Genetic Profiling of Glucocorticoid (NR3C1) and Mineralocorticoid (NR3C2) Receptor Polymorphisms before Starting Therapy with Androgen Receptor Inhibitors: A Study of a Patient Who Developed Toxic Myocarditis after Enzalutamide Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient’s and Offspring’s Clinical History
2.2. Leukocyte Isolation
2.3. White Cell mRNA Extraction and Transcriptome Sequencing
3. Results
3.1. Analysis of Genetic Variants of Glucocorticoid and Mineralocorticoid Receptor Genes
3.2. Analysis of Alternative Splicing of Glucocorticoid and Mineralocorticoid Receptor Genes
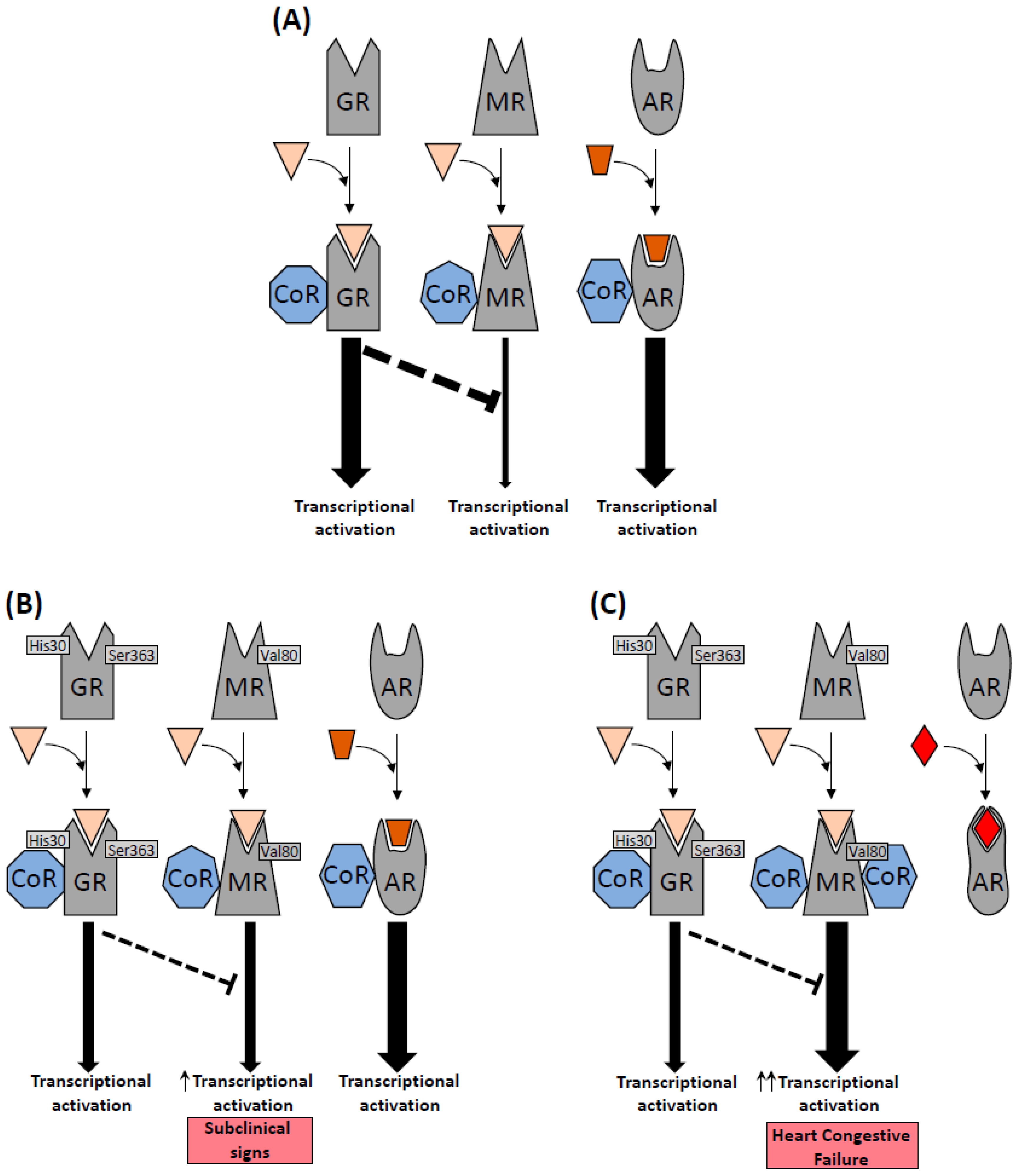
4. Discussion
4.1. Mineralocorticoid Receptor: rs5522 Genetic Variant
4.2. Glucocorticoid Receptor: rs143711342 and rs56149945 Genetics Variants
4.3. Physiological Effects of GR and MR Genetic Variants in Cardiovascular System
4.4. MR/GR Polymorphisms and the Effect of Enzalutamide on Cardiac Health
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tran, C.; Ouk, S.; Clegg, N.J.; Chen, Y.; Watson, P.A.; Arora, V.; Wongvipat, J.; Smith-Jones, P.M.; Yoo, D.; Kwon, A.; et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 2009, 324, 787–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, Y.; Sadar, M.D. Enzalutamide and blocking androgen receptor in advanced prostate cancer: Lessons learnt from the history of drug development of antiandrogens. Res. Rep. Urol. 2018, 10, 23–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scher, H.I.; Beer, T.M.; Higano, C.S.; Anand, A.; Taplin, M.E.; Efstathiou, E.; Rathkopf, D.; Shelkey, J.; Evan, Y.Y.; Alumkal, J.; et al. Antitumour activity of MDV3100 in castra-tion-resistant prostate cancer: A phase 1–2 study. Lancet 2010, 375, 1437–1446. [Google Scholar] [CrossRef] [Green Version]
- Shiota, M.; Fujimoto, N.; Higashijima, K.; Imada, K.; Kashiwagi, E.; Takeuchi, A.; Inokuchi, J.; Tatsugami, K.; Kajioka, S.; Uchiumi, T.; et al. Mineralocorticoid receptor signal-ing affects therapeutic effect of enzalutamide. Prostate 2018, 78, 1045–1052. [Google Scholar] [CrossRef]
- Scailteux, L.M.; Despas, F.; Balusson, F.; Campillo-Gimenez, B.; Mathieu, R.; Vincendeau, S.; Happe, A.; Nowak, E.; Kerbrat, S.; Oger, E. Hospitalization for adverse events under abiraterone or enzalutamide exposure in real-world setting: A French population-based study on prostate cancer patients. Br. J. Clin. Pharmacol. 2022, 88, 336–346. [Google Scholar] [CrossRef]
- Hu, J.; Aprikian, A.G.; Vanhuyse, M.; Dragomir, A. Comparative Cardiovascular Safety of Novel Hormonal Agents in Metastatic Castration-Resistant Prostate Cancer Using Real-World Data. Clin. Genitourin. Cancer 2022, 20, 17–24. [Google Scholar] [CrossRef]
- Mancini, M.; Zazzara, M.; Zattoni, F. Stem cells, biomarkers and genetic profiling: Approaching future challenges in Urology. Urologia 2016, 83, 4–13. [Google Scholar] [CrossRef]
- Ramos Reyes, V.; Laynez Carnicero, A.; Morales, M. Acute myocarditis by enzalutamide. Med. Clínica 2021, 156, 359. [Google Scholar] [CrossRef]
- Petrovich, E.; Asher, C.; Garty, H. Induction of FKBP51 by aldosterone in intestinal epithelium. J. Steroid Biochem. Mol. Biol. 2014, 139, 78–87. [Google Scholar] [CrossRef]
- Young, M.J.; Rickard, A.J. Mechanisms of mineralocorticoid salt-induced hypertension and cardiac fibrosis. Mol. Cell. Endocrinol. 2011, 350, 248–255. [Google Scholar] [CrossRef]
- Yang, J.; Young, M.J. The mineralocorticoid receptor and its coregulators. J. Mol. Endocrinol. 2009, 43, 53–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farman, N.; Rafestin-Oblin, M.E. Multiple aspects of mineralocorticoid selectivity. Am. J. Physiol. Renal Physiol. 2001, 280, F181–F192. [Google Scholar] [CrossRef] [PubMed]
- Bridgham, J.T.; Carroll, S.M.; Thornton, J.W. Evolution of hormone-receptor complexity by molecular exploitation. Science 2006, 312, 97–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Gasparo, M.; Joss, U.; Ramjoue, H.P.; Whitebread, S.E.; Haenni, H.; Schenkel, L.; Kraehenbuehl, C.; Biollaz, M.; Grob, J.; Schmidlin, J. Three new epoxy-spirolactone derivatives: Characterization in vivo and in vitro. J. Pharmacol. Exp. Ther. 1987, 240, 650–656. [Google Scholar]
- Struthers, A.; Krum, H.; Williams, G.H. A Comparison of the Aldosterone-blocking Agents Eplerenone and Spironolac-tone. Clin. Cardiol. 2008, 31, 153–158. [Google Scholar] [CrossRef]
- Bitting, R.L.; Healy, P.; George, D.J.; Anand, M.; Kim, S.; Mayer, T.; Winters, C.; Riggan, C.; Rasmussen, J.; Wilder, R.; et al. Phase II Trial of Enzalutamide and Androgen Dep-rivation Therapy with Salvage Radiation in Men with High-risk Prostate-specific Antigen Recurrent Prostate Can-cer: The STREAM Trial. Eur. Urol. Oncol. 2021, 4, 948–954. [Google Scholar] [CrossRef] [Green Version]
- Grossmann, C.; Almeida-Prieto, B.; Nolze, A.; Alvarez de la Rosa, D. Structural and molecular determinants of mineralocorticoid receptor signalling. Br. J. Pharmacol. 2021. [Google Scholar] [CrossRef]
- Huyet, J.; Pinon, G.M.; Fay, M.R.; Rafestin-Oblin, M.E.; Fagart, J. Structural determinants of ligand binding to the miner-alocorticoid receptor. Mol. Cell. Endocrinol. 2012, 350, 187–195. [Google Scholar] [CrossRef]
- De, G.; Brandine, S.; Smith, A.D.; Nilsson, R.H. Falco: High-speed FastQC emulation for quality control of sequencing data. F1000Research 2021, 8, 1874. [Google Scholar] [CrossRef]
- Goldstein, L.D.; Cao, Y.; Pau, G.; Lawrence, M.; Wu, T.D.; Seshagiri, S.; Gentleman, R. Prediction and Quantification of Splice Events from RNA-Seq Data. PLoS ONE 2016, 11, e0156132. [Google Scholar] [CrossRef]
- Fischer, K.; Kelly, S.M.; Watt, K.; Price, N.C.; McEwan, I.J. Conformation of the mineralocorticoid receptor N-terminal domain: Evidence for induced and stable structure. Mol. Endocrinol. 2010, 24, 1935–1948. [Google Scholar] [CrossRef] [PubMed]
- Fuse, H.; Kitagawa, H.; Kato, S. Characterization of transactivational property and coactivator mediation of rat mineralocorticoid receptor activation function-1 (AF-1). Mol. Endocrinol. 2000, 14, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Le Tallec, L.; Kirsh, O.; Lecomte, M.C.; Viengchareun, S.; Zennaro, M.C.; Dejean, A.; Lombès, M. Protein inhibitor of activated signal transducer and activator of transcription 1 interacts with the N-terminal domain of mineralocorticoid re-ceptor and represses its transcriptional activity: Implication of small ubiquitin-related modifier 1 modification. Mol. Endocrinol. 2003, 17, 2529–2542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dougherty, E.J.; Elinoff, J.M.; Ferreyra, G.A.; Hou, A.; Cai, R.; Sun, J.; Blaine, K.P.; Wang, S.; Danner, R.L. Mineralocorticoid Receptor (MR) trans-Activation of Inflammatory AP-1 Signaling: Dependence on DNA Sequence, MR Conformation, and AP-1 Family Member Expression. J. Biol. Chem. 2016, 291, 23628–23644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Planey, S.L.; Derfoul, A.; Steplewski, A.; Robertson, N.M.; Litwack, G. Inhibition of glucocorticoid-induced apoptosis in 697 pre-B lymphocytes by the mineralocorticoid receptor N-terminal domain. J. Biol. Chem. 2002, 277, 42188–42196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arai, K.; Nakagomi, Y.; Iketani, M.; Shimura, Y.; Amemiya, S.; Ohyama, K.; Shibasaki, T. Functional polymorphisms in the miner-alocorticoid receptor and amirolide-sensitive sodium channel genes in a patient with sporadic pseudohypoaldos-teronism. Hum. Genet. 2003, 112, 91–97. [Google Scholar] [CrossRef]
- Zennaro, M.C.; Fernandes-Rosa, F. 30 Years of the mineralocorticoid receptor: Mineralocorticoid receptor mutations. J. Endocrinol. 2017, 234, T93–T106. [Google Scholar] [CrossRef]
- Govindan, M.V.; Warriar, N. Reconstitution of the N-terminal transcription activation function of human mineral-ocorticoid receptor in a defective human glucocorticoid receptor. J. Biol. Chem. 1998, 273, 24439–24447. [Google Scholar] [CrossRef] [Green Version]
- Shiota, M.; Fujimoto, N.; Imada, K.; Kashiwagi, E.; Takeuchi, A.; Inokuchi, J.; Tatsugami, K.; Kajioka, S.; Uchiumi, T.; Eto, M. Prognostic Impact of Genetic Poly-morphism in Mineralocorticoid Receptor and Comorbidity with Hypertension in Androgen-Deprivation Therapy. Front. Oncol. 2018, 8, 635. [Google Scholar] [CrossRef]
- Terock, J.; van der Auwera, S.; Janowitz, D.; Wittfeld, K.; Teumer, A.; Grabe, H.J. Functional polymorphisms of the min-eralocorticoid receptor gene NR3C2 are associated with diminished memory decline: Results from a longitudinal general-population study. Mol. Genet. Genomic Med. 2020, 8, e1345. [Google Scholar] [CrossRef]
- Kumsta, R.; Kliegel, D.; Linden, M.; DeRijk, R.; de Kloet, E.R. Genetic variation of the mineralocorticoid receptor gene (MR, NR3C2) is associated with a conceptual endophenotype of “CRF-hypoactivity”. Psychoneuroendocrinology 2019, 105, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, N.; Bellingrath, S.; de Kloet, E.R.; Zitman, F.G.; DeRijk, R.H.; Kudielka, B.M.; Wüst, S. Human mineralocorticoid receptor (MR) gene haplotypes modulate MR expression and transactivation: Implication for the stress re-sponse. Psychoneuroendocrinology 2011, 36, 699–709. [Google Scholar] [CrossRef] [PubMed]
- DeRijk, R.H.; Wüst, S.; Meijer, O.C.; Zennaro, M.C.; Federenko, I.S.; Hellhammer, D.H.; Giacchetti, G.; Vreugdenhil, E.; Zitman, F.G.; de Kloet, E.R. A common polymorphism in the mineralocorticoid receptor modulates stress responsiveness. J. Clin. Endocrinol. Metab. 2006, 91, 5083–5089. [Google Scholar] [CrossRef] [Green Version]
- Panek, M.; Jonakowski, M.; Zioło, J.; Wieteska, Ł.; Małachowska, B.; Pietras, T.; Szemraj, J.; Kuna, P. A novel approach to understand-ing the role of polymorphic forms of the NR3C1 and TGF-β1 genes in the modulation of the expression of IL-5 and IL-15 mRNA in asthmatic inflammation. Mol. Med. Rep. 2016, 13, 4879–4887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, R.C.Y.; Wang, X.L.; Morris, B.J. Association of coronary artery disease with glucocorticoid receptor N363S variant. Hypertension 2003, 41, 404–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Funder, J.W.; Pearce, P.T.; Smith, R.; Smith, A.I. Mineralocorticoid action: Target tissue specificity is enzyme, not recep-tor, mediated. Science 1988, 242, 583–585. [Google Scholar] [CrossRef]
- Young, M.J.; Kanki, M.; Karthigan, N.; Konstandopoulos, P. The Role of the Mineralocorticoid Receptor and Mineralo-corticoid Receptor–Directed Therapies in Heart Failure. Endocrinology 2021, 162, bqab105. [Google Scholar] [CrossRef]
- Oakley, R.H.; Cruz-Topete, D.; He, B.O.; Foley, J.F.; Myers, P.H.; Xu, X.; Gomez-Sanchez, C.E.; Chambon, P.; Willis, M.S.; Cidlowski, J.A. Cardiomyocyte glucocorticoid and mineralocor-ticoid receptors directly and antagonistically regulate heart disease in mice. Sci. Signal. 2019, 12, eaau9685. [Google Scholar] [CrossRef]
- Bauersachs, J.; López-Andrés, N. Mineralocorticoid receptor in cardiovascular diseases-Clinical trials and mecha-nistic insights. Br. J. Pharmacol. 2021. [Google Scholar] [CrossRef]
- Fejes-Tóth, G.; Náray-Fejes-Tóth, A. Early aldosterone-regulated genes in cardiomyocytes: Clues to cardiac re-modeling? Endocrinology 2007, 148, 1502–1510. [Google Scholar] [CrossRef] [Green Version]
- Nabeebaccus, A.; Zhang, M.; Shah, A.M. NADPH oxidases and cardiac remodelling. Heart Fail. Rev. 2010, 16, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Ibarrola, J.; Garcia-Peña, A.; Matilla, L.; Bonnard, B.; Sádaba, R.; Arrieta, V.; Alvarez, V.; Fernández-Celis, A.; Gainza, A.; Navarro, A.; et al. A New Role for the Aldoste-rone/Mineralocorticoid Receptor Pathway in the Development of Mitral Valve Prolapse. Circ. Res. 2020, 127, e80–e93. [Google Scholar] [CrossRef] [PubMed]
- Rude, M.K.; Duhaney, T.A.S.; Kuster, G.M.; Judge, S.; Heo, J.; Colucci, W.S.; Siwik, D.A.; Sam, F. Aldosterone stimulates matrix metallopro-teinases and reactive oxygen species in adult rat ventricular cardiomyocytes. Hypertension 2005, 46, 555–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, H.; Kobara, M.; Abe, M.; Tanaka, N.; Gouda, E.; Toba, H.; Yamada, H.; Tatsumi, T.; Nakata, T.; Matsubara, H. Aldosterone nongenomically produces NADPH oxidase-dependent reactive oxygen species and induces myocyte apoptosis. Hypertens. Res. 2008, 31, 363–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rickard, A.J.; Young, M.J. Corticosteroid receptors, macrophages and cardiovascular disease. J. Mol. Endocrinol. 2009, 42, 449–459. [Google Scholar] [CrossRef]
- Bene, N.C.; Alcaide, P.; Wortis, H.H.; Jaffe, I.Z. Mineralocorticoid receptors in immune cells: Emerging role in cardio-vascular disease. Steroids 2014, 91, 38–45. [Google Scholar] [CrossRef] [Green Version]
- Dumeny, L.; Vardeny, O.; Edelmann, F.; Pieske, B.; Duarte, J.D.; Cavallari, L.H. NR3C2 genotype is associated with re-sponse to spironolactone in diastolic heart failure patients from the Aldo-DHF trial. Pharmacotherapy 2021, 41, 978–987. [Google Scholar] [CrossRef]
- Ritter, A.M.V.; Fontana, V.; de Faria, A.P.C.; Modolo, R.; Barbaro, N.R.; Sabbatini, A.R.; Peres, H.; Biagi, C.; Silva, P.S.; Lopes, P.C.; et al. Association of Mineralocorti-coid Receptor Polymorphism I180V With Left Ventricular Hypertrophy in Resistant Hypertension. Am. J. Hypertens. 2016, 29, 245–250. [Google Scholar] [CrossRef] [Green Version]
- Alyamani, M.; Li, J.; Patel, M.; Taylor, S.; Nakamura, F.; Berk, M.; Przybycin, C.; Posadas, E.M.; Madan, R.A.; Gulley, J.L.; et al. Deep androgen receptor suppression in prostate cancer exploits sexually dimorphic renal expression for systemic glucocorticoid exposure. Ann. Oncol. 2020, 31, 369–376. [Google Scholar] [CrossRef] [Green Version]
- Kaochar, S.; Mitsiades, N. Glucocorticoids mediate adverse events of deep androgen receptor axis inhibition in prostate cancer patients. Ann. Oncol. 2020, 31, 323–325. [Google Scholar] [CrossRef]
- Gomez-Sanchez, C.E.; Gomez-Sanchez, E.P. The Mineralocorticoid Receptor and the Heart. Endocrinology 2021, 162, bqab131. [Google Scholar] [CrossRef] [PubMed]


| Gene | refSNP | European Allele Frequency | Sample | nt | Allele | aa | Allele |
|---|---|---|---|---|---|---|---|
| MR (NR3C2) | rs5522 | A = 0.889731/G = 0.110269 | Patient | nt 538 | G/G | aa 180 | Val/Val |
| Son 1 | A/G | Ile/Val | |||||
| Son 2 | A/G | Ile/Val | |||||
| Daughter | A/G | Ile/Val | |||||
| GR (NR3C1) | rs143711342 | A = 0.999740/G = 0.000260 | Patient | nt 88 | A/G | aa 30 | Tyr/His |
| Son 1 | A/A | Tyr/Tyr | |||||
| Son 2 | A/G | Tyr/His | |||||
| Daughter | A/A | Tyr/Tyr | |||||
| GR (NR3C1) | rs56149945 | T = 0.967909/C = 0.032091 | Patient | nt 1088 | T/C | aa 363 | Asn/Ser |
| Son 1 | T/C | Asn/Ser | |||||
| Son 2 | T/T | Asn/Asn | |||||
| Daughter | T/C | Asn/Ser |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales, M.; Martín-Vasallo, P.; Ávila, J. Genetic Profiling of Glucocorticoid (NR3C1) and Mineralocorticoid (NR3C2) Receptor Polymorphisms before Starting Therapy with Androgen Receptor Inhibitors: A Study of a Patient Who Developed Toxic Myocarditis after Enzalutamide Treatment. Biomedicines 2022, 10, 1271. https://doi.org/10.3390/biomedicines10061271
Morales M, Martín-Vasallo P, Ávila J. Genetic Profiling of Glucocorticoid (NR3C1) and Mineralocorticoid (NR3C2) Receptor Polymorphisms before Starting Therapy with Androgen Receptor Inhibitors: A Study of a Patient Who Developed Toxic Myocarditis after Enzalutamide Treatment. Biomedicines. 2022; 10(6):1271. https://doi.org/10.3390/biomedicines10061271
Chicago/Turabian StyleMorales, Manuel, Pablo Martín-Vasallo, and Julio Ávila. 2022. "Genetic Profiling of Glucocorticoid (NR3C1) and Mineralocorticoid (NR3C2) Receptor Polymorphisms before Starting Therapy with Androgen Receptor Inhibitors: A Study of a Patient Who Developed Toxic Myocarditis after Enzalutamide Treatment" Biomedicines 10, no. 6: 1271. https://doi.org/10.3390/biomedicines10061271






