C-Reactive Protein and Serum Albumin Ratio: A Feasible Prognostic Marker in Hospitalized Patients with COVID-19
Abstract
:1. Introduction
2. Materials and Methods
2.1. Type of Study
2.2. Procedures
2.3. Exposure and Outcome
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
3.2. COVID-19-Related Characteristics
3.3. Outcomes
3.4. Univariate Analysis
3.5. Multivariate Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. List of the SEMI-COVID-19 Network Members
Appendix B. List of the Analyzed Variables
| Variable | Definition | Type of Variable and Values |
|---|---|---|
| Demographics | ||
| Age | Age in years at hospital admission. | Continuous. Numerical. |
| Race | Race of the patient. | Categorical. Caucasian, Latin, or other. |
| Sex | Sex of patient at birth. | Categorical. Male or female. |
| Comorbidities | ||
| Age-adjusted Charlson index | Charlson Comorbidity Index calculated at hospital admission, based on comorbidity previously reported in medical history adjusted for age. | Continuous. Numerical, 0–48. |
| AMI | If acute myocardial infarction was previously reported in the medical history. | Categorical. Yes or no. |
| Alcohol abuse | If alcohol abuse was reported in the medical history. | Categorical. Yes or no. |
| Angina | If angina was previously reported in the medical history. | Categorical. Yes or no. |
| Asthma | If asthma was previously reported in the medical history. | Categorical. Yes or no. |
| Atrial fibrillation | If atrial fibrillation was previously reported in the medical history. | Categorical. Yes or no. |
| Cancer | If active cancer was reported in the medical history. | Categorical. Yes or no. |
| Comorbidity | Age-adjusted Charlson score value ≥ 3 | Categorical. Yes or no. |
| COPD | If chronic obstructive pulmonary disease was previously reported in the medical history. | Categorical. Yes or no. |
| Dependence status | What was the degree of dependence before hospital admission reported in the medical history according to the Barthel index? | Categorical. Absent or mild dependence, moderate dependence, or severe dependence. |
| Depression | If chronic depression was previously reported in the medical history. | Categorical. Yes or no. |
| CHF | If chronic heart failure was previously reported in the medical history. | Categorical. Yes or no. |
| CHT | If chronic hypertension was previously reported in the medical history. | Categorical. Yes or no. |
| CKD | If chronic kidney disease, defined as MDRD4 <60 mL/min (1.73 m2), was previously reported in the medical history. | Categorical. Yes or no. |
| CLD | If chronic liver disease was previously reported in the medical history. | Categorical. Yes or no. |
| CTD | If a connective tissue disease (e.g., systemic lupus erythematosus and scleroderma) was previously reported in the medical history. | Categorical. Yes or no. |
| DM | If diabetes mellitus was previously reported in the medical history. | Categorical. Yes or no. |
| Dyslipidemia | If dyslipidemia was previously reported in the medical history. | Categorical. Yes or no. |
| IS | If ischemic stroke was previously reported in the medical history. | Categorical. Yes or no. |
| Neurodegenerative disease | If a neurodegenerative disease (e.g., Alzheimer disease, Parkinson disease, and Huntington disease) was previously reported in the medical history. | Categorical. Yes or no. |
| Obesity | If obesity, defined as a body mass index >30 kg/m2, was previously reported in the medical history. | Categorical. Yes or no. |
| OSAS | If obstructive sleep apnea syndrome was previously reported in the medical history. | Categorical. Yes or no. |
| PVD | If peripheral vascular disease was previously reported in the medical history. | Categorical. Yes or no. |
| Smoking | If smoking was previously reported in the medical history. | Categorical. Never smoked, former smoker, or current smoker. |
| TIA | If transient ischemic accident was previously reported in the medical history. | Categorical. Yes or no. |
| Baseline pharmacological treatments | ||
| ACEi | If maintained use of angiotensin-converting enzyme inhibitors was previously reported in the medical history. | Categorical. Yes or no. |
| Acenocoumarol | If maintained use of acenocoumarol was previously reported in the medical history. | Categorical. Yes or no. |
| ARB | If maintained use of angiotensin receptor blockers was previously reported in the medical history. | Categorical. Yes or no. |
| Aspirin | If maintained use of aspirin was previously reported in the medical history. | Categorical. Yes or no. |
| Direct anticoagulants | If maintained use of direct anticoagulants was previously reported in the medical history. | Categorical. Yes or no. |
| DPP4i | If maintained use of dipeptidyl peptidase-4 inhibitors was previously reported in the medical history. | Categorical. Yes or no. |
| Inhaled corticosteroids | If maintained use of inhaled corticosteroids was previously reported in the medical history. | Categorical. Yes or no. |
| Insulin | If maintained use of insulin was previously reported in the medical history. | Categorical. Yes or no. |
| LMWH | If maintained use of low-molecular-weight heparin was previously reported in the medical history. | Categorical. Yes or no. |
| Metformin | If maintained use of metformin was previously reported in the medical history. | Categorical. Yes or no. |
| Monoclonal antibodies | If maintained use of monoclonal antibodies was previously reported in the medical history. | Categorical. Yes or no. |
| SGLT2i | If maintained use of sodium-glucose transporter (SGLT) 2 inhibitors was previously reported in the medical history. | Categorical. Yes or no. |
| Statins | If maintained use of statins was previously reported in the medical history. | Categorical. Yes or no. |
| Systemic corticosteroids | If maintained use of systemic corticosteroids was previously reported in the medical history. | Categorical. Yes or no. |
| COVID-19-related variables | ||
| General variables | ||
| Origin of infection | Suspected site of infection. | Categorical. Community, healthcare staff, or others. |
| Duration of symptoms | Days since onset of symptoms until admission. | Numerical. Days. |
| Clinical manifestations | ||
| Abdominal pain | If abdominal pain was present at admission or during the days before according to the medical history. | Categorical. Yes or no. |
| Ageusia | If ageusia was present at admission or during the days before according to the medical history. | Categorical. Yes or no. |
| Anosmia | If anosmia was present at admission or during the days before according to the medical history. | Categorical. Yes or no. |
| Anorexia | If anorexia was present at admission or during the days before according to the medical history. | Categorical. Yes or no. |
| Asthenia | If asthenia was present at admission or during the days before according to the medical history. | Categorical. Yes or no. |
| Cough | If cough was present at admission or during the days before according to the medical history. | Categorical. No cough, dry cough, or wet cough. |
| Diarrhea | If diarrhea was present at admission or during the days before according to the medical history. | Categorical. Yes or no. |
| Dyspnea | If dyspnea was present at admission or during the days before according to the medical history. | Categorical. Yes or no. |
| Headache | If headache was present at admission or during the days before according to the medical history. | Categorical. Yes or no. |
| Myalgia | If myalgia was present at admission or during the days before according to the medical history. | Categorical. Yes or no. |
| Nausea | If nausea was present at admission or during the days before according to the medical history. | Categorical. Yes or no. |
| Odynophagia | If odynophagia was present at admission or during the days before according to the medical history. | Categorical. Yes or no. |
| Vomiting | If vomiting was present at admission or during the days before according to the medical history. | Categorical. Yes or no. |
| Physical examination at admission | ||
| Baseline O2 saturation | Oxygen saturation, in percentage, at hospital admission, without supplementary oxygen therapy. | Continuous. Numerical. |
| Baseline O2 saturation < 93% | If oxygen saturation without supplementary oxygen at hospital admission was less than 93%. | Categorical. Yes or no. |
| Confusion | If confusion was present at admission or during the days before, according to the medical history. | Categorical. Yes or no. |
| Crackles | If crackles were present at admission. | Categorical. Yes or no. |
| DBP | Diastolic blood pressure, in millimeters of mercury, at hospital admission. | Continuous. Numerical. |
| Heart rate | Heart rate, in beats per minute, at hospital admission. | Continuous. Numerical. |
| SBP | Systolic blood pressure, in millimeters of mercury, at hospital admission. | Continuous. Numerical. |
| Tachypnea | If tachypnea, defined as breathing rate greater than 22 breaths per minute, was present at admission according to the medical history. | Categorical. Yes or no. |
| Rhonchi | If rhonchi were present at hospital admission. | Categorical. Yes or no. |
| Temperature | Temperature, in degrees Celsius, at hospital admission. | Continuous. Numerical. |
| Temperature ≥ 38 °C | If temperature at hospital admission was greater than 38 °C. | Categorical. Yes or no. |
| Wheezing | If wheezing was present at hospital admission. | Categorical. Yes or no. |
| Radiological findings | ||
| Alveolar condensation | Alveolar condensation in the plain chest radiograph at admission. | Categorical. Absent, unilateral, or bilateral. |
| Interstitial infiltrate | Interstitial infiltrate in the plain chest radiograph at admission. | Categorical. Absent, unilateral, or bilateral. |
| Pleural effusion | Pleural effusion in the plain chest radiograph at admission. | Categorical. Absent, unilateral, or bilateral. |
| Analytical findings | ||
| Albumin | Value of serum albumin at admission in g/dL. | Continuous. Numerical. |
| Creatinine | Value of plasma creatinine at admission in mg/dL. | Continuous. Numerical. |
| CRP | Value of C-reactive protein at admission in mg/L. | Continuous. Numerical. |
| Glucose | Value of plasma glucose at admission in mg/dL. | Continuous. Numerical. |
| Leukocytes | Number of leukocytes’ 106 per liter at hospital admission. | Continuous. Numerical. |
| Hemoglobin | Value of hemoglobin at admission in g/dL. | Continuous. Numerical. |
| Lymphocytes | Number of lymphocytes’ 106 per liter at hospital admission. | Continuous. Numerical. |
| Neutrophils | Number of neutrophils’ 106 per liter at hospital admission. | Continuous. Numerical. |
| Platelets | Number of platelets’ 106 per liter in blood test at hospital admission. | Continuous. Numerical. |
| Plasma sodium | Value of plasma sodium at admission in mEq/L. | Continuous. Numerical. |
| Plasma potassium | Value of plasma potassium at admission in mEq/L. | Continuous. Numerical. |
| Outcomes | ||
| In-hospital mortality | If the patient died during hospitalization due to SARS-CoV-2 infection, or 30 days after hospital discharge. | Categorical. Yes or no. |
| Length of stay | More or less than 14 days. | Categorical. Yes or no. |
| Need for NIMV | If during hospitalization the patient has received continuous positive airway pressure (CPAP) and/or bilevel positive airway pressure (BiPAP) and/or high-flow cannula oxygen therapy. | Categorical. Yes or no. |
| ICU admission | If the patient has required admission to the intensive care unit. | Categorical. Yes or no. |
References
- Moore, B.J.B.; June, C.H. Cytokine release syndrome in severe COVID-19. Science 2020, 368, 473–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khadke, S.; Ahmed, N.; Ahmed, N.; Ratts, R.; Raju, S.; Gallogly, M.; De Lima, M.; Sohail, M.R. Harnessing the immune system to overcome cytokine storm and reduce viral load in COVID-19: A review of the phases of illness and therapeutic agents. Virol. J. 2020, 17, 154. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72,314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Berenguer, J.; Ryan, P.; Rodríguez-Baño, J.; Jarrín, I.; Carratalà, J.; Pachón, J.; Yllescas, M.; Arriba, J.R.; Muñoz, E.A.; COVID-19@Spain Study Group; et al. Characteristics and predictors of death among 4035 consecutively hospitalized patients with COVID-19 in Spain. Clin. Microbiol. Infect. 2020, 26, 1525–1536. [Google Scholar] [CrossRef] [PubMed]
- Jutzeler, C.R.; Bourguignon, L.; Weis, C.V.; Tong, B.; Wong, C.; Rieck, B.; Pargger, H.; Tschudin-Sutter, S.; Egli, A.; Borgwardt, K.; et al. Comorbidities, clinical signs and symptoms, laboratory findings, imaging features, treatment strategies, and outcomes in adult and pediatric patients with COVID-19: A systematic review and meta-analysis. Travel Med. Infect. Dis. 2020, 37, 101825. [Google Scholar] [CrossRef]
- Nandy, K.; Salunke, A.; Pathak, S.K.; Pandey, A.; Doctor, C.; Puj, K.; Sharma, M.; Jain, A.; Warikoo, V. Coronavirus disease (COVID-19): A systematic review and meta-analysis to evaluate the impact of various comorbidities on serious events. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 1017–1025. [Google Scholar] [CrossRef]
- Agbehadji, I.E.; Awuzie, B.O.; Ngowi, A.B.; Millham, R.C. Review of big data analytics, artificial intelligence and nature-inspired computing models towards accurate detection of COVID-19 pandemic cases and contact tracing. Int. J. Environ. Res. Public Health 2020, 17, 5330. [Google Scholar] [CrossRef]
- Wang, S.; Zha, Y.; Li, W.; Wu, Q.; Li, X.; Niu, M.; Wang, M.; Qiu, X.; Li, H.; Yu, H.; et al. A fully automatic deep learning system for COVID-19 diagnostic and prognostic analysis. Eur. Respir. J. 2020, 56, 2000775. [Google Scholar] [CrossRef]
- Soeters, P.B.; Wolfe, R.R.; Shenkin, A. Hypoalbuminemia: Pathogenesis and Clinical Significance. J. Parenter. Enter. Nutr. 2018, 43, 181–193. [Google Scholar] [CrossRef] [Green Version]
- Eckart, A.; Struja, T.; Kutz, A.; Baumgartner, A.; Baumgartner, T.; Zurfluh, S.; Neeser, O.; Huber, A.; Stanga, Z.; Mueller, B.; et al. Relationship of Nutritional Status, Inflammation, and Serum Albumin Levels During Acute Illness: A Prospective Study. Am. J. Med. 2020, 133, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, M.; Zheng, S.; Li, M.; Zhang, M.; Sun, M.; Li, X.; Deng, A.; Cai, Y.; Zhang, H. Plasma albumin levels predict risk for nonsurvivors in critically ill patients with COVID-19. Biomarkers Med. 2020, 14, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Horton, R. Offline: COVID-19 is not a pandemic. Lancet 2020, 396, 874. [Google Scholar] [CrossRef]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H.; et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Investig. 2020, 130, 2620–2629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouyang, S.-M.; Zhu, H.-Q.; Xie, Y.-N.; Zou, Z.-S.; Zuo, H.-M.; Rao, Y.-W.; Liu, X.-Y.; Zhong, B.; Chen, X. Temporal changes in laboratory markers of survivors and non-survivors of adult inpatients with COVID-19. BMC Infect. Dis. 2020, 20, 952. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Cheng, A.; Kumar, R.; Fang, Y.; Chen, G.; Zhu, Y.; Lin, S. Hypoalbuminemia predicts the outcome of COVID-19 independent of age and co-morbidity. J. Med. Virol. 2020, 92, 2152–2158. [Google Scholar] [CrossRef]
- Liu, W.; Tao, Z.-W.; Wang, L.; Yuan, M.-L.; Liu, K.; Zhou, L.; Wei, S.; Deng, Y.; Liu, J.; Liu, H.-G.; et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin. Med. J. 2020, 133, 1032–1038. [Google Scholar] [CrossRef]
- Bonetti, G.; Manelli, F.; Patroni, A.; Bettinardi, A.; Borrelli, G.; Fiordalisi, G.; Marino, A.; Menolfi, A.; Saggini, S.; Volpi, R.; et al. Laboratory predictors of death from coronavirus disease 2019 (COVID-19) in the area of Valcamonica, Italy. Clin. Chem. Lab. Med. (CCLM) 2020, 58, 1100–1105. [Google Scholar] [CrossRef]
- De La Rica, R.; Borges, M.; Aranda, M.; Del Castillo, A.; Socias, A.; Payeras, A.; Rialp, G.; Socias, L.; Masmiquel, L.; Gonzalez-Freire, M. Low Albumin Levels Are Associated with Poorer Outcomes in a Case Series of COVID-19 Patients in Spain: A Retrospective Cohort Study. Microorganisms 2020, 8, 1106. [Google Scholar] [CrossRef]
- Kheir, M.; Saleem, F.; Wang, C.; Mann, A.; Chua, J. Higher albumin levels on admission predict better prognosis in patients with confirmed COVID-19. PLoS ONE 2021, 16, e0248358. [Google Scholar] [CrossRef]
- Matin, S.; Fouladi, N.; Pahlevan, Y.; Asghariazar, V.; Molaei, S.; Khiavi, H.A.; Negaresh, M.; Safarzadeh, E. The sufficient vitamin D and albumin level have a protective effect on COVID-19 infection. Arch. Microbiol. 2021, 203, 5153–5162. [Google Scholar] [CrossRef] [PubMed]
- Acharya, R.; Poudel, D.; Patel, A.; Schultz, E.; Bourgeois, M.; Paswan, R.; Stockholm, S.; Batten, M.; Kafle, S.; Atkinson, A.; et al. Low serum albumin and the risk of hospitalization in COVID-19 infection: A retrospective case-control study. PLoS ONE 2021, 16, e0250906. [Google Scholar] [CrossRef]
- Sanson, G.; De Nicolò, A.; Zerbato, V.; Segat, L.; Koncan, R.; Di Bella, S.; Cusato, J.; di Masi, A.; Palermo, A.; Caironi, P.; et al. A combined role for low vitamin D and low albumin circulating levels as strong predictors of worse outcome in COVID-19 patients. Ir. J. Med. Sci. 2022, 3, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Uyar, E.; Merdin, A.; Yamanyar, S.; Ezgü, M.C.; Artuk, C.; Taşkin, G.; Arslan, Y.; Ceritli, S. Could serum albumin value and thrombocyte/lymphocyte ratio be an important prognostic factor in determining the severity of COVID 19? Turk. J. Med. Sci. 2021, 51, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Paliogiannis, P.; Mangoni, A.A.; Cangemi, M.; Fois, A.G.; Carru, C.; Zinellu, A. Serum albumin concentrations are associated with disease severity and outcomes in coronavirus 19 disease (COVID-19): A systematic review and meta-analysis. Clin. Exp. Med. 2021, 21, 343–354. [Google Scholar] [CrossRef]
- Hariyanto, T.I.; Japar, K.V.; Kwenandar, F.; Damay, V.; Siregar, J.I.; Lugito, N.P.H.; Tjiang, M.M.; Kurniawan, A. Inflammatory and hematologic markers as predictors of severe outcomes in COVID-19 infection: A systematic review and meta-analysis. Am. J. Emerg. Med. 2020, 41, 110–119. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.; Song, C.; Lu, R.; Zhao, Y.; Lin, F.; Han, D.; Chen, L.; Pan, P.; Dai, M. Early Prediction of Disease Progression in Patients with Severe COVID-19 Using C-Reactive Protein to Albumin Ratio. Dis. Markers 2021, 2021, 6304189. [Google Scholar] [CrossRef]
- Karakoyun, I.; Colak, A.; Turken, M.; Altin, Z.; Arslan, F.D.; Iyilikci, V.; Yilmaz, N.; Kose, S. Diagnostic utility of C-reactive protein to albumin ratio as an early warning sign in hospitalized severe COVID-19 patients. Int. Immunopharmacol. 2020, 91, 107285. [Google Scholar] [CrossRef]
- El-Shabrawy, M.; Alsadik, M.E.; El-Shafei, M.; Abdelmoaty, A.A.; Alazzouni, A.S.; Esawy, M.M.; Shabana, M.A. Interleukin-6 and C-reactive protein/albumin ratio as predictors of COVID-19 severity and mortality. Egypt. J. Bronchol. 2021, 15, 5. [Google Scholar] [CrossRef]
- Güney, B.Ç.; Taştan, Y.Ö.; Doğantekin, B.; Serindağ, Z.; Yeniçeri, M.; Çiçek, V.; Kılıç, Ş.; Şeker, M.; Çınar, T.; Hayiroglu, M.İ.; et al. Predictive Value of CAR for In-Hospital Mortality in Patients with COVID-19 Pneumonia: A Retrospective Cohort Study. Arch. Med. Res. 2021, 52, 554–560. [Google Scholar] [CrossRef]
- Ayrancı, M.K.; Küçükceran, K.; Dundar, Z.D. NLR and CRP to albumin ratio as a predictor of in-hospital mortality in the geriatric ED patients. Am. J. Emerg. Med. 2021, 44, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Torun, A.; Çakırca, T.D.; Çakırca, G.; Portakal, R.D. The value of C-reactive protein/albumin, fibrinogen/albumin, and neutrophil/lymphocyte ratios in predicting the severity of COVID-19. Rev. Assoc. Médica Bras. 2021, 67, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Kalyon, S.; Gültop, F.; Şimşek, F.; Adaş, M. Relationships of the neutrophil–lymphocyte and CRP–albumin ratios with the duration of hospitalization and fatality in geriatric patients with COVID-19. J. Int. Med. Res. 2021, 49. [Google Scholar] [CrossRef] [PubMed]
- Lucijanić, M.; Stojić, J.; Atić, A.; Čikara, T.; Osmani, B.; Barišić-Jaman, M.; Andrilović, A.; Bistrović, P.; Vrkljan, A.Z.; Lagančić, M.; et al. Clinical and prognostic significance of C-reactive protein to albumin ratio in hospitalized coronavirus disease 2019 (COVID-19) patients: Data on 2309 patients from a tertiary center and validation in an independent cohort. Wien. Klin. Wochenschr. 2022, 134, 377–384. [Google Scholar] [CrossRef]
- Qin, R.; He, L.; Yang, Z.; Jia, N.; Chen, R.; Xie, J.; Fu, W.; Chen, H.; Lin, X.; Huang, R.; et al. Identification of Parameters Representative of Immune Dysfunction in Patients with Severe and Fatal COVID-19 Infection: A Systematic Review and Meta-analysis. Clin. Rev. Allergy Immunol. 2022, 1–33. [Google Scholar] [CrossRef]
- Casas-Rojo, J.M.; Antón-Santos, J.M.; Millán-Núñez-Cortés, J.; Lumbreras-Bermejo, C.; Ramos-Rincón, J.M.; Roy-Vallejo, E.; Artero-Mora, A.; Arnalich-Fernández, F.; García-Bruñén, J.M.; SEMI-COVID-19 Network Group; et al. Clinical characteristics of patients hospitalized with COVID-19 in Spain: Results from the SEMI-COVID-19 Registry. Rev. Clin. Esp. 2020, 220, 480–494. [Google Scholar] [CrossRef]
- World Health Organization. Laboratory Testing for Coronavirus Disease 2019 (COVID-19) in Suspected Human Cases: Interim Guidance; World Health Organization: Geneva, Switzerland, 2020; pp. 1–7. [Google Scholar]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. PLoS Med. 2007, 4, e296. [Google Scholar] [CrossRef] [Green Version]
- McCabe, R.; Schmit, N.; Christen, P.; D’Aeth, J.C.; Løchen, A.; Rizmie, D.; Nayagam, S.; Miraldo, M.; Aylin, P.; Bottle, A.; et al. Adapting hospital capacity to meet changing demands during the COVID-19 pandemic. BMC Med. 2020, 18, 329. [Google Scholar] [CrossRef]
- Klein, M.G.; Cheng, C.J.; Lii, E.; Mao, K.; Mesbahi, H.; Zhu, T.; Muckstadt, J.A.; Hupert, N. COVID-19 Models for Hospital Surge Capacity Planning: A Systematic Review. Disaster Med. Public Health Prep. 2020, 16, 390–397. [Google Scholar] [CrossRef]
- Olivas-Martínez, A.; Cárdenas-Fragoso, J.L.; Jiménez, J.V.; Lozano-Cruz, O.A.; Ortiz-Brizuela, E.; Tovar-Méndez, V.H.; Medrano-Borromeo, C.; Martínez-Valenzuela, A.; Román-Montes, C.M.; Martínez-Guerra, B.; et al. In-hospital mortality from severe COVID-19 in a tertiary care center in Mexico City; causes of death, risk factors and the impact of hospital saturation. PLoS ONE 2021, 16, e0245772. [Google Scholar] [CrossRef] [PubMed]
- Ghahramani, S.; Tabrizi, R.; Lankarani, K.B.; Kashani, S.M.A.; Rezaei, S.; Zeidi, N.; Akbari, M.; Heydari, S.T.; Akbari, H.; Nowrouzi-Sohrabi, P.; et al. Laboratory features of severe vs. non-severe COVID-19 patients in Asian populations: A systematic review and meta-analysis. Eur. J. Med. Res. 2020, 25, 30. [Google Scholar] [CrossRef] [PubMed]
- Izcovich, A.; Ragusa, M.A.; Tortosa, F.; Marzio, M.A.L.; Agnoletti, C.; Bengolea, A.; Ceirano, A.; Espinosa, F.; Saavedra, E.; Sanguine, V.; et al. Prognostic factors for severity and mortality in patients infected with COVID-19: A systematic review. PLoS ONE 2020, 15, e0241955. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.; Zhang, D.; Xu, J.; Chen, Z.; Yang, T.; Zhao, P.; Chen, G.; Cheng, G.; Wang, Y.; Bi, J.; et al. Prediction for Progression Risk in Patients With COVID-19 Pneumonia: The CALL Score. Clin. Infect. Dis. 2020, 71, 1393–1399. [Google Scholar] [CrossRef] [Green Version]
- Knight, S.R.; Ho, A.; Pius, R.; Buchan, I.; Carson, G.; Drake, T.M.; Dunning, J.; Fairfield, C.J.; Gamble, C.; ISARIC4C Investigators; et al. Risk stratification of patients admitted to hospital with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol: Development and validation of the 4C Mortality Score. BMJ 2020, 370, m3339. [Google Scholar] [CrossRef]
- Gupta, R.K.; Harrison, E.M.; Ho, A.; Docherty, A.B.; Knight, S.R.; van Smeden, M.; Abubakar, I.; Lipman, M.; Quartagno, M.; Pius, R.; et al. Development and validation of the ISARIC 4C Deterioration model for adults hospitalised with COVID-19: A prospective cohort study. Lancet Respir. Med. 2021, 9, 349–359. [Google Scholar] [CrossRef]
- Quan, H.; Li, B.; Couris, C.M.; Fushimi, K.; Graham, P.; Hider, P.; Januel, J.-M.; Sundararajan, V. Updating and Validating the Charlson Comorbidity Index and Score for Risk Adjustment in Hospital Discharge Abstracts Using Data From 6 Countries. Am. J. Epidemiol. 2011, 173, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Huang, X.; Sun, J.; Xie, T.; Lei, Y.; Muhammad, J.; Li, X.; Zeng, X.; Zhou, F.; Qin, H.; et al. Clinical Characteristics and Immune Injury Mechanisms in 71 Patients with COVID-19. mSphere 2020, 5, e00362-20. [Google Scholar] [CrossRef]
- Ranzani, O.T.; Zampieri, F.G.; Forte, D.N.; Azevedo, L.C.P.; Park, M. C-Reactive Protein/Albumin Ratio Predicts 90-Day Mortality of Septic Patients. PLoS ONE 2013, 8, e59321. [Google Scholar] [CrossRef]
- Oh, J.; Kim, S.H.; Park, K.N.; Oh, S.H.; Kim, Y.-M.; Kim, H.J.; Youn, C.S. High-sensitivity C-reactive protein/albumin ratio as a predictor of in-hospital mortality in older adults admitted to the emergency department. Clin. Exp. Emerg. Med. 2017, 4, 19–24. [Google Scholar] [CrossRef]
- Guner, A.; Kim, H.-I. Biomarkers for Evaluating the Inflammation Status in Patients with Cancer. J. Gastric Cancer 2019, 19, 254–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, T.K.; Ji, E.; Na, H.-S.; Min, B.; Jeon, Y.-T.; Do, S.-H.; Song, I.-A.; Park, H.-P.; Hwang, J.-W. C-Reactive Protein to Albumin Ratio Predicts 30-Day and 1-Year Mortality in Postoperative Patients after Admission to the Intensive Care Unit. J. Clin. Med. 2018, 7, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Uranga, A.; Guzmán-Martínez, J.; Esteve-Atiénzar, P.J.; Wikman-Jorgensen, P.; Núñez-Cruz, J.M.; Espinosa-Del-Barrio, L.; Hernández-Isasi, I.; Pomares-Gómez, F.J.; Perelló-Camacho, E.; Fernández-García, N.; et al. Nutritional and Functional Impact of Acute SARS-CoV-2 Infection in Hospitalized Patients. J. Clin. Med. 2022, 11, 2424. [Google Scholar] [CrossRef] [PubMed]
- Artero, A.; Madrazo, M.; Fernández-Garcés, M.; Miguez, A.M.; García, A.G.; Vieitez, A.C.; Guijarro, E.G.; Aizpuru, E.M.F.; Gómez, M.G.; Manrique, M.A.; et al. SEMI-COVID-19 Network. Severity Scores in COVID-19 Pneumonia: A Multicenter, Retrospective, Cohort Study. J. Gen. Intern. Med. 2021, 36, 1338–1345. [Google Scholar] [CrossRef] [PubMed]
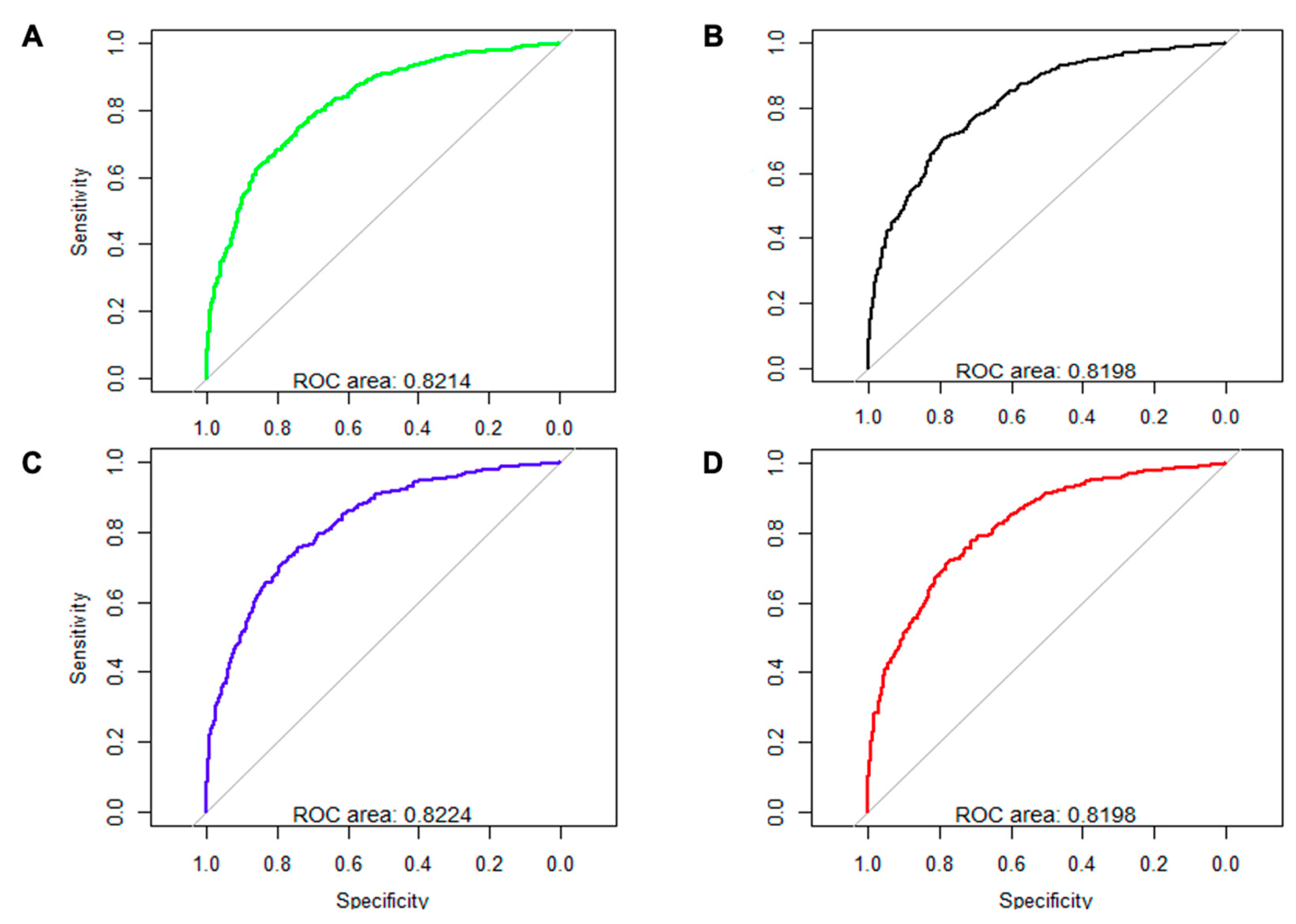
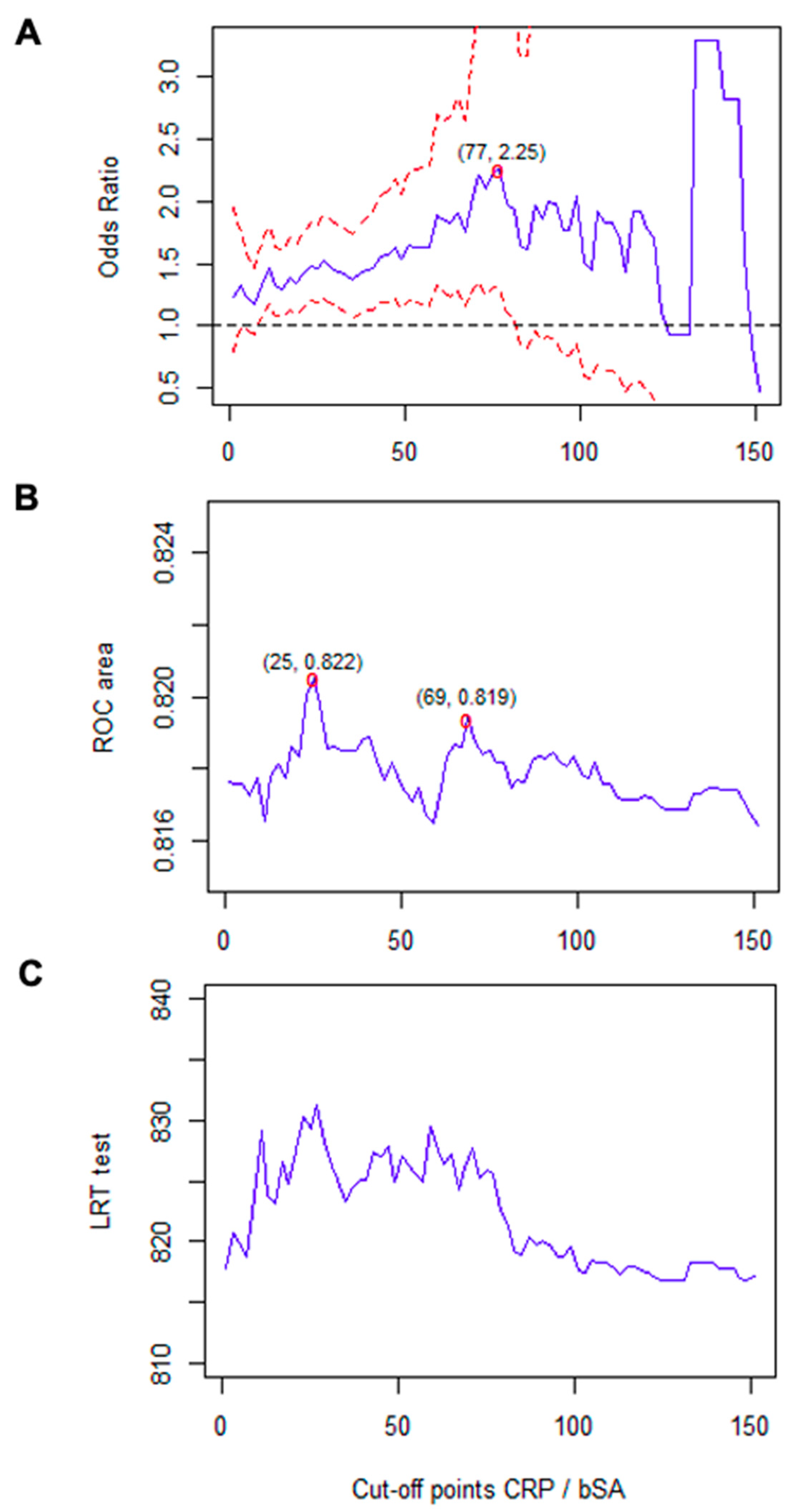
| Variable | All Patients (n = 3471) | Outcome Group (n = 1425) | Non-Outcome Group (n = 2046) | p * |
|---|---|---|---|---|
| Demographic Characteristics | ||||
| Age, years, mean (SD) | 66.2 (16.0) | 72.0 (14.7) | 62.1 (15.6) | <0.001 |
| Male sex, n (%) | 2000 (57.6) | 877 (61.5) | 1123 (54.9) | <0.001 |
| Race, n (%) | 0.001 | |||
| Caucasian | 3100 (89.3) | 1306 (91.6) | 1794 (87.7) | |
| Latin American | 322 (9.3) | 100 (7.0) | 222 (10.8) | |
| Others | 49 (1.4) | 19 (1.3) | 30 (1.5) | |
| Baseline comorbidities | ||||
| CHT, n (%) | 1718 (49.5) | 865 (60.7) | 853 (41.7) | <0.001 |
| Dyslipidemia, n (%) | 1375 (39.5) | 663 (46.5) | 712 (34.8) | <0.001 |
| Smoking, n (%) | <0.001 | |||
| Former smoker | 897 (25.6) | 437 (30.7) | 460 (22.5) | |
| Current smoker | 200 (5.8) | 80 (5.6) | 120 (5.8) | |
| Obesity, n (%) | 843 (24.3) | 392 (27.5) | 451 (31.6) | <0.001 |
| DM, n (%) | 655 (18.9) | 345 (24.2) | 311 (15.2) | <0.001 |
| Previous TIA, n (%) | 265 (7.6) | 63 (3.1) | 89 (6.2) | <0.001 |
| Previous IS, n (%) | 152 (4.4) | 54 (3.8) | 37 (2.6) | <0.001 |
| Depression, n (%) | 364 (10.5) | 163 (11.4) | 201 (9.8) | 0.127 |
| Atrial fibrillation, n (%) | 361 (10.4) | 220 (15.4) | 141 (6.9) | <0.001 |
| PVD, n (%) | 304 (8.8) | 99 (6.9) | 61 (3.0) | <0.001 |
| Chronic anxiety, n (%) | 299 (8.6) | 137 (9.6) | 162 (7.9) | 0.091 |
| COPD, n (%) | 225 (6.5) | 142 (10.0) | 90 (4.4) | <0.001 |
| Cancer, n (%) | 223 (6.4) | 91 (6.4) | 93 (4.5) | 0.017 |
| Asthma, n (%) | 199 (5.7) | 95 (6.7) | 170 (8.3) | 0.073 |
| CTD, n (%) | 188 (5.4) | 51 (3.6) | 53 (2.6) | 0.093 |
| Neurodeg. disease, n (%) | 184 (5.3) | 172 (12.0) | 99 (4.8) | <0.001 |
| Alcohol abuse, n (%) | 175 (5.0) | 89 (6.2) | 86 (4.2) | 0.007 |
| Angina, n (%) | 166 (4.8) | 53 (3.7) | 44 (2.2) | 0.006 |
| AMI, n (%) | 271 (7.8) | 96 (6.7) | 70 (3.4) | <0.001 |
| CLD, n (%) | 160 (4.6) | 35 (2.4) | 45 (2.2) | 0.620 |
| CHF, n (%) | 97 (2.8) | 146 (10.2) | 79 (3.9) | <0.001 |
| OSAS, n (%) | 89 (2.6) | 122 (8.6) | 109 (5.3) | <0.001 |
| CKD, n (%) | 80 (2.3) | 144 (10.1) | 79 (3.9) | <0.001 |
| Dependence status, n (%) | <0.001 | |||
| Absent/mild | 2980 (85.9) | 1104 (77.5) | 1876 (91.7) | |
| Moderate | 296 (8.5) | 191 (13.4) | 105 (5.1) | |
| Severe | 195 (5.6) | 130 (9.1) | 65 (3.2) | |
| CCI, points, mean (SD) | 3.4 (2.6) | 4.4 (2.7) | 2.7 (2.3) | <0.001 |
| CCI ≥ 3, n (%) | 2885 (57.6) | 1590 (55.1) | 1295 (44.9) | <0.001 |
| Baseline pharmacological treatments | ||||
| Immunosuppressants, n (%) | ||||
| Systemic corticosteroids | 156 (4.5) | 101 (7.1) | 55 (2.7) | <0.001 |
| Inhaled corticosteroids | 337 (9.7) | 175 (12.3) | 162 (7.9) | <0.001 |
| Monoclonal antibodies | 34 (1.0) | 15 (1.1) | 19 (0.9) | 0.715 |
| Cardiovascular agents, n (%) | ||||
| ACEi | 567 (16.3) | 284 (19.9) | 283 (13.8) | <0.001 |
| ARB | 652 (18.8) | 327 (22.9) | 325 (15.9) | <0.001 |
| Statins | 1131 (32.6) | 542 (38.0) | 589 (28.8) | <0.001 |
| Aspirin | 477 (13.7) | 253 (17.7) | 224 (10.9) | <0.001 |
| Acenocoumarol | 185 (5.3) | 125 (8.8) | 60 (2.9) | <0.001 |
| Direct anticoagulants | 149 (4.3) | 79 (5.5) | 70 (3.4) | |
| LMWH | 23 (0.7) | 18 (1.3) | 5 (0.2) | |
| Antidiabetic agents, n (%) | ||||
| Insulin | 202 (5.8) | 115 (8.1) | 87 (4.3) | <0.001 |
| Metformin | 455 (13.1) | 227 (15.9) | 228 (11.1) | <0.001 |
| SGLT2i | 78 (2.2) | 30 (2.1) | 48 (2.3) | 0.638 |
| DPP4i | 242 (7.0) | 125 (8.8) | 117 (5.7) | 0.001 |
| Variable | All Patients (n = 3471) | Outcome Group (n = 1425) | Non-Outcome Group (n = 2046) | p * |
|---|---|---|---|---|
| Hospitalization | ||||
| Origin of infection, n (%) | <0.001 | |||
| Community | 2983 (85.9) | 1151 (80.8) | 1832 (89.5) | |
| Healthcare staff | 245 (7.1) | 142 (10.0) | 103 (5.0) | |
| Others | 243 (7.0) | 132 (9.3) | 111 (5.4) | |
| Duration of symptoms before admission, days, mean (SD) | 6.9 (4.4) | 6.2 (4.5) | 7.5 (4.3) | <0.001 |
| Clinical symptoms | ||||
| Cough, n (%) | <0.001 | |||
| Absent | 849 (24.5) | 396 (27.8) | 453 (22.1) | |
| Dry | 2072 (59.7) | 781 (54.8) | 1291 (63.1) | |
| Wet | 550 (15.8) | 248 (17.4) | 302 (14.8) | |
| Dyspnea, n (%) | 2021 (58.2) | 949 (66.6) | 1072 (52.4) | <0.001 |
| Asthenia, n (%) | 1499 (43.2) | 561 (39.4) | 938 (45.8) | <0.001 |
| Myalgia, n (%) | 1153 (33.2) | 375 (26.3) | 778 (38.0) | <0.001 |
| Diarrhea, n (%) | 878 (25.3) | 289 (20.3) | 589 (28.8) | <0.001 |
| Anorexia, n (%) | 634 (18.3) | 282 (19.8) | 352 (17.2) | 0.058 |
| Nausea, n (%) | 438 (12.6) | 158 (11.1) | 280 (13.7) | 0.027 |
| Headache, n (%) | 414 (11.9) | 138 (9.7) | 276 (13.5) | 0.001 |
| Odynophagia, n (%) | 318 (9.2) | 110 (7.7) | 208 (10.2) | 0.014 |
| Ageusia, n (%) | 276 (8.0) | 49 (3.4) | 227 (11.1) | <0.001 |
| Vomiting, n (%) | 263 (7.6) | 107 (7.5) | 156 (7.6) | 0.951 |
| Anosmia, n (%) | 240 (6.9) | 44 (3.1) | 196 (9.6) | <0.001 |
| Abdominal pain, n (%) | 215 (6.2) | 84 (5.9) | 131 (6.4) | 0.590 |
| Physical examination | ||||
| Confusion, n (%) | 334 (9.6) | 244 (17.1) | 90 (4.4) | <0.001 |
| Tachypnea, n (%) | 1128 (32.5) | 669 (46.9) | 459 (22.4) | <0.001 |
| SBP, mmHg, mean (SD) | 128.7 (20.5) | 128.6 (21.3) | 128.8 (19.9) | 0.815 |
| DBP, mmHg, mean (SD) | 74.4 (12.8) | 72.7 (12.9) | 75.6 (12.7) | <0.001 |
| Heart rate, bpm, mean (SD) | 88.9 (17.2) | 89.0 (16.8) | 88.8 (17.7) | 0.736 |
| Temperature, °C, mean (SD) | 37.1 (1.0) | 37.0 (0.9) | 37.3 (1.0) | <0.001 |
| Temperature ≥ 38 ° C, n (%) | 2238 (64.5) | 910 (63.9) | 1328 (64.9) | 0.019 |
| Crackles, n (%) | 1806 (52.0) | 812 (57.0) | 994 (48.6) | <0.001 |
| Wheezing, n (%) | 223 (11.7) | 124 (8.7) | 99 (4.8) | <0.001 |
| Rhonchi, n (%) | 406 (11.7) | 232 (16.3) | 174 (8.5) | <0.001 |
| Baseline O2 saturation, %, mean (SD) | 93.3 (5.5) | 94.9 (3.4) | 90.9 (6.9) | <0.001 |
| Baseline O2 saturation <93%, n (%) | 1598 (31.9) | 829 (58.2) | 554 (27.1) | <0.001 |
| Thoracic radiological findings | ||||
| Alveolar condensation, n (%) | <0.001 | |||
| Absent | 1782 (51.3) | 683 (47.9) | 1099 (53.7) | |
| Unilateral | 586 (16.9) | 215 (15.1) | 371 (18.1) | |
| Bilateral | 1103 (31.8) | 527 (37.0) | 576 (28.2) | |
| Interstitial infiltrate, n (%) | <0.001 | |||
| Absent | 1208 (34.8) | 497 (34.9) | 711 (34.8) | |
| Unilateral | 367 (10.6) | 111 (7.8) | 256 (12.5) | |
| Bilateral | 1896 (54.6) | 817 (57.3) | 1079 (52.7) | |
| Pleural effusion, n (%) | 0.001 | |||
| Absent | 3344 (96.3) | 1352 (94.9) | 1992 (97.4) | |
| Unilateral | 87 (2.5) | 50 (3.5) | 37 (1.8) | |
| Bilateral | 40 (1.2) | 23 (1.6) | 17 (0.8) | |
| Analytical findings | ||||
| Glucose, mg/dL, mean (SD) | 124.8 (49.9) | 136.7 (59.4) | 116.6 (40.1) | <0.001 |
| Creatinine, mg/dL, mean (SD) | 1.1 (0.83) | 1.28 (1.0) | 0.95 (0.62) | <0.001 |
| Sodium, mEq/L, mean (SD) | 137.6 (4.4) | 137.6 (5.1) | 137.6 (3.8) | 0.922 |
| Potassium, mEq/L, mean (SD) | 4.1 (0.5) | 4.2 (0.6) | 4.1 (0.5) | <0.001 |
| Hemoglobin, g/dL, mean (SD) | 13.8 (1.9) | 13.4 (2.0) | 14.0 (1.7) | <0.001 |
| Leukocytes’ 106/L, mean (SD) | 7074.0 (4668.6) | 7629.7 (4702.5) | 6687.0 (4606.5) | <0.001 |
| Lymphocytes’ 106/L, mean (SD) | 1080.5 (888.6) | 1001.6 (1119.2) | 1135.5 (678.4) | <0.001 |
| Neutrophils’ 106/L, mean (SD) | 5216.4 (3031.6) | 593 (3.46) | 400.7 (2.6) | <0.001 |
| Platelets’ 109/L, mean (SD) | 20,5182.7 (85,858.2) | 200.0 (91.6) | 208.7 (81.5) | 0.004 |
| CRP, mg/L, mean (SD) | 86.3 (84.2) | 108.9 (96.5) | 70.6 (70.3) | <0.001 |
| Albumin, g/dL, mean (SD) | 3.7 (0.5) | 3.5 (0.6) | 3.8 (0.5) | <0.001 |
| CRP/SA, mean (SD) | 24.4 (25.1) | 31.9 (29.2) | 19.2 (20.2) | <0.001 |
| Variable | Odds Ratio (95% CI) * | p |
|---|---|---|
| Age | 1.015 (1.004–1.026) | 0.008 |
| Baseline SA | 0.674 (0.551–0.826) | <0.001 |
| CRP | 1.002 (1.001–1.004) | 0.003 |
| Community origin | 2.469 (1.654–3.685) | <0.001 |
| Dyspnea | 1.273 (1.034–1.568) | 0.023 |
| Confusion | 1.843 (1.278–2.659) | 0.001 |
| Tachypnea | 1.591 (1.272–1.991) | <0.001 |
| Baseline systemic corticosteroids | 2.288 (1.441–3.632) | <0.001 |
| Days with symptoms | 0.939 (0.917–0.961) | <0.001 |
| Age-adjusted Charlson score | 1.100 (1.029–1.175) | 0.005 |
| Temperature | 1.279 (1.152–1.420) | <0.001 |
| Baseline 02 saturation | 0.889 (0.866–0.912) | <0.001 |
| Platelet count | 0.997 (0.996–0.998) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giner-Galvañ, V.; Pomares-Gómez, F.J.; Quesada, J.A.; Rubio-Rivas, M.; Tejada-Montes, J.; Baltasar-Corral, J.; Taboada-Martínez, M.L.; Sánchez-Mesa, B.; Arnalich-Fernández, F.; Del Corral-Beamonte, E.; et al. C-Reactive Protein and Serum Albumin Ratio: A Feasible Prognostic Marker in Hospitalized Patients with COVID-19. Biomedicines 2022, 10, 1393. https://doi.org/10.3390/biomedicines10061393
Giner-Galvañ V, Pomares-Gómez FJ, Quesada JA, Rubio-Rivas M, Tejada-Montes J, Baltasar-Corral J, Taboada-Martínez ML, Sánchez-Mesa B, Arnalich-Fernández F, Del Corral-Beamonte E, et al. C-Reactive Protein and Serum Albumin Ratio: A Feasible Prognostic Marker in Hospitalized Patients with COVID-19. Biomedicines. 2022; 10(6):1393. https://doi.org/10.3390/biomedicines10061393
Chicago/Turabian StyleGiner-Galvañ, Vicente, Francisco José Pomares-Gómez, José Antonio Quesada, Manuel Rubio-Rivas, Javier Tejada-Montes, Jesús Baltasar-Corral, María Luisa Taboada-Martínez, Blanca Sánchez-Mesa, Francisco Arnalich-Fernández, Esther Del Corral-Beamonte, and et al. 2022. "C-Reactive Protein and Serum Albumin Ratio: A Feasible Prognostic Marker in Hospitalized Patients with COVID-19" Biomedicines 10, no. 6: 1393. https://doi.org/10.3390/biomedicines10061393







