The Reduction in Microtubule Arrays Caused by the Dysplasia of the Non-Centrosomal Microtubule-Organizing Center Leads to a Malformed Organ of Corti in the Cx26-Null Mouse
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mouse Models
- Cx26 (F): 5′-ACAGAAATGTGTTGGTGATGG-3′,
- Cx26 (R): 5′-CTTTCCAATGCTGGTGGAGTG-3′,
- Rosa26Cre(F): 5’-AGCTAAACATGCTTCATCGTCGGTC-3’,
- Rosa26Cre(R): 5’-TATCCAGGTTACGGATATAGTTCATG-3’.
2.2. Transmission Electron Microscopy (TEM)
2.3. RNA Preparation and Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
- GAPDH (F): 5’-GAAGGTCGGTGTGAACGGAT-3’;
- GAPDH (R): 5’-CTCGCTCCTGGAAGATGGTG-3’;
- AKAP450 (F): 5’-AGCCCAATATGATGGGGACAT-3’;
- AKAP450 (R): 5’-CTGAGAACTTTCCGAGCAGAG-3’;
- Cdk5rap2 (F): 5’-CTCGGGGATGGAAGAGGAAG-3’;
- Cdk5rap2 (R): 5’-AAGCCAGAAGTGTCACTGATG-3’;
- GM130 (F): 5’-GGGCCTCACATCTTCCAACAT-3’;
- GM130 (R): 5’-GACACCAGGATGCCTATGGTC-3’;
- Camsap1 (F): 5’-CCTATGGCCTAGACAACATCCC-3’;
- Camsap1 (R): 5’-ATAACGGGTGGCTTAATGTGC-3’;
- Camsap2 (F): 5’-GGCCAAAATCGCCTGCAATC-3’;
- Camsap2 (R): 5’-GTCTGTGTAAAATGGGTCGCC-3’;
- Camsap3 (F): 5’-CCACTGCGGAGGACTTTCC-3’;
- Camsap3 (R): 5’-ACTGATCGGTGTAGAAGGGTT-3’;
- Clasp1 (F): 5’-CCTTGAGCACGACCAGACC-3’;
- Clasp1 (R): 5’-CGATTTGCGCCTTGAACCG-3’;
- Clasp2 (F): 5’-TGGATGGAAATAGGCCGTCG-3’;
- Clasp2 (R): 5’-CCTCCAACCTTAGGGCCAC-3’;
- Mtcl1 (F): 5’-AGCCTGAAAGTGGCGGAAAC-3’;
- Mtcl1 (R): 5’-CGAGTTCGTGGTGTAATCTGAC-3’.
2.4. Immunofluorescence Staining
2.5. Data Analysis
3. Results
3.1. Impaired Degradation of Organelles in PCs and Hypertrophy of PCs in Cx26-Null Mice
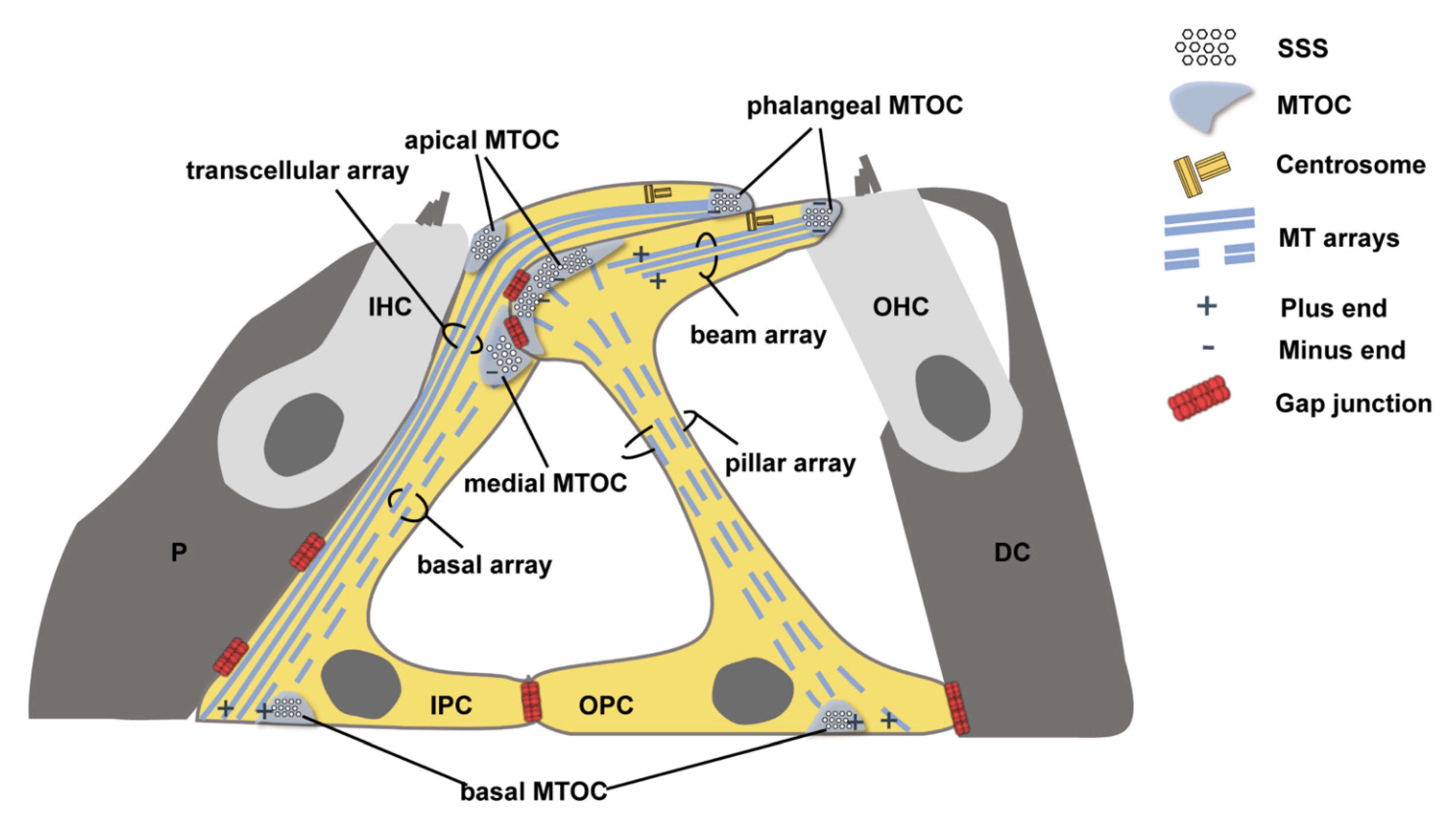
3.2. MT Arrays Anchored at the MTOCs Away from the Centrosome Rather Than MTOCs near the Centrosome Were Significantly Reduced in the Cx26-Null Group
3.3. Ultrastructural Changes of PCs’ Non-centrosomal MTOCs in Cx26-Null Mice
3.4. No Changes Were Found in the mRNA Expression Level of Non-Centrosomal MT Nucleation and Anchorage-Associated Proteins
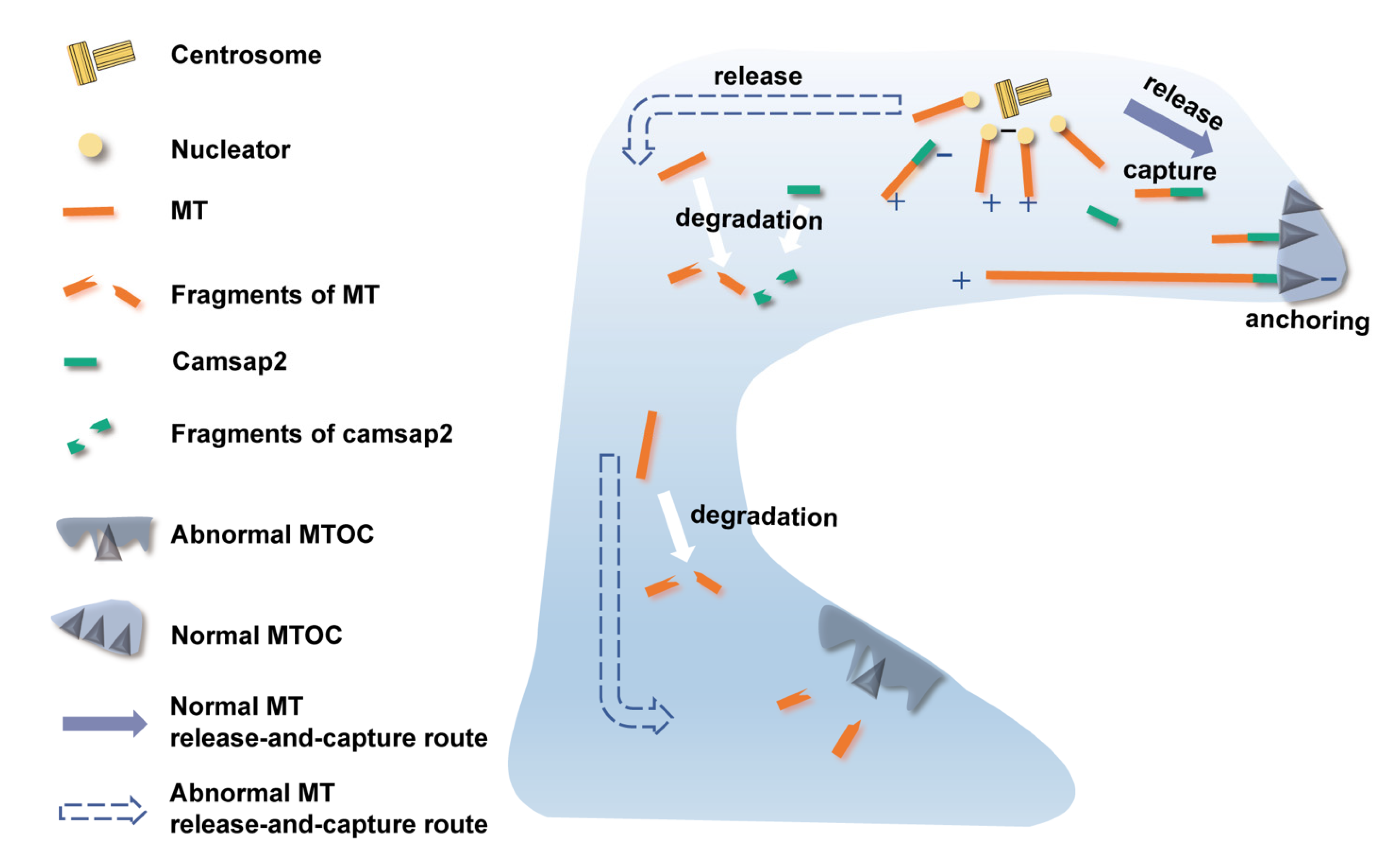
3.5. Reduced Protein Expression Level of Camsap2 in PCs in the Cx26-Null Group
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Chang, Q.; Tang, W.; Sun, Y.; Zhou, B.; Li, H.; Lin, X. Targeted connexin26 ablation arrests postnatal development of the organ of Corti. Biochem. Biophys. Res. Commun. 2009, 385, 33–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mammano, F. Inner Ear Connexin Channels: Roles in Development and Maintenance of Cochlear Function. Cold Spring Harb. Perspect. Med. 2019, 9, a033233. [Google Scholar] [CrossRef] [PubMed]
- Wingard, J.C.; Zhao, H.B. Cellular and Deafness Mechanisms Underlying Connexin Mutation-Induced Hearing Loss—A Common Hereditary Deafness. Front Cell Neurosci. 2015, 9, 202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inoshita, A.; Iizuka, T.; Okamura, H.O.; Minekawa, A.; Kojima, K.; Furukawa, M.; Kusunoki, T.; Ikeda, K. Postnatal development of the organ of Corti in dominant-negative Gjb2 transgenic mice. Neuroscience 2008, 156, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xie, L.; Xu, K.; Cao, H.Y.; Wu, X.; Xu, X.X.; Sun, Y.; Kong, W.J. Developmental abnormalities in supporting cell phalangeal processes and cytoskeleton in the Gjb2 knockdown mouse model. Dis. Model Mech. 2018, 11, dmm033019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, L.; Chen, S.; Xu, K.; Cao, H.Y.; Du, A.N.; Bai, X.; Sun, Y.; Kong, W.J. Reduced postnatal expression of cochlear Connexin26 induces hearing loss and affects the developmental status of pillar cells in a dose-dependent manner. Neurochem. Int. 2019, 128, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Bieniussa, L.; Jain, I.; Bosch Grau, M.; Juergens, L.; Hagen, R.; Janke, C.; Rak, K. Microtubule and auditory function—An underestimated connection. Semin. Cell Dev. Biol. 2022, S1084-9521(22)00041-6. [Google Scholar] [CrossRef] [PubMed]
- Paz, J.; Luders, J. Microtubule-Organizing Centers: Towards a Minimal Parts List. Trends Cell Biol. 2018, 28, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Meiring, J.C.M.; Shneyer, B.I.; Akhmanova, A. Generation and regulation of microtubule network asymmetry to drive cell polarity. Curr. Opin. Cell Biol. 2020, 62, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Rivero, S.; Cardenas, J.; Bornens, M.; Rios, R.M. Microtubule nucleation at the cis-side of the Golgi apparatus requires AKAP450 and GM130. EMBO J. 2009, 28, 1016–1028. [Google Scholar] [CrossRef] [PubMed]
- Henderson, C.G.; Tucker, J.B.; Mogensen, M.M.; Mackie, J.B.; Chaplin, M.A.; Slepecky, N.B.; Leckie, L.M. Three microtubule-organizing centres collaborate in a mouse cochlear epithelial cell during supracellularly coordinated control of microtubule positioning. J. Cell Sci. 1995, 108, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Tucker, J.B.; Mogensen, M.M.; Paton, C.C.; Mackie, J.B.; Henderson, C.G.; Leckie, L.M. Formation of two microtubule-nucleating sites which perform differently during centrosomal reorganization in a mouse cochlear epithelial cell. J. Cell Sci. 1995, 108, 1333–1345. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Z.; Jin, Y.; Chen, S.; Xu, K.; Xie, L.; Qiu, Y.; Wang, X.H.; Sun, Y.; Kong, W.J. F-Actin Dysplasia Involved in Organ of Corti Deformity in Gjb2 Knockdown Mouse Model. Front. Mol. Neurosci. 2021, 14, 808553. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Chen, S.; Xie, L.; Qiu, Y.; Bai, X.; Liu, X.Z.; Zhang, H.M.; Wang, X.H.; Jin, Y.; Sun, Y.; et al. Local Macrophage-Related Immune Response Is Involved in Cochlear Epithelial Damage in Distinct Gjb2-Related Hereditary Deafness Models. Front. Cell Dev. Biol. 2020, 8, 597769. [Google Scholar] [CrossRef] [PubMed]
- Goldspink, D.A.; Rookyard, C.; Tyrrell, B.J.; Gadsby, J.; Perkins, J.; Lund, E.K.; Galjart, N.; Thomas, P.; Wileman, T.; Mogensen, M.M. Ninein is essential for apico-basal microtubule formation and CLIP-170 facilitates its redeployment to non-centrosomal microtubule organizing centres. Open Biol. 2017, 7, 160274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mogensen, M.M.; Malik, A.; Piel, M.; Bouckson-Castaing, V.; Bornens, M. Microtubule minus-end anchorage at centrosomal and non-centrosomal sites: The role of ninein. J. Cell Sci. 2000, 113, 3013–3023. [Google Scholar] [CrossRef] [PubMed]
- Moss, D.K.; Bellett, G.; Carter, J.M.; Liovic, M.; Keynton, J.; Prescott, A.R.; Lane, E.B.; Mogensen, M.M. Ninein is released from the centrosome and moves bi-directionally along microtubules. J. Cell Sci. 2007, 120, 3064–3074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.; Ding, X.; Xie, M.; Huang, Z.; Han, P.; Tian, D.; Xia, L. CAMSAP2-mediated noncentrosomal microtubule acetylation drives hepatocellular carcinoma metastasis. Theranostics 2020, 10, 3749–3766. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Akhmanova, A. Coming into Focus: Mechanisms of Microtubule Minus-End Organization. Trends Cell Biol. 2018, 28, 574–588. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Meng, W.; Nagae, S.; Takeichi, M. Nezha/CAMSAP3 and CAMSAP2 cooperate in epithelial-specific organization of noncentrosomal microtubules. Proc. Natl. Acad. Sci. USA 2012, 109, 20029–20034. [Google Scholar] [CrossRef] [PubMed] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, Y.; Xu, K.; Xie, L.; Chen, S.; Sun, Y. The Reduction in Microtubule Arrays Caused by the Dysplasia of the Non-Centrosomal Microtubule-Organizing Center Leads to a Malformed Organ of Corti in the Cx26-Null Mouse. Biomedicines 2022, 10, 1364. https://doi.org/10.3390/biomedicines10061364
Qiu Y, Xu K, Xie L, Chen S, Sun Y. The Reduction in Microtubule Arrays Caused by the Dysplasia of the Non-Centrosomal Microtubule-Organizing Center Leads to a Malformed Organ of Corti in the Cx26-Null Mouse. Biomedicines. 2022; 10(6):1364. https://doi.org/10.3390/biomedicines10061364
Chicago/Turabian StyleQiu, Yue, Kai Xu, Le Xie, Sen Chen, and Yu Sun. 2022. "The Reduction in Microtubule Arrays Caused by the Dysplasia of the Non-Centrosomal Microtubule-Organizing Center Leads to a Malformed Organ of Corti in the Cx26-Null Mouse" Biomedicines 10, no. 6: 1364. https://doi.org/10.3390/biomedicines10061364
APA StyleQiu, Y., Xu, K., Xie, L., Chen, S., & Sun, Y. (2022). The Reduction in Microtubule Arrays Caused by the Dysplasia of the Non-Centrosomal Microtubule-Organizing Center Leads to a Malformed Organ of Corti in the Cx26-Null Mouse. Biomedicines, 10(6), 1364. https://doi.org/10.3390/biomedicines10061364






