scholar]
- Chiu, M.L.; Goulet, D.R.; Teplyakov, A.; Gilliland, G.L. Antibody Structure and Function: The Basis for Engineering Therapeutics. Antibodies 2019, 8, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riechmann, L.; Clark, M.; Waldmann, H.; Winter, G. Reshaping Human Antibodies for Therapy. Nature 1988, 332, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.-B.; Wang, Z.; Liu, Y.Y.; Stavrou, S.; Mathias, A.; Goodwin, K.J.; Thomas, J.M.; Neville, D.M. A Fold-Back Single-Chain Diabody Format Enhances the Bioactivity of an Anti-Monkey CD3 Recombinant Diphtheria Toxin-Based Immunotoxin. Protein Eng. Des. Sel. 2007, 20, 425–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, J.; Stavrou, S.; Weetall, M.; Hexham, J.M.; Digan, M.E.; Wang, Z.; Woo, J.H.; Yu, Y.; Mathias, A.; Liu, Y.Y.; et al. Improved Binding of a Bivalent Single-Chain Immunotoxin Results in Increased Efficacy for in Vivo T-Cell Depletion. Protein Eng. Des. Sel. 2001, 14, 1035–1041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woo, J.H.; Bour, S.H.; Dang, T.; Lee, Y.-J.; Park, S.K.; Andreas, E.; Kang, S.H.; Liu, J.-S.; Neville, D.M.; Frankel, A.E. Preclinical Studies in Rats and Squirrel Monkeys for Safety Evaluation of the Bivalent Anti-Human T Cell Immunotoxin, A-DmDT390–BisFv(UCHT1). Cancer Immunol. Immunother. 2008, 57, 1225–1239. [Google Scholar] [CrossRef]
- Hamawy, M.M.; Tsuchida, M.; Manthei, E.R.; Dong, Y.; Fechner, J.H.; Knechtle, S.J. Activation of T Lymphocytes for Adhesion and Cytokine Expression by Toxin-Conjugated Anti-CD3 Monoclonal Antibodies. Transplantation 1999, 68, 693–698. [Google Scholar] [CrossRef]
- Smallshaw, J.E.; Ghetie, V.; Rizo, J.; Fulmer, J.R.; Trahan, L.L.; Ghetie, M.-A.; Vitetta, E.S. Genetic Engineering of an Immunotoxin to Eliminate Pulmonary Vascular Leak in Mice. Nat. Biotechnol. 2003, 21, 387–391. [Google Scholar] [CrossRef]
- Weldon, J.E.; Xiang, L.; Chertov, O.; Margulies, I.; Kreitman, R.J.; FitzGerald, D.J.; Pastan, I. A Protease-Resistant Immunotoxin against CD22 with Greatly Increased Activity against CLL and Diminished Animal Toxicity. Blood 2009, 113, 3792–3800. [Google Scholar] [CrossRef] [Green Version]
- Weldon, J.E.; Xiang, L.; Zhang, J.; Beers, R.; Walker, D.A.; Onda, M.; Hassan, R.; Pastan, I. A Recombinant Immunotoxin against the Tumor-Associated Antigen Mesothelin Reengineered for High Activity, Low Off-Target Toxicity, and Reduced Antigenicity. Mol. Cancer Ther. 2013, 12, 48–57. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Song, S.; Kou, G.; Li, B.; Zhang, D.; Hou, S.; Qian, W.; Dai, J.; Tian, L.; Zhao, J.; et al. Treatment of Hepatocellular Carcinoma in a Mouse Xenograft Model with an Immunotoxin Which Is Engineered to Eliminate Vascular Leak Syndrome. Cancer Immunol. Immunother. 2007, 56, 1775–1783. [Google Scholar] [CrossRef]
- Mühlen, K.; Schümann, J.; Wittke, F.; Stenger, S.; Van Rooijen, N.; Kaer, L.; Tiegs, G. NK Cells, but Not NKT Cells, Are Involved in Pseudomonas Aeruginosa Exotoxin A-Induced Hepatotoxicity in Mice. J. Immunol. 2004, 172, 3034–3041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegall, C.B.; Liggitt, D.; Chace, D.; Tepper, M.A.; Fell, H.P. Prevention of Immunotoxin-Mediated Vascular Leak Syndrome in Rats with Retention of Antitumor Activity. Proc. Natl. Acad. Sci. USA 1994, 91, 9514–9518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schümann, J.; Angermüller, S.; Bang, R.; Lohoff, M.; Tiegs, G. Acute Hepatotoxicity of Pseudomonas Aeruginosa Exotoxin A in Mice Depends on T Cells and TNF. J. Immunol. 1998, 161, 5745–5754. [Google Scholar]
- Baluna, R.; Rizo, J.; Gordon, B.E.; Ghetie, V.; Vitetta, E.S. Evidence for a Structural Motif in Toxins and Interleukin-2 That May Be Responsible for Binding to Endothelial Cells and Initiating Vascular Leak Syndrome. Proc. Natl. Acad. Sci. USA 1999, 96, 3957–3962. [Google Scholar] [CrossRef] [Green Version]
- Thorgersen, E.B.; Asvall, J.; Frøysnes, I.S.; Schjalm, C.; Larsen, S.G.; Dueland, S.; Andersson, Y.; Fodstad, Ø.; Mollnes, T.E.; Flatmark, K. Increased Local Inflammatory Response to MOC31PE Immunotoxin After Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. Ann. Surg. Oncol. 2021, 28, 5252–5262. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Xiao, L.; Joo, K.-I.; Liu, Y.; Zhang, C.; Liu, S.; Conti, P.S.; Li, Z.; Wang, P. A Potent Immunotoxin Targeting Fibroblast Activation Protein for Treatment of Breast Cancer in Mice: Immunotoxin Targeting Fibroblast Activation Protein. Int. J. Cancer 2016, 138, 1013–1023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, R.V.P.; Ku, T.; Wang, A.; Pires, L.; Ferreira, V.H.; Michaelsen, V.; Ali, A.; Galasso, M.; Moshkelgosha, S.; Gazzalle, A.; et al. Ex Vivo Treatment of Cytomegalovirus in Human Donor Lungs Using a Novel Chemokine-Based Immunotoxin. J. Heart Lung Transplant. 2022, 41, 287–297. [Google Scholar] [CrossRef]
- Anderson, K.G.; Mayer-Barber, K.; Sung, H.; Beura, L.; James, B.R.; Taylor, J.J.; Qunaj, L.; Griffith, T.S.; Vezys, V.; Barber, D.L.; et al. Intravascular Staining for Discrimination of Vascular and Tissue Leukocytes. Nat. Protoc. 2014, 9, 209–222. [Google Scholar] [CrossRef] [Green Version]
- Potter, E.L.; Gideon, H.P.; Tkachev, V.; Fabozzi, G.; Chassiakos, A.; Petrovas, C.; Darrah, P.A.; Lin, P.L.; Foulds, K.E.; Kean, L.S.; et al. Measurement of Leukocyte Trafficking Kinetics in Macaques by Serial Intravascular Staining. Sci. Transl. Med. 2021, 13, eabb4582. [Google Scholar] [CrossRef]
- Reutershan, J.; Basit, A.; Galkina, E.V.; Ley, K. Sequential Recruitment of Neutrophils into Lung and Bronchoalveolar Lavage Fluid in LPS-Induced Acute Lung Injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005, 289, L807–L815. [Google Scholar] [CrossRef] [Green Version]
- Reutershan, J.; Morris, M.; Burcin, T.; Smith, D.; Chang, D.; Saprito, M.; Ley, K. Critical Role of Endothelial CXCR2 in LPS-Induced Neutrophil Migration into the Lung. J. Clin. Investig. 2006, 116, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Giansanti, F.; Flavell, D.J.; Angelucci, F.; Fabbrini, M.S.; Ippoliti, R. Strategies to Improve the Clinical Utility of Saporin-Based Targeted Toxins. Toxins 2018, 10, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ancheta, L.R.; Shramm, P.A.; Bouajram, R.; Higgins, D.; Lappi, D.A. Saporin as a Commercial Reagent: Its Uses and Unexpected Impacts in the Biological Sciences—Tools from the Plant Kingdom. Toxins 2022, 14, 184. [Google Scholar] [CrossRef] [PubMed]
- Bonardi, M.A.; Bell, A.; French, R.R.; Gromo, G.; Hamblin, T.; Modena, D.; Tutt, A.L.; Glennie, M.J. Initial Experience in Treating Human Lymphoma with a Combination of Bispecific Antibody and Saporin. Int. J. Cancer Suppl. 1992, 7, 73–77. [Google Scholar]
- French, R.R.; Bell, A.J.; Hamblin, T.J.; Tutt, A.L.; Glennie, M.J. Response of B-Cell Lymphoma to a Combination of Bispecific Antibodies and Saporin. Leuk Res. 1996, 20, 607–617. [Google Scholar] [CrossRef]
- Falini, B.; Bolognesi, A.; Flenghi, L.; Tazzari, P.L.; Broe, M.K.; Stein, H.; Dürkop, H.; Aversa, F.; Corneli, P.; Pizzolo, G. Response of Refractory Hodgkin’s Disease to Monoclonal Anti-CD30 Immunotoxin. Lancet 1992, 339, 1195–1196. [Google Scholar] [CrossRef]
- Carlring, J.; Barr, T.A.; Buckle, A.-M.; Heath, A.W. Anti-CD28 Has a Potent Adjuvant Effect on the Antibody Response to Soluble Antigens Mediated through CTLA-4 by-Pass. Eur. J. Immunol. 2003, 33, 135–142. [Google Scholar] [CrossRef]
- Buch, T.; Heppner, F.L.; Tertilt, C.; Heinen, T.J.A.J.; Kremer, M.; Wunderlich, F.T.; Jung, S.; Waisman, A. A Cre-Inducible Diphtheria Toxin Receptor Mediates Cell Lineage Ablation after Toxin Administration. Nat. Methods 2005, 2, 419–426. [Google Scholar] [CrossRef]
- Kumar, R.K.; Jin, Y.; Watts, S.W.; Rockwell, C.E. Naïve, Regulatory, Activated, and Memory Immune Cells Co-Exist in PVATs That Are Comparable in Density to Non-PVAT Fats in Health. Front. Physiol. 2020, 11, 58. [Google Scholar] [CrossRef] [Green Version]
- Palchaudhuri, R.; Saez, B.; Hoggatt, J.; Schajnovitz, A.; Sykes, D.B.; Tate, T.A.; Czechowicz, A.; Kfoury, Y.; Ruchika, F.; Rossi, D.J.; et al. Non-Genotoxic Conditioning for Hematopoietic Stem Cell Transplantation Using a Hematopoietic-Cell-Specific Internalizing Immunotoxin. Nat. Biotechnol. 2016, 34, 738–745. [Google Scholar] [CrossRef] [Green Version]
- Peng, B.; Ye, P.; Rawlings, D.J.; Ochs, H.D.; Miao, C.H. Anti-CD3 Antibodies Modulate Anti–Factor VIII Immune Responses in Hemophilia A Mice after Factor VIII Plasmid-Mediated Gene Therapy. Blood 2009, 114, 4373–4382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penaranda, C.; Tang, Q.; Bluestone, J.A. Anti-CD3 Therapy Promotes Tolerance by Selectively Depleting Pathogenic Cells While Preserving Regulatory T Cells. J. Immunol. 2011, 187, 2015–2022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, K.; Sung, H.; Skon, C.N.; Lefrançois, L.; Deisinger, A.; Vezys, V.; Masopust, D. Cutting Edge: Intravascular Staining Redefines Lung CD8 T Cell Responses. J. Immunol. 2012, 189, 2702–2706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.-R.A.; O’Koren, E.G.; Hotten, D.F.; Kan, M.J.; Kopin, D.; Nelson, E.R.; Que, L.; Gunn, M.D. A Protocol for the Comprehensive Flow Cytometric Analysis of Immune Cells in Normal and Inflamed Murine Non-Lymphoid Tissues. PLoS ONE 2016, 11, e0150606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Misharin, A.V.; Morales-Nebreda, L.; Mutlu, G.M.; Budinger, G.R.S.; Perlman, H. Flow Cytometric Analysis of Macrophages and Dendritic Cell Subsets in the Mouse Lung. Am. J. Respir. Cell Mol. Biol. 2013, 49, 503–510. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.Y.; Wang, Z.; Thomas, J.; Goodwin, K.J.; Stavrou, S.; Neville, D.M., Jr. Polymorphisms of CD3ε in Cynomolgus and Rhesus Monkeys and Their Relevance to Anti-CD3 Antibodies and Immunotoxins. Immunol. Cell Biol. 2007, 85, 357–362. [Google Scholar] [CrossRef]
- Wang, Z.; Kim, G.-B.; Woo, J.-H.; Liu, Y.Y.; Mathias, A.; Stavrou, S.; Neville, D.M. Improvement of a Recombinant Anti-Monkey Anti-CD3 Diphtheria Toxin Based Immunotoxin by Yeast Display Affinity Maturation of the ScFv. Bioconjug. Chem. 2007, 18, 947–955. [Google Scholar] [CrossRef]
- Steffens, S.; Burger, F.; Pelli, G.; Dean, Y.; Elson, G.; Kosco-Vilbois, M.; Chatenoud, L.; Mach, F. Short-Term Treatment With Anti-CD3 Antibody Reduces the Development and Progression of Atherosclerosis in Mice. Circulation 2006, 114, 1977–1984. [Google Scholar] [CrossRef] [Green Version]
- Kuhn, C.; Weiner, H. Therapeutic Anti-CD3 Monoclonal Antibodies: From Bench to Bedside. Immunotherapy 2016, 8, 889–906. [Google Scholar] [CrossRef] [Green Version]
- Mehta, D.S.; Christmas, R.A.; Waldmann, H.; Rosenzweig, M. Partial and Transient Modulation of the CD3-T-Cell Receptor Complex, Elicited by Low-Dose Regimens of Monoclonal Anti-CD3, Is Sufficient to Induce Disease Remission in Non-Obese Diabetic Mice. Immunology 2010, 130, 103–113. [Google Scholar] [CrossRef]
- Wamala, I.; Matar, A.J.; Farkash, E.; Wang, Z.; Huang, C.A.; Sachs, D.H. Recombinant Anti-Monkey CD3 Immunotoxin Depletes Peripheral Lymph Node T Lymphocytes More Effectively than Rabbit Anti-Thymocyte Globulin in Naïve Baboons. Transpl. Immunol. 2013, 29, 60–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nobre, C.F.; Newman, M.J.; DeLisa, A.; Newman, P. Moxetumomab Pasudotox-Tdfk for Relapsed/Refractory Hairy Cell Leukemia: A Review of Clinical Considerations. Cancer Chemother. Pharm. 2019, 84, 255–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kollmorgen, G.; Palme, K.; Seidl, A.; Scheiblich, S.; Birzele, F.; Wilson, S.; Clemens, C.; Voss, E.; Kaufmann, M.; Hirzel, K.; et al. A Re-Engineered Immunotoxin Shows Promising Preclinical Activity in Ovarian Cancer. Sci. Rep. 2017, 7, 18086. [Google Scholar] [CrossRef] [PubMed]
- Dépis, F.; Hatterer, E.; Ballet, R.; Daubeuf, B.; Cons, L.; Glatt, S.; Reith, W.; Kosco-Vilbois, M.; Dean, Y. Characterization of a Surrogate Murine Antibody to Model Anti-Human CD3 Therapies. mAbs 2013, 5, 555–564. [Google Scholar] [CrossRef] [Green Version]
- Meijer, R.T.; Koopmans, R.P.; Ten Berge, I.J.M.; Schellekens, P.T.A. Pharmacokinetics of Murine Anti-Human CD3 Antibodies in Man Are Determined by the Disappearance of Target Antigen. J. Pharm. Exp. Ther. 2002, 300, 346–353. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Roller, J.; Slotta, J.E.; Zhang, S.; Luo, L.; Rahman, M.; Syk, I.; Menger, M.D.; Thorlacius, H. Distinct Patterns of Leukocyte Recruitment in the Pulmonary Microvasculature in Response to Local and Systemic Inflammation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013, 304, L298–L305. [Google Scholar] [CrossRef]
- Miyazaki, S.; Ishikawa, F.; Fujikawa, T.; Nagata, S.; Yamaguchi, K. Intraperitoneal Injection of Lipopolysaccharide Induces Dynamic Migration of Gr-1high Polymorphonuclear Neutrophils in the Murine Abdominal Cavity. Clin. Diagn. Lab. Immunol. 2004, 11, 452–457. [Google Scholar] [CrossRef] [Green Version]
- Assier, E.; Jullien, V.; Lefort, J.; Moreau, J.-L.; Di Santo, J.P.; Vargaftig, B.B.; Lapa e Silva, J.R.; Thèze, J. NK Cells and Polymorphonuclear Neutrophils Are Both Critical for IL-2-Induced Pulmonary Vascular Leak Syndrome. J. Immunol. 2004, 172, 7661–7668. [Google Scholar] [CrossRef] [Green Version]
- Hirsch, R.; Gress, R.E.; Pluznik, D.H.; Eckhaus, M.; Bluestone, J.A. Effects of In Vivo Administration of Anti-CD3 Monoclonal Antibody on T Cell Function in Mice. II. In Vivo Activation of T Cells. J. Immunol. 1989, 142, 737–743. [Google Scholar]
- Blazar, B.R.; Hirsch, R.; Gress, R.E.; Carroll, S.F.; Vallera, D.A. In Vivo Administration of Anti-CD3 Monoclonal Antibodies or Immunotoxins in Murine Recipients of Allogeneic T Cell-Depleted Marrow for the Promotion of Engraftment. J. Immunol. 1991, 147, 1492–1503. [Google Scholar]
- Schenkel, J.M.; Fraser, K.A.; Masopust, D. Cutting Edge: Resident Memory CD8 T Cells Occupy Frontline Niches in Secondary Lymphoid Organs. J. Immunol. 2014, 192, 2961–2964. [Google Scholar] [CrossRef] [PubMed]
- Marriott, C.L.; Dutton, E.E.; Tomura, M.; Withers, D.R. Retention of Ag-Specific Memory CD4+ T Cells in the Draining Lymph Node Indicates Lymphoid Tissue Resident Memory Populations. Eur. J. Immunol. 2017, 47, 860–871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masopust, D.; Soerens, A.G. Tissue-Resident T Cells and Other Resident Leukocytes. Annu. Rev. Immunol. 2019, 37, 521–546. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, R.; Gehad, A.; Yang, C.; Campbell, L.; Teague, J.E.; Schlapbach, C.; Elco, C.; Huang, V.; Matos, T.R.; Kupper, T.S.; et al. Human Skin Is Protected by Four Functionally and Phenotypically Discrete Populations of Resident and Recirculating Memory T Cells. Sci. Transl. Med. 2015, 7, 279ra39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alipour, M.; Pucaj, K.; Smith, M.G.; Suntres, Z.E. Toxicity of Ricin Toxin A Chain in Rats. Drug Chem. Toxicol. 2013, 36, 224–230. [Google Scholar] [CrossRef]
- Vallera, D.A.; Panoskaltsis-Mortari, A.; Blazar, B.R. Renal Dysfunction Accounts for the Dose Limiting Toxicity of DT390anti- CD3sFv, a Potential New Recombinant Anti-GVHD Immunotoxin. Protein Eng. Des. Sel. 1997, 10, 1071–1076. [Google Scholar] [CrossRef] [Green Version]
- Makarem, R.; Humphries, M.J. LDV: A Novel Cell Adhesion Motif Recognized by the Integrin A4β1. Biochem. Soc. Trans. 1991, 19, 380S. [Google Scholar] [CrossRef]
- Wang, Z.; Duran-Struuck, R.; Crepeau, R.; Matar, A.; Hanekamp, I.; Srinivasan, S.; Neville, D.M.; Sachs, D.H.; Huang, C.A. Development of a Diphtheria Toxin Based Antiporcine CD3 Recombinant Immunotoxin. Bioconjug. Chem. 2011, 22, 2014–2020. [Google Scholar] [CrossRef] [Green Version]
- Lesch, H.P.; Kaikkonen, M.U.; Pikkarainen, J.T.; Ylä-Herttuala, S. Avidin-Biotin Technology in Targeted Therapy. Expert Opin. Drug Deliv. 2010, 7, 551–564. [Google Scholar] [CrossRef]
- Rosebrough, S.F.; Hartley, D.F. Biochemical Modification of Streptavidin and Avidin: In Vitro and In Vivo Analysis. J. Nucl. Med. 1996, 37, 1380–1384. [Google Scholar]
- Bubb, M.O.; Green, F.; Conradie, J.D.; Tchernyshev, B.; Bayer, E.A.; Wilchek, M. Natural Antibodies to Avidin in Human Serum. Immunol. Lett. 1993, 35, 277–280. [Google Scholar] [CrossRef]
- Cox, J.E.; Cheng, T.L. Egg-Based Vaccines. Pediatrics Rev. 2006, 27, 118–119. [Google Scholar] [CrossRef] [PubMed]
- Petronzelli, F.; Pelliccia, A.; Anastasi, A.M.; Lindstedt, R.; Manganello, S.; Ferrari, L.E.; Albertoni, C.; Leoni, B.; Rosi, A.; D’Alessio, V.; et al. Therapeutic Use of Avidin Is Not Hampered by Antiavidin Antibodies in Humans. Cancer Biother. Radiopharm. 2010, 25, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Díaz, R.; Pallarès, V.; Cano-Garrido, O.; Serna, N.; Sánchez-García, L.; Falgàs, A.; Pesarrodona, M.; Unzueta, U.; Sánchez-Chardi, A.; Sánchez, J.M.; et al. Selective CXCR4+ Cancer Cell Targeting and Potent Antineoplastic Effect by a Nanostructured Version of Recombinant Ricin. Small 2018, 14, e1800665. [Google Scholar] [CrossRef]
- Cheung, L.S.; Fu, J.; Kumar, P.; Kumar, A.; Urbanowski, M.E.; Ihms, E.A.; Parveen, S.; Bullen, C.K.; Patrick, G.J.; Harrison, R.; et al. Second-Generation IL-2 Receptor-Targeted Diphtheria Fusion Toxin Exhibits Antitumor Activity and Synergy with Anti–PD-1 in Melanoma. Proc. Natl. Acad. Sci. USA 2019, 116, 3100–3105. [Google Scholar] [CrossRef] [Green Version]
- Bolognesi, A.; Tazzari, P.L.; Tassi, C.; Gromo, G.; Gobbi, M.; Stirpe, F. A Comparison of Anti-Lymphocyte Immunotoxins Containing Different Ribosome-Inactivating Proteins and Antibodies. Clin. Exp. Immunol. 1992, 89, 341–346. [Google Scholar] [CrossRef]
- Foss, F.M.; Bacha, P.; Osann, K.E.; Demierre, M.F.; Bell, T.; Kuzel, T. Biological Correlates of Acute Hypersensitivity Events with DAB(389)IL-2 (Denileukin Diftitox, ONTAK) in Cutaneous T-Cell Lymphoma: Decreased Frequency and Severity with Steroid Premedication. Clin. Lymphoma 2001, 1, 298–302. [Google Scholar] [CrossRef]
- McCann, S.; Akilov, O.E.; Geskin, L. Adverse Effects of Denileukin Diftitox and Their Management in Patients with Cutaneous T-Cell Lymphoma. Clin. J. Oncol. Nurs. 2012, 16, E164–E172. [Google Scholar] [CrossRef] [Green Version]
- Ohmachi, K.; Ando, K.; Ogura, M.; Uchida, T.; Tobinai, K.; Maruyama, D.; Namiki, M.; Nakanishi, T. E7777 in Japanese Patients with Relapsed/Refractory Peripheral and Cutaneous T-Cell Lymphoma: A Phase I Study. Cancer Sci. 2018, 109, 794–802. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Shukla, R.K.; Kim, E.; Cressman, S.G.; Yu, H.; Baek, A.; Choi, H.; Kim, A.; Sharma, A.; Wang, Z.; et al. Comparison of CD3e Antibody and CD3e-sZAP Immunotoxin Treatment in Mice Identifies sZAP as the Main Driver of Vascular Leakage. Biomedicines 2022, 10, 1221. https://doi.org/10.3390/biomedicines10061221
Kim S, Shukla RK, Kim E, Cressman SG, Yu H, Baek A, Choi H, Kim A, Sharma A, Wang Z, et al. Comparison of CD3e Antibody and CD3e-sZAP Immunotoxin Treatment in Mice Identifies sZAP as the Main Driver of Vascular Leakage. Biomedicines. 2022; 10(6):1221. https://doi.org/10.3390/biomedicines10061221
Chicago/Turabian StyleKim, Shihyoung, Rajni Kant Shukla, Eunsoo Kim, Sophie G. Cressman, Hannah Yu, Alice Baek, Hyewon Choi, Alan Kim, Amit Sharma, Zhirui Wang, and et al. 2022. "Comparison of CD3e Antibody and CD3e-sZAP Immunotoxin Treatment in Mice Identifies sZAP as the Main Driver of Vascular Leakage" Biomedicines 10, no. 6: 1221. https://doi.org/10.3390/biomedicines10061221
APA StyleKim, S., Shukla, R. K., Kim, E., Cressman, S. G., Yu, H., Baek, A., Choi, H., Kim, A., Sharma, A., Wang, Z., Huang, C. A., Reneau, J. C., Boyaka, P. N., Liyanage, N. P. M., & Kim, S. (2022). Comparison of CD3e Antibody and CD3e-sZAP Immunotoxin Treatment in Mice Identifies sZAP as the Main Driver of Vascular Leakage. Biomedicines, 10(6), 1221. https://doi.org/10.3390/biomedicines10061221





