Development of Nomograms for Predicting Prognosis of Pancreatic Cancer after Pancreatectomy: A Multicenter Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Clinical Variables for Analysis
2.3. Statistical Analysis
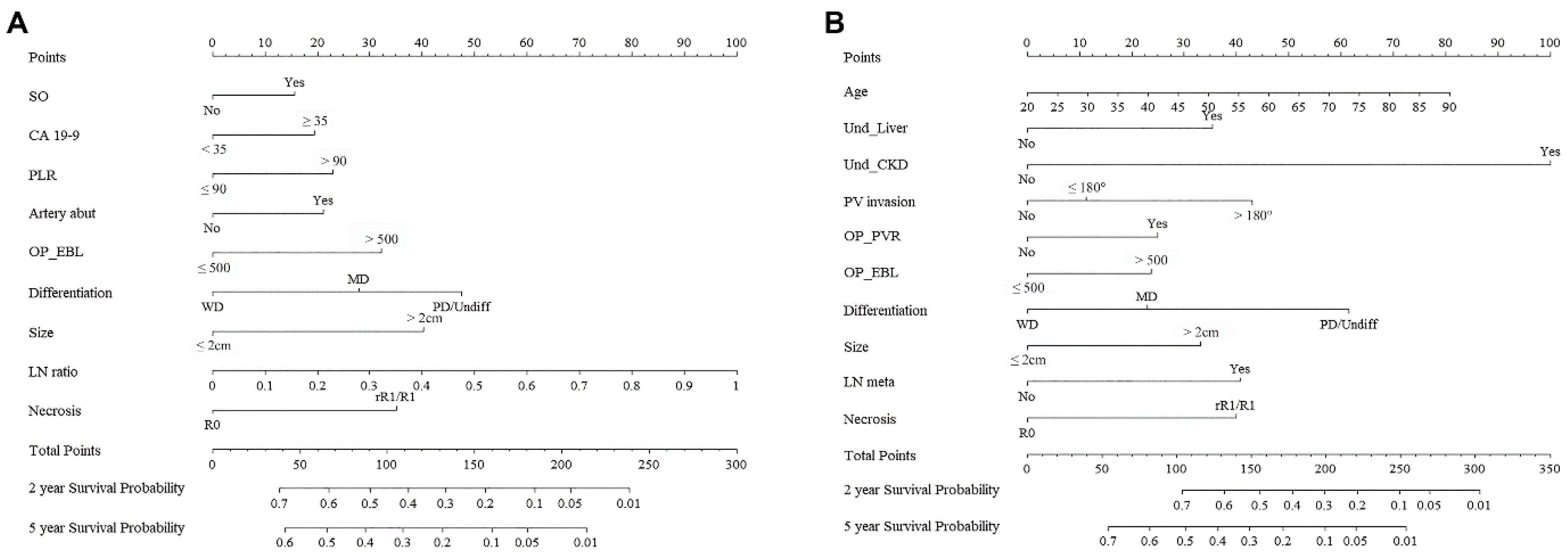
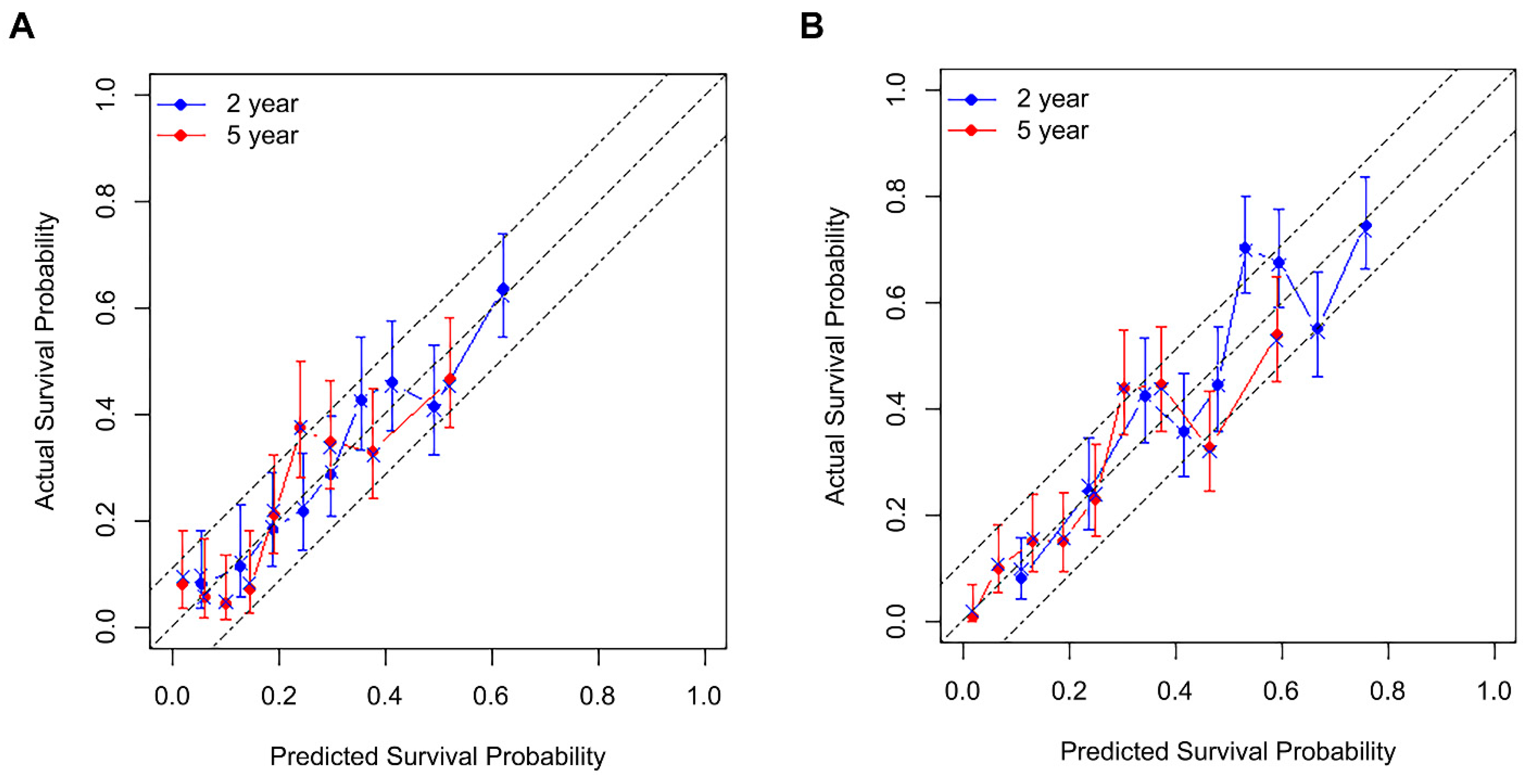
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PDAC | pancreatic ductal adenocarcinoma |
| AJCC | the American Joint Committee on Cancer |
| CA 19–9 | carbohydrate antigen 19–9 |
| NLR | neutrophil-to-lymphocyte |
| EBL | estimated blood loss |
| LNR | lymph node ratio |
| PLR | platelet-to-lymphocyte ratio |
| CT | computed tomography |
| SMI | skeletal muscle index |
| SO | Sarcopenic obesity |
| VFA | visceral fat area |
| CHA | common hepatic artery |
| SMA | superior mesenteric artery |
| PV | portal vein |
| SMV | superior mesenteric vein |
| LN | lymph node |
| PET | positron emission tomography |
| RFS | recurrence-free survival |
| OS | overall survival |
| SD | standard deviation |
| HR | hazard ratio |
| CI | confidence interval |
| ROC | curve, receiver operating characteristic curve |
| AUC | area under the curve |
| SE | standard error |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.J.; Shin, S.H.; Yoon, S.K.; Jung, J.H.; You, Y.; Han, I.W.; Choi, D.W.; Heo, J.S. Appraisal of 5-year recurrence-free survival after surgery in pancreatic ductal adenocarcinoma. J. Hepatobiliary Pancreat. Sci. 2021, 28, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Edge, S.; Greene, F.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. AJCC Cancer Staging Manual, 8th ed.; Springer: New York, NY, USA, 2017. [Google Scholar]
- Allen, P.J.; Kuk, D.; Castillo, C.F.; Basturk, O.; Wolfgang, C.L.; Cameron, J.L.; Lillemoe, K.D.; Ferrone, C.R.; Morales-Oyarvide, V.; He, J.; et al. Multi-institutional Validation Study of the American Joint Commission on Cancer (8th Edition) Changes for T and N Staging in Patients with Pancreatic Adenocarcinoma. Ann. Surg. 2017, 265, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Van Roessel, S.; Kasumova, G.G.; Verheij, J.; Najarian, R.M.; Maggino, L.; de Pastena, M.; Malleo, G.; Marchegiani, G.; Salvia, R.; Ng, S.C.; et al. International Validation of the Eighth Edition of the American Joint Committee on Cancer (AJCC) TNM Staging System in Patients with Resected Pancreatic Cancer. JAMA Surg. 2018, 153, e183617. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.J.; Hu, Z.G.; Shi, W.X.; Deng, T.; He, S.Q.; Yuan, S.G. Prognostic significance of neutrophil to lymphocyte ratio in pancreatic cancer: A meta-analysis. World J. Gastroenterol. 2015, 21, 2807–2815. [Google Scholar] [CrossRef] [PubMed]
- Hoshimoto, S.; Hishinuma, S.; Shirakawa, H.; Tomikawa, M.; Ozawa, I.; Ogata, Y. Validation and clinical usefulness of pre- and postoperative systemic inflammatory parameters as prognostic markers in patients with potentially resectable pancreatic cancer. Pancreatology 2020, 20, 239–246. [Google Scholar] [CrossRef]
- Ryu, Y.; Shin, S.H.; Kim, J.H.; Jeong, W.K.; Park, D.J.; Kim, N.; Heo, J.S.; Choi, D.W.; Han, I.W. The effects of sarcopenia and sarcopenic obesity after pancreaticoduodenectomy in patients with pancreatic head cancer. HPB 2020, 22, 1782–1792. [Google Scholar] [CrossRef]
- Tamagawa, H.; Aoyama, T.; Yamamoto, N.; Kamiya, M.; Murakawa, M.; Atsumi, Y.; Numata, M.; Kazama, K.; Hara, K.; Yukawa, N.; et al. The Impact of Intraoperative Blood Loss on the Survival of Patients with Stage II/III Pancreatic Cancer. In Vivo 2020, 34, 1469–1474. [Google Scholar] [CrossRef]
- Aoyama, T.; Yamamoto, N.; Kamiya, M.; Murakawa, M.; Tamagawa, H.; Sawazaki, S.; Numata, M.; Shiozawa, M.; Kobayashi, S.; Ueno, M.; et al. The Lymph Node Ratio Is an Independent Prognostic Factor in Pancreatic Cancer Patients Who Receive Curative Resection Followed by Adjuvant Chemotherapy. Anticancer Res. 2018, 38, 4877–4882. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Ikoma, H.; Morimura, R.; Konishi, H.; Murayama, Y.; Komatsu, S.; Shiozaki, A.; Kuriu, Y.; Kubota, T.; Nakanishi, M.; et al. The clinical impact of the lymph node ratio as a prognostic factor after resection of pancreatic cancer. Anticancer Res. 2014, 34, 2389–2394. [Google Scholar]
- Brennan, M.F.; Kattan, M.W.; Klimstra, D.; Conlon, K. Prognostic nomogram for patients undergoing resection for adenocarcinoma of the pancreas. Ann. Surg. 2004, 240, 293–298. [Google Scholar] [CrossRef]
- Song, W.; Miao, D.L.; Chen, L. Nomogram for predicting survival in patients with pancreatic cancer. Onco. Targets Ther. 2018, 11, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.H.; Kim, K.M.; Kim, J.H.; Moon, J.H.; Choi, S.H.; Lim, S.; Lim, J.Y.; Kim, K.W.; Park, K.S.; Jang, H.C. Predictive Values of the New Sarcopenia Index by the Foundation for the National Institutes of Health Sarcopenia Project for Mortality among Older Korean Adults. PLoS ONE 2016, 11, e0166344. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.M.; Wells, J.C.; Smith, S.R.; Stephan, B.C.; Siervo, M. Sarcopenic obesity: A Critical appraisal of the current evidence. Clin. Nutr. 2012, 31, 583–601. [Google Scholar] [CrossRef] [PubMed]
- Storz, P.; Crawford, H.C. Carcinogenesis of Pancreatic Ductal Adenocarcinoma. Gastroenterology 2020, 158, 2072–2081. [Google Scholar] [CrossRef] [PubMed]
- Shibutani, M.; Maeda, K.; Nagahara, H.; Ohtani, H.; Iseki, Y.; Ikeya, T.; Sugano, K.; Hirakawa, K. The prognostic significance of a postoperative systemic inflammatory response in patients with colorectal cancer. World J. Surg. Oncol. 2015, 13, 194. [Google Scholar] [CrossRef][Green Version]
- Da Silva Alexandre, T.; de Oliveira Duarte, Y.A.; Ferreira Santos, J.L.; Wong, R.; Lebrão, M.L. Sarcopenia according to the european working group on sarcopenia in older people (EWGSOP) versus Dynapenia as a risk factor for disability in the elderly. J. Nutr. Health Aging 2014, 18, 547–553. [Google Scholar] [CrossRef]
- Vetrano, D.L.; Landi, F.; Volpato, S.; Corsonello, A.; Meloni, E.; Bernabei, R.; Onder, G. Association of sarcopenia with short- and long-term mortality in older adults admitted to acute care wards: Results from the CRIME study. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 1154–1161. [Google Scholar] [CrossRef]
- Elliott, J.A.; Doyle, S.L.; Murphy, C.F.; King, S.; Guinan, E.M.; Beddy, P.; Ravi, N.; Reynolds, J.V. Sarcopenia: Prevalence, and Impact on Operative and Oncologic Outcomes in the Multimodal Management of Locally Advanced Esophageal Cancer. Ann. Surg. 2017, 266, 822–830. [Google Scholar] [CrossRef]
- Li, S.; Li, H.; Hu, Y.; Zhu, S.; Xu, Z.; Zhang, Q.; Yang, Y.; Wang, Z.; Xu, J. Ultrasound for Measuring the Cross-Sectional Area of Biceps Brachii Muscle in Sarcopenia. Int. J. Med. Sci. 2020, 17, 2947–2953. [Google Scholar] [CrossRef]
- Schwarz, R.E.; Smith, D.D. Extent of lymph node retrieval and pancreatic cancer survival: Information from a large US population database. Ann. Surg. Oncol. 2006, 13, 1189–1200. [Google Scholar] [CrossRef] [PubMed]
- Tol, J.A.; Gouma, D.J.; Bassi, C.; Dervenis, C.; Montorsi, M.; Adham, M.; Andrén-Sandberg, A.; Asbun, H.J.; Bockhorn, M.; Büchler, M.W.; et al. Definition of a standard lymphadenectomy in surgery for pancreatic ductal adenocarcinoma: A consensus statement by the International Study Group on Pancreatic Surgery (ISGPS). Surgery 2014, 156, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Pedrazzoli, S.; DiCarlo, V.; Dionigi, R.; Mosca, F.; Pederzoli, P.; Pasquali, C.; Klöppel, G.; Dhaene, K.; Michelassi, F. Standard versus extended lymphadenectomy associated with pancreatoduodenectomy in the surgical treatment of adenocarcinoma of the head of the pancreas: A multicenter, prospective, randomized study. Lymphadenectomy Study Group. Ann. Surg. 1998, 228, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Worni, M.; Castleberry, A.W.; Clary, B.M.; Gloor, B.; Carvalho, E.; Jacobs, D.O.; Pietrobon, R.; Scarborough, J.E.; White, R.R. Concomitant vascular reconstruction during pancreatectomy for malignant disease: A propensity score-adjusted, population-based trend analysis involving 10,206 patients. JAMA Surg. 2013, 148, 331–338. [Google Scholar] [CrossRef]
- Roch, A.M.; House, M.G.; Cioffi, J.; Ceppa, E.P.; Zyromski, N.J.; Nakeeb, A.; Schmidt, C.M. Significance of Portal Vein Invasion and Extent of Invasion in Patients Undergoing Pancreatoduodenectomy for Pancreatic Adenocarcinoma. J. Gastrointest. Surg. 2016, 20, 479–487. [Google Scholar] [CrossRef]
- Xie, Z.B.; Li, J.; Gu, J.C.; Jin, C.; Zou, C.F.; Fu, D.L. Pancreatoduodenectomy with portal vein resection favors the survival time of patients with pancreatic ductal adenocarcinoma: A propensity score matching analysis. Oncol. Lett. 2019, 18, 4563–4572. [Google Scholar] [CrossRef]
- Ansari, D.; Nilsson, J.; Andersson, R.; Regnér, S.; Tingstedt, B.; Andersson, B. Artificial neural networks predict survival from pancreatic cancer after radical surgery. Am. J. Surg. 2013, 205, 1–7. [Google Scholar] [CrossRef]
- Alabi, R.O.; Mäkitie, A.A.; Pirinen, M.; Elmusrati, M.; Leivo, I.; Almangush, A. Comparison of nomogram with machine learning techniques for prediction of overall survival in patients with tongue cancer. Int. J. Med. Inform. 2021, 145, 104313. [Google Scholar] [CrossRef]
- Chen, S.; Jiang, L.; Zhang, E.; Hu, S.; Wang, T.; Gao, F.; Zhang, N.; Wang, X.; Zheng, J. A Novel Nomogram Based on Machine Learning-Pathomics Signature and Neutrophil to Lymphocyte Ratio for Survival Prediction of Bladder Cancer Patients. Front. Oncol. 2021, 11, 703033. [Google Scholar] [CrossRef]

| Variable | N (%) or Mean (±SD) | Variable | N (%) or Mean (±SD) |
|---|---|---|---|
| Age at operation | 63.1 (±10.3) | Operation | |
| Sex, male | 520 (58.8%) | Pancreatoduodenectomy | 585 (66.1%) |
| BMI | 22.9 (±3.1) | Left-sided pancreatectomy | 295 (33.3%) |
| ASA score | Total pancreatectomy | 5 (0.6%) | |
| I | 176 (19.9%) | Combined PV/SMV resection | 144 (16.3%) |
| II | 632 (71.4%) | EBL > 500 mL | 301 (34.0%) |
| III | 77 (8.7%) | Intraop. RBC transfusion | 149 (16.8%) |
| Underlying disease | |||
| Cardiovascular | 371 (41.9%) | Tumor differentiation | |
| Respiratory | 66 (7.5%) | Well | 85 (9.6%) |
| Liver | 41 (4.6%) | Moderate | 521 (58.9%) |
| CKD | 6 (0.7%) | Poor/Undifferentiated | 279 (31.5%) |
| DM | 347 (39.2%) | Tumor size, cm | 3.14 (±1.3) |
| Neoadjuvant treatment | 33 (3.7%) | LN metastasis | 562 (63.5%) |
| Sarcopenia, yes | 99 (11.2%) | LN ratio | 0.1 (±0.15) |
| Sarcopenic obesity, yes | 285 (32.2%) | Tumor necrosis, yes | 149 (16.8%) |
| Preop. elevated CA 19–9 a | 617 (69.7%) | R0 resection | 671 (75.8%) |
| Preop. serum albumin | 4.0 (±0.5) | ||
| NLR > 2 | 451 (51.0%) | Length of stay, days | 15.1 (±11.9) |
| PLR > 90 | 739 (83.5%) | Major complication, yes d | 158 (17.9%) |
| Preop. CHA/SMA abutment b | 166 (18.8%) | Adjuvant chemotherapy | 508 (57.4%) |
| Preop. PV/SMV abutment b | 181 (20.5%) | ||
| Preop. PV/SMV invasion c | 79 (8.9%) |
| Variable | Univariable Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Age at operation | 0.96 | 0.89–1.03 | 0.261 | |||
| BMI | 0.99 | 0.97–1.02 | 0.560 | |||
| ASA score (ref. I) | ||||||
| II | 1.05 | 0.86–1.28 | 0.634 | |||
| III | 0.89 | 0.63–1.24 | 0.479 | |||
| Underlying liver disease | 1.28 | 0.89–1.83 | 0.186 | |||
| Underlying CKD | 1.77 | 0.73–4.26 | 0.205 | |||
| Underlying DM | 1.05 | 0.89–1.23 | 0.563 | |||
| Sarcopenia | 1.03 | 0.80–1.32 | 0.821 | |||
| Sarcopenic obesity | 1.22 | 1.03–1.43 | 0.020 | 1.22 | 1.03–1.44 | 0.020 |
| Preop. Albumin | 0.85 | 0.71–1.01 | 0.056 | |||
| Preop. elevated CA 19–9 a | 1.46 | 1.23–1.74 | <0.001 | 1.28 | 1.07–1.53 | 0.006 |
| NLR > 2 | 1.19 | 1.01–1.39 | 0.033 | |||
| PLR > 90 | 1.43 | 1.14–1.78 | 0.002 | 1.34 | 1.07–1.68 | 0.010 |
| Preop. CHA/SMA abutment b | 1.30 | 1.07–1.58 | 0.007 | 1.31 | 1.08–1.59 | 0.007 |
| Preop. PV/SMV abutment b | 1.07 | 0.88–1.31 | 0.475 | |||
| Preop. PV/SMV invasion c | 1.44 | 1.10–1.89 | 0.009 | |||
| Operation type (ref. PD) | ||||||
| DP | 0.88 | 0.74–1.04 | 0.132 | |||
| TP | 1.83 | 0.76–4.42 | 0.181 | |||
| PV/SMV resection | 1.22 | 0.99–1.51 | 0.062 | |||
| EBL > 500 mL | 1.55 | 1.31–1.82 | <0.001 | 1.51 | 1.28–1.78 | <0.001 |
| RBC transfusion | 1.38 | 1.13–1.70 | 0.002 | |||
| Major complication (CD ≥ 3) | 1.05 | 0.85–1.29 | 0.669 | |||
| Differentiation (ref. Well) | ||||||
| Moderate | 1.38 | 1.04–1.83 | 0.027 | 1.43 | 1.07–1.90 | 0.015 |
| Poor/Undifferentiated | 1.86 | 1.38–2.50 | <0.001 | 1.83 | 1.35–2.49 | <0.001 |
| Tumor size > 2 cm | 1.91 | 1.53–2.39 | <0.001 | 1.67 | 1.33–2.09 | <0.001 |
| LN metastasis | 1.53 | 1.30–1.81 | <0.001 | |||
| LN ratio | 1.16 | 1.10–1.22 | <0.001 | 1.14 | 1.08–1.20 | <0.001 |
| Tumor necrosis | 1.76 | 1.44–2.16 | <0.001 | 1.56 | 1.27–1.93 | <0.001 |
| R1 resection (including rR1) | 1.14 | 0.95–1.37 | 0.165 | |||
| Variable | Univariable Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Age at operation | 1.07 | 0.99–1.15 | 0.088 | 1.16 | 1.07–1.25 | <0.001 |
| BMI | 0.97 | 0.95–1.00 | 0.031 | |||
| ASA score (ref. I) | ||||||
| II | 1.19 | 0.98–1.44 | 0.076 | |||
| III | 1.00 | 0.73–1.37 | 0.992 | |||
| Underlying liver disease | 1.40 | 0.99–1.96 | 0.055 | 1.58 | 1.12–2.22 | 0.009 |
| Underlying CKD | 2.25 | 1.01–5.03 | 0.049 | 3.62 | 1.58–8.28 | 0.002 |
| Underlying DM | 1.17 | 1.01–1.37 | 0.040 | |||
| Sarcopenia | 1.07 | 0.84–1.35 | 0.605 | |||
| Sarcopenic obesity | 1.06 | 0.90–1.24 | 0.499 | |||
| Preop. Albumin | 0.92 | 0.78–1.08 | 0.300 | |||
| Preop. elevated CA 19–9 a | 1.27 | 1.08–1.51 | 0.005 | |||
| NLR > 2 | 1.09 | 0.94–1.27 | 0.244 | |||
| PLR > 90 | 1.18 | 0.96–1.45 | 0.113 | |||
| Preop. CHA/SMA abutment b | 1.20 | 1.00–1.45 | 0.052 | |||
| Preop. PV/SMV abutment b | 1.25 | 1.04–1.51 | 0.016 | 1.16 | 0.95–1.41 | 0.152 |
| Preop. PV/SMV invasion c | 1.99 | 1.56–2.56 | < 0.001 | 1.74 | 1.32–2.29 | <0.001 |
| Operation type (ref. PD) | ||||||
| DP | 0.87 | 0.74–1.02 | 0.092 | |||
| TP | 0.80 | 0.30–2.13 | 0.649 | |||
| PV/SMV resection | 1.65 | 1.36–2.00 | <0.001 | 1.38 | 1.10–1.72 | 0.005 |
| EBL > 500 cc | 1.38 | 1.18–1.61 | <0.001 | 1.36 | 1.16–1.59 | <0.001 |
| RBC transfusion | 1.09 | 0.89–1.33 | 0.421 | |||
| Major complication (CD ≥ 3) | 1.06 | 0.88–1.29 | 0.536 | |||
| Differentiation (ref. Well) | ||||||
| Moderate | 1.42 | 1.08–1.88 | 0.013 | 1.34 | 1.01–1.78 | 0.040 |
| Poor/Undifferentiated | 2.34 | 1.75–3.12 | <0.001 | 2.20 | 1.64–2.96 | <0.001 |
| Tumor size > 2 cm | 1.73 | 1.40–2.13 | <0.001 | 1.53 | 1.24–1.89 | <0.001 |
| LN metastasis | 1.73 | 1.47–2.04 | <0.001 | 1.69 | 1.42–1.99 | <0.001 |
| LN ratio | 1.10 | 1.05–1.15 | <0.001 | |||
| Tumor necrosis | 1.90 | 1.57–2.30 | <0.001 | 1.67 | 1.37–2.04 | <0.001 |
| R1 resection (including rR1) | 1.20 | 1.01–1.43 | 0.038 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, S.J.; Park, B.; Kwon, J.; Lim, C.-S.; Shin, Y.C.; Jung, W.; Shin, S.H.; Heo, J.S.; Han, I.W. Development of Nomograms for Predicting Prognosis of Pancreatic Cancer after Pancreatectomy: A Multicenter Study. Biomedicines 2022, 10, 1341. https://doi.org/10.3390/biomedicines10061341
Yoon SJ, Park B, Kwon J, Lim C-S, Shin YC, Jung W, Shin SH, Heo JS, Han IW. Development of Nomograms for Predicting Prognosis of Pancreatic Cancer after Pancreatectomy: A Multicenter Study. Biomedicines. 2022; 10(6):1341. https://doi.org/10.3390/biomedicines10061341
Chicago/Turabian StyleYoon, So Jeong, Boram Park, Jaewoo Kwon, Chang-Sup Lim, Yong Chan Shin, Woohyun Jung, Sang Hyun Shin, Jin Seok Heo, and In Woong Han. 2022. "Development of Nomograms for Predicting Prognosis of Pancreatic Cancer after Pancreatectomy: A Multicenter Study" Biomedicines 10, no. 6: 1341. https://doi.org/10.3390/biomedicines10061341
APA StyleYoon, S. J., Park, B., Kwon, J., Lim, C.-S., Shin, Y. C., Jung, W., Shin, S. H., Heo, J. S., & Han, I. W. (2022). Development of Nomograms for Predicting Prognosis of Pancreatic Cancer after Pancreatectomy: A Multicenter Study. Biomedicines, 10(6), 1341. https://doi.org/10.3390/biomedicines10061341







