Increased Abundances of CD16+ Non-Classical Monocytes Accompany with Elevated Monocytic PD-L1 and CD4+ T Cell Disturbances in Oropharyngeal Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Blood Collection and Patient Data
2.3. Profiling of Monocyte Subsets in Whole Blood
2.4. Profiling of T Cell Subsets in Isolated PBMCs
2.5. Flow Cytometry Analysis
2.6. Statistical Analysis
3. Results
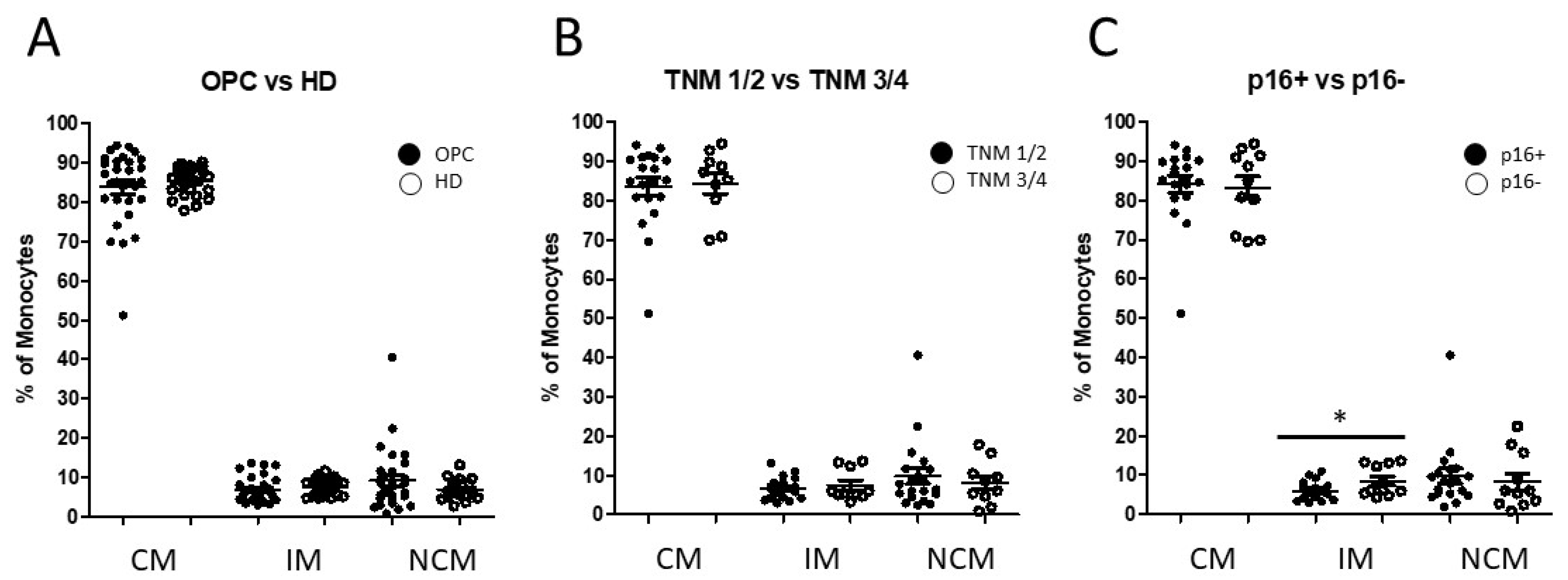
3.1. Distribution of Circulating Monocyte Subsets in Oropharyngeal Cancer Patients
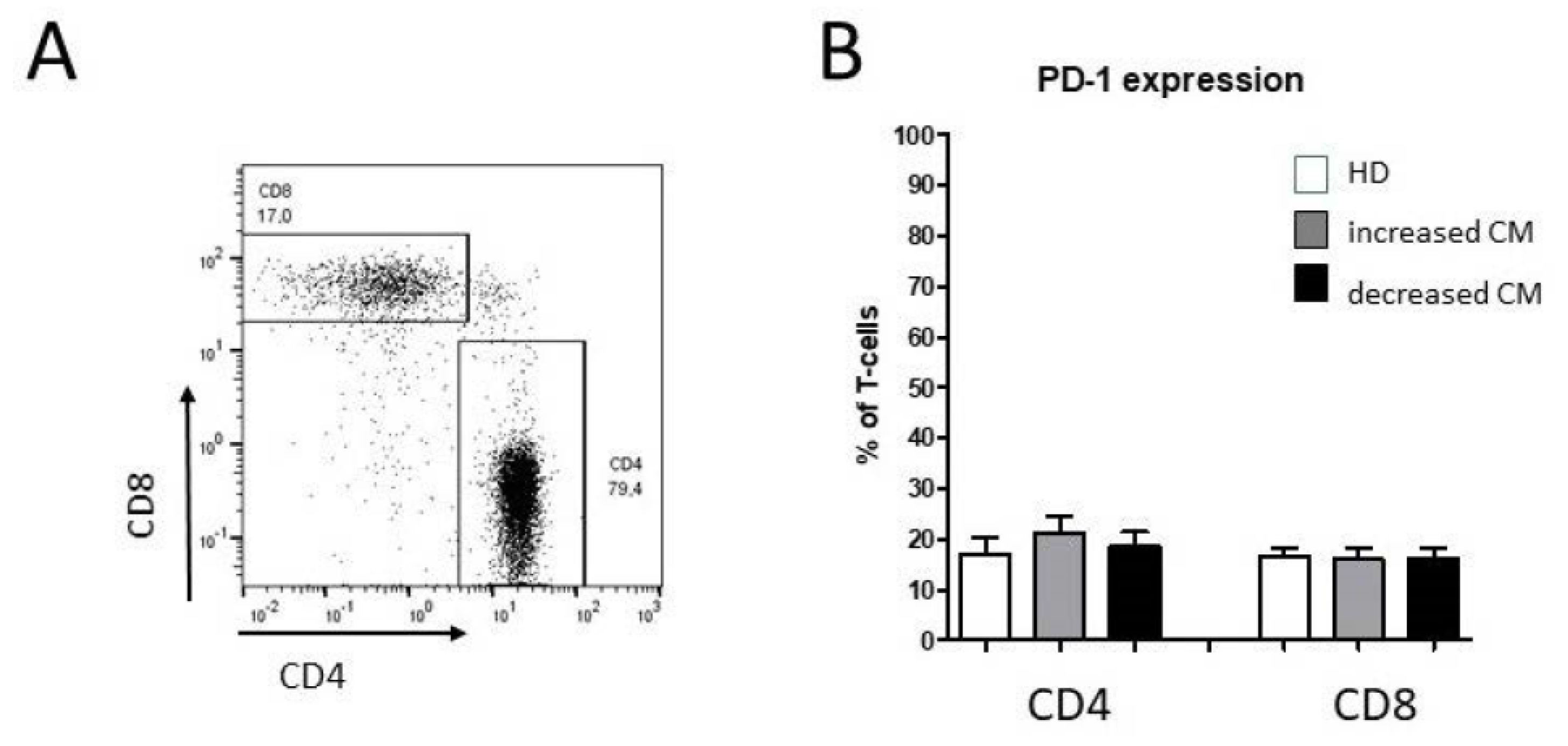
3.2. Alteration of Circulating CD4+ T Cell Subsets in Oropharyngeal Cancer Patients
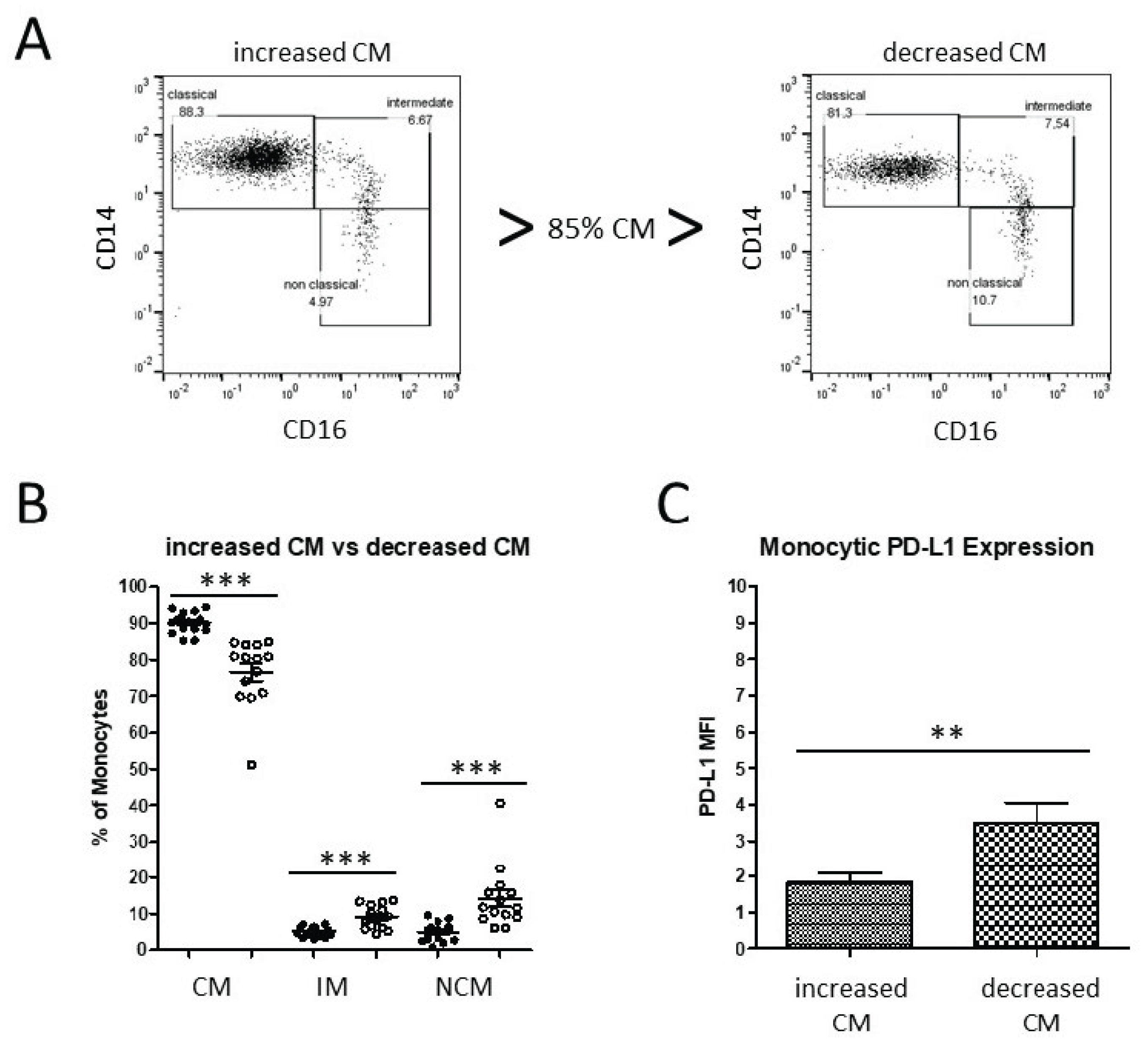
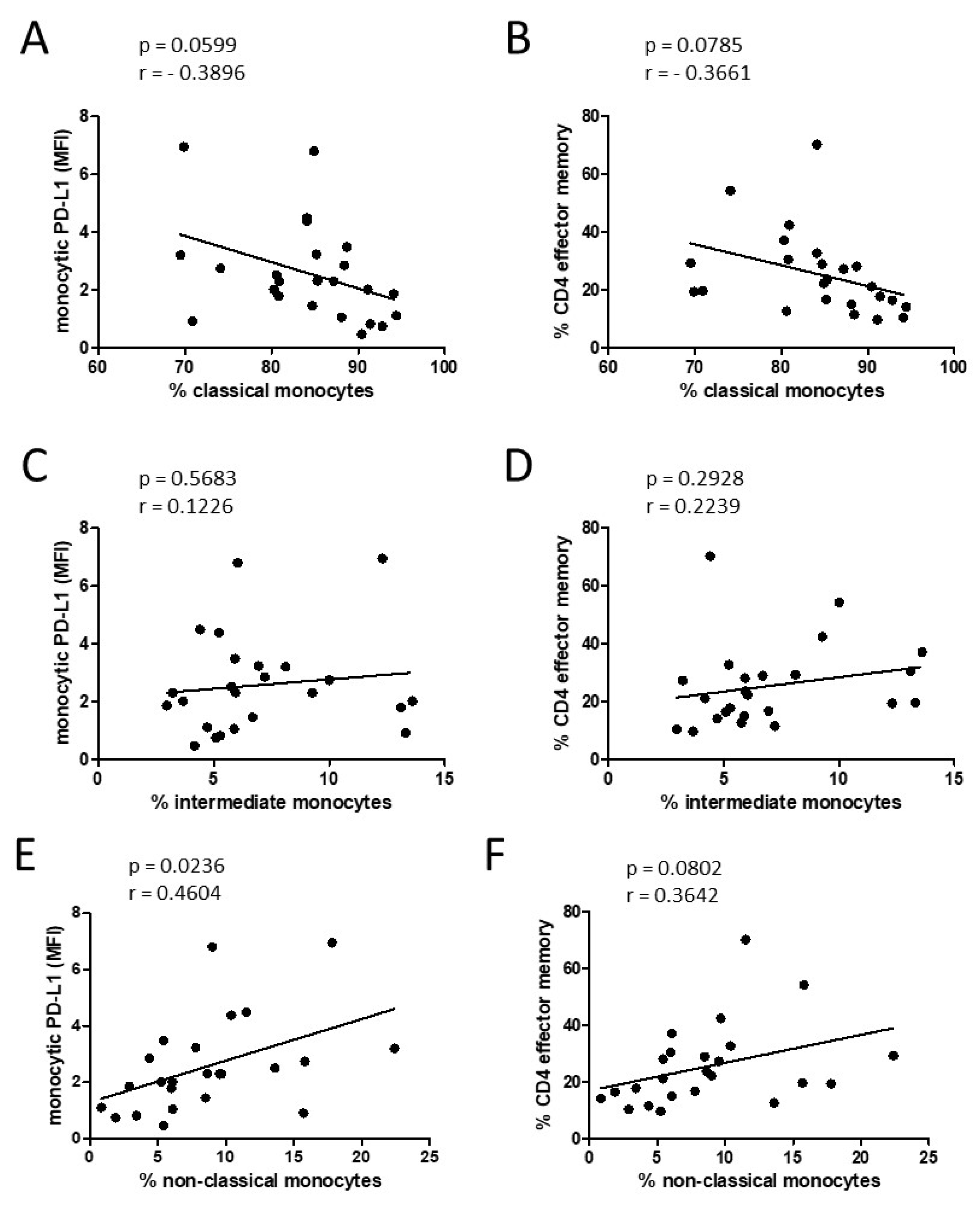
3.3. Alteration of Monocyte Subsets and Accompanying Consequences
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, P.; Li, S.; Zhang, T.; Cui, F.; Shi, J.H.; Zhao, F.; Sheng, X. Characterization of Molecular Subtypes in Head and Neck Squamous Cell Carcinoma with Distinct Prognosis and Treatment Responsiveness. Front. Cell Dev. Biol. 2021, 9, 711348. [Google Scholar] [CrossRef] [PubMed]
- Alsahafi, E.; Begg, K.; Amelio, I.; Raulf, N.; Lucarelli, P.; Sauter, T.; Tavassoli, M. Clinical update on head and neck cancer: Molecular biology and ongoing challenges. Cell Death Dis. 2019, 10, 540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canning, M.; Guo, G.; Yu, M.; Myint, C.; Groves, M.W.; Byrd, J.K.; Cui, Y. Heterogeneity of the Head and Neck Squamous Cell Carcinoma Immune Landscape and Its Impact on Immunotherapy. Front. Cell Dev. Biol. 2019, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Leemans, C.R.; Snijders, P.J.F.; Brakenhoff, R.H. The molecular landscape of head and neck cancer. Nat. Reviews. Cancer 2018, 18, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Gregoire, V.; Lefebvre, J.L.; Licitra, L.; Felip, E.; On behalf of the EHNS–ESMO–ESTRO Guidelines Working Group. Squamous cell carcinoma of the head and neck: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2010, 21, v184–v186. [Google Scholar] [CrossRef]
- Puram, S.V.; Tirosh, I.; Parikh, A.S.; Patel, A.P.; Yizhak, K.; Gillespie, S.; Rodman, C.; Luo, C.L.; Mroz, E.A.; Emerick, K.S.; et al. Single-Cell Transcriptomic Analysis of Primary and Metastatic Tumor Ecosystems in Head and Neck Cancer. Cell 2017, 171, 1611–1624. [Google Scholar] [CrossRef] [Green Version]
- Cavalieri, S.; Serafini, M.S.; Carenzo, A.; Canevari, S.; Brakenhoff, R.H.; Leemans, C.R.; Nauta, I.H.; Hoebers, F.; van den Hout, M.; Scheckenbach, K.; et al. Clinical Validity of a Prognostic Gene Expression Cluster-Based Model in Human Papillomavirus-Positive Oropharyngeal Carcinoma. JCO Precis. Oncol. 2021, 5, 1666–1676. [Google Scholar]
- Golusinski, P.; Corry, J.; Poorten, V.V.; Simo, R.; Sjogren, E.; Makitie, A.; Kowalski, L.P.; Langendijk, J.; Braakhuis, B.J.M.; Takes, R.P.; et al. De-escalation studies in HPV-positive oropharyngeal cancer: How should we proceed? Oral Oncol. 2021, 123, 105620. [Google Scholar] [CrossRef]
- Gillison, M.L.; Lowy, D.R. A causal role for human papillomavirus in head and neck cancer. Lancet 2004, 363, 1488–1489. [Google Scholar] [CrossRef]
- Gillison, M.L. Human papillomavirus-associated head and neck cancer is a distinct epidemiologic, clinical, and molecular entity. Semin. Oncol. 2004, 31, 744–754. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, M.; Liu, Y.; Wang, X.; Li, Y.; Hu, X.; Qiu, Y.; Liang, W.; Wei, Y.; Zhong, Y. HPV Positive Status Is a Favorable Prognostic Factor in Non-Nasopharyngeal Head and Neck Squamous Cell Carcinoma Patients: A Retrospective Study From the Surveillance, Epidemiology, and End Results Database. Front. Oncol. 2021, 11, 688615. [Google Scholar] [CrossRef] [PubMed]
- Syrjanen, S. The role of human papillomavirus infection in head and neck cancers. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2010, 21, vii243–vii245. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.J.; Thirdborough, S.M.; Mellows, T.; Riley, C.; Harris, S.; Suchak, K.; Webb, A.; Hampton, C.; Patel, N.N.; Randall, C.J.; et al. Tumour-infiltrating lymphocytes predict for outcome in HPV-positive oropharyngeal cancer. Br. J. Cancer. 2014, 110, 489–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Partlova, S.; Boucek, J.; Kloudova, K.; Lukesova, E.; Zabrodsky, M.; Grega, M.; Fucikova, J.; Truxova, I.; Tachezy, R.; Spisek, R.; et al. Distinct patterns of intratumoral immune cell infiltrates in patients with HPV-associated compared to non-virally induced head and neck squamous cell carcinoma. Oncoimmunology 2015, 4, e965570. [Google Scholar] [CrossRef] [PubMed]
- Jung, A.C.; Guihard, S.; Krugell, S.; Ledrappier, S.; Brochot, A.; Dalstein, V.; Job, S.; de Reynies, A.; Noel, G.; Wasylyk, B.; et al. CD8-alpha T-cell infiltration in human papillomavirus-related oropharyngeal carcinoma correlates with improved patient prognosis. Int. J. Cancer 2013, 132, E26–E36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.F.; Wang, S.S.; Tang, Y.J.; Chen, Y.; Zheng, M.; Tang, Y.L.; Liang, X.H. The Double-Edged Sword-How Human Papillomaviruses Interact with Immunity in Head and Neck Cancer. Front. Immunol. 2019, 10, 653. [Google Scholar] [CrossRef] [PubMed]
- Sakakura, K.; Takahashi, H.; Motegi, S.I.; Yokobori-Kuwabara, Y.; Oyama, T.; Chikamatsu, K. Immunological features of circulating monocyte subsets in patients with squamous cell carcinoma of the head and neck. Clin. Immunol. 2021, 225, 108677. [Google Scholar] [CrossRef]
- Takahashi, H.; Sakakura, K.; Tada, H.; Kaira, K.; Oyama, T.; Chikamatsu, K. Prognostic significance and population dynamics of peripheral monocytes in patients with oropharyngeal squamous cell carcinoma. Head Neck 2019, 41, 1880–1888. [Google Scholar] [CrossRef]
- Lang, S.; Lauffer, L.; Clausen, C.; Lohr, I.; Schmitt, B.; Holzel, D.; Wollenberg, B.; Gires, O.; Kastenbauer, E.; Zeidler, R. Impaired monocyte function in cancer patients: Restoration with a cyclooxygenase-2 inhibitor. FASEB J. 2003, 17, 286–288. [Google Scholar] [CrossRef]
- Aarstad, H.J.; Vintermyr, O.K.; Ulvestad, E.; Aarstad, H.H.; Kross, K.W.; Heimdal, J.H. Peripheral blood monocyte and T-lymphocyte activation levels at diagnosis predict long-term survival in head and neck squamous cell carcinoma patients. APMIS Acta Pathol. Microbiol. Immunol. Scand. 2015, 123, 305–314. [Google Scholar] [CrossRef]
- Wong, K.L.; Yeap, W.H.; Tai, J.J.; Ong, S.M.; Dang, T.M.; Wong, S.C. The three human monocyte subsets: Implications for health and disease. Immunol. Res. 2012, 53, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.A.; Zhang, Y.; Fullerton, J.N.; Boelen, L.; Rongvaux, A.; Maini, A.A.; Bigley, V.; Flavell, R.A.; Gilroy, D.W.; Asquith, B.; et al. The fate and lifespan of human monocyte subsets in steady state and systemic inflammation. J. Exp. Med. 2017, 214, 1913–1923. [Google Scholar] [CrossRef] [PubMed]
- Ziegler-Heitbrock, L. Blood Monocytes and Their Subsets: Established Features and Open Questions. Front. Immunol. 2015, 6, 423. [Google Scholar] [CrossRef] [PubMed]
- Boyette, L.B.; Macedo, C.; Hadi, K.; Elinoff, B.D.; Walters, J.T.; Ramaswami, B.; Chalasani, G.; Taboas, J.M.; Lakkis, F.G.; Metes, D.M. Phenotype, function, and differentiation potential of human monocyte subsets. PLoS ONE 2017, 12, e0176460. [Google Scholar]
- Cros, J.; Cagnard, N.; Woollard, K.; Patey, N.; Zhang, S.Y.; Senechal, B.; Puel, A.; Biswas, S.K.; Moshous, D.; Picard, C.; et al. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity 2010, 33, 375–386. [Google Scholar] [CrossRef] [Green Version]
- Ancuta, P.; Liu, K.Y.; Misra, V.; Wacleche, V.S.; Gosselin, A.; Zhou, X.; Gabuzda, D. Transcriptional profiling reveals developmental relationship and distinct biological functions of CD16+ and CD16− monocyte subsets. BMC Genom. 2009, 10, 403. [Google Scholar] [CrossRef] [Green Version]
- Jakubzick, C.V.; Randolph, G.J.; Henson, P.M. Monocyte differentiation and antigen-presenting functions. Nat. Reviews. Immunol. 2017, 17, 349–362. [Google Scholar] [CrossRef]
- Coillard, A.; Segura, E. In vivo Differentiation of Human Monocytes. Front. Immunol. 2019, 10, 1907. [Google Scholar] [CrossRef]
- Kapellos, T.S.; Bonaguro, L.; Gemund, I.; Reusch, N.; Saglam, A.; Hinkley, E.R.; Schultze, J.L. Human Monocyte Subsets and Phenotypes in Major Chronic Inflammatory Diseases. Front. Immunol. 2019, 10, 2035. [Google Scholar] [CrossRef] [Green Version]
- Israr, M.; DeVoti, J.A.; Lam, F.; Abramson, A.L.; Steinberg, B.M.; Bonagura, V.R. Altered Monocyte and Langerhans Cell Innate Immunity in Patients with Recurrent Respiratory Papillomatosis (RRP). Front. Immunol. 2020, 11, 336. [Google Scholar] [CrossRef]
- Gissmann, L.; Wolnik, L.; Ikenberg, H.; Koldovsky, U.; Schnurch, H.G.; Hausen, H.z. Human papillomavirus types 6 and 11 DNA sequences in genital and laryngeal papillomas and in some cervical cancers. Proc. Natl. Acad. Sci. USA 1983, 80, 560–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polasky, C.; Steffen, A.; Loyal, K.; Lange, C.; Bruchhage, K.L.; Pries, R. Redistribution of Monocyte Subsets in Obstructive Sleep Apnea Syndrome Patients Leads to an Imbalanced PD-1/PD-L1 Cross-Talk with CD4/CD8 T Cells. J. Immunol. 2021, 206, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Levovitz, C.; Chen, D.; Ivansson, E.; Gyllensten, U.; Finnigan, J.P.; Alshawish, S.; Zhang, W.; Schadt, E.E.; Posner, M.R.; Genden, E.M.; et al. TGFbeta receptor 1: An immune susceptibility gene in HPV-associated cancer. Cancer Res. 2014, 74, 6833–6844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moniuszko, M.; Bodzenta-Lukaszyk, A.; Kowal, K.; Lenczewska, D.; Dabrowska, M. Enhanced frequencies of CD14++CD16+, but not CD14+CD16+, peripheral blood monocytes in severe asthmatic patients. Clin. Immunol. 2009, 130, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Ge, L.; Chen, D.; Zhang, M.; Zhao, L.; Liu, W.; Chen, S.; Wang, J.; Zhou, C.; Zhao, X.; et al. Increased Frequency of Circulating Classical Monocytes in Patients with Rosacea. Clin. Cosmet. Investig. Dermatol. 2021, 14, 1629–1636. [Google Scholar] [CrossRef]
- Korenfeld, D.; Roussak, K.; Dinkel, S.; Vogel, T.P.; Pollack, H.; Levy, J.; Leiding, J.W.; Milner, J.; Cooper, M.; Klechevsky, E. STAT3 Gain-of-Function Mutations Underlie Deficiency in Human Nonclassical CD16+ Monocytes and CD141+ Dendritic Cells. J. Immunol. 2021, 207, 2423–2432. [Google Scholar] [CrossRef]
- Freeman, G.J.; Long, A.J.; Iwai, Y.; Bourque, K.; Chernova, T.; Nishimura, H.; Fitz, L.J.; Malenkovich, N.; Okazaki, T.; Byrne, M.C.; et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000, 192, 1027–1034. [Google Scholar] [CrossRef] [Green Version]
- Joyce, J.A.; Fearon, D.T. T cell exclusion, immune privilege, and the tumor microenvironment. Science 2015, 348, 74–80. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Allison, J.P. The future of immune checkpoint therapy. Science 2015, 348, 56–61. [Google Scholar] [CrossRef]
- Kuang, D.M.; Peng, C.; Zhao, Q.; Wu, Y.; Chen, M.S.; Zheng, L. Activated monocytes in peritumoral stroma of hepatocellular carcinoma promote expansion of memory T helper 17 cells. Hepatology 2010, 51, 154–164. [Google Scholar] [CrossRef]
- Heeren, A.M.; Punt, S.; Bleeker, M.C.; Gaarenstroom, K.N.; van der Velden, J.; Kenter, G.G.; de Gruijl, T.D.; Jordanova, E.S. Prognostic effect of different PD-L1 expression patterns in squamous cell carcinoma and adenocarcinoma of the cervix, Modern pathology: An official journal of the United States and Canadian Academy of Pathology. Mod. Pathol. 2016, 29, 753–763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.R.; Ha, S.J.; Hong, M.H.; Heo, S.J.; Koh, Y.W.; Choi, E.C.; Kim, E.K.; Pyo, K.H.; Jung, I.; Seo, D.; et al. PD-L1 expression on immune cells, but not on tumor cells, is a favorable prognostic factor for head and neck cancer patients. Sci. Rep. 2016, 6, 36956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sallusto, F.; Geginat, J.; Lanzavecchia, A. Central memory and effector memory T cell subsets: Function, generation, and maintenance. Annu. Rev. Immunol. 2004, 22, 745–763. [Google Scholar] [CrossRef] [PubMed]
- Turksma, A.W.; Bontkes, H.J.; van den Heuvel, H.; de Gruijl, T.D.; von Blomberg, B.M.; Braakhuis, B.J.; Leemans, C.R.; Bloemena, E.; Meijer, C.J.; Hooijberg, E. Effector memory T-cell frequencies in relation to tumour stage, location and HPV status in HNSCC patients. Oral Dis. 2013, 19, 577–584. [Google Scholar] [CrossRef]
- Klussmann, J.P.; Weissenborn, S.J.; Wieland, U.; Dries, V.; Kolligs, J.; Jungehuelsing, M.; Eckel, H.E.; Dienes, H.P.; Pfister, H.J.; Fuchs, P.G. Prevalence, distribution, and viral load of human papillomavirus 16 DNA in tonsillar carcinomas. Cancer 2001, 92, 2875–2884. [Google Scholar] [CrossRef]
- Wansom, D.; Light, E.; Thomas, D.; Worden, F.; Prince, M.; Urba, S.; Chepeha, D.; Kumar, B.; Cordell, K.; Eisbruch, A.; et al. Program, Infiltrating lymphocytes and human papillomavirus-16—Associated oropharyngeal cancer. Laryngoscope 2012, 122, 121–127. [Google Scholar] [CrossRef] [Green Version]
- Rossol, M.; Kraus, S.; Pierer, M.; Baerwald, C.; Wagner, U. The CD14(bright) CD16+ monocyte subset is expanded in rheumatoid arthritis and promotes expansion of the Th17 cell population. Arthritis Rheum. 2012, 64, 671–677. [Google Scholar] [CrossRef]

| Characteristics | Patients (n = 32) | |
|---|---|---|
| n | % | |
| Sex | ||
| m | 26 | 81 |
| f | 6 | 19 |
| Age (years) | ||
| ≤ 60 | 9 | 28 |
| > 60 | 23 | 72 |
| Tumor Stage | ||
| T1–T2 | 21 | 66 |
| T3–T4 | 11 | 34 |
| Nodal Stage | ||
| N0 | 6 | 19 |
| N1 | 11 | 34 |
| N2 | 10 | 31 |
| N3 | 5 | 16 |
| Distant Metastasis | ||
| M0 | 32 | 100 |
| HPV status | ||
| Positive | 21 | 66 |
| Negative | 11 | 34 |
| UICC stage | ||
| I | 1 | 3 |
| II | 4 | 13 |
| III | 7 | 22 |
| Obsolete | 20 | 63 |
| Alcohol abuse | ||
| Yes | 6 | 19 |
| No | 26 | 81 |
| Tobacco consumption | ||
| Yes | 24 | 75 |
| No | 8 | 25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Idel, C.; Polasky, C.; Ribbat-Idel, J.; Loyal, K.; Perner, S.; Rades, D.; Bruchhage, K.-L.; Pries, R. Increased Abundances of CD16+ Non-Classical Monocytes Accompany with Elevated Monocytic PD-L1 and CD4+ T Cell Disturbances in Oropharyngeal Cancer. Biomedicines 2022, 10, 1363. https://doi.org/10.3390/biomedicines10061363
Idel C, Polasky C, Ribbat-Idel J, Loyal K, Perner S, Rades D, Bruchhage K-L, Pries R. Increased Abundances of CD16+ Non-Classical Monocytes Accompany with Elevated Monocytic PD-L1 and CD4+ T Cell Disturbances in Oropharyngeal Cancer. Biomedicines. 2022; 10(6):1363. https://doi.org/10.3390/biomedicines10061363
Chicago/Turabian StyleIdel, Christian, Christina Polasky, Julika Ribbat-Idel, Kristin Loyal, Sven Perner, Dirk Rades, Karl-Ludwig Bruchhage, and Ralph Pries. 2022. "Increased Abundances of CD16+ Non-Classical Monocytes Accompany with Elevated Monocytic PD-L1 and CD4+ T Cell Disturbances in Oropharyngeal Cancer" Biomedicines 10, no. 6: 1363. https://doi.org/10.3390/biomedicines10061363






