BPC 157, L-NAME, L-Arginine, NO-Relation, in the Suited Rat Ketamine Models Resembling “Negative-Like” Symptoms of Schizophrenia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Drugs
2.3. Novel Object Recognition Test
2.4. Social Interaction Test
2.5. Open Field Test
2.6. Anhedonia (Sucrose Preference Test)
2.7. Gene Expression Analysis
2.8. Statistical Analysis
3. Results
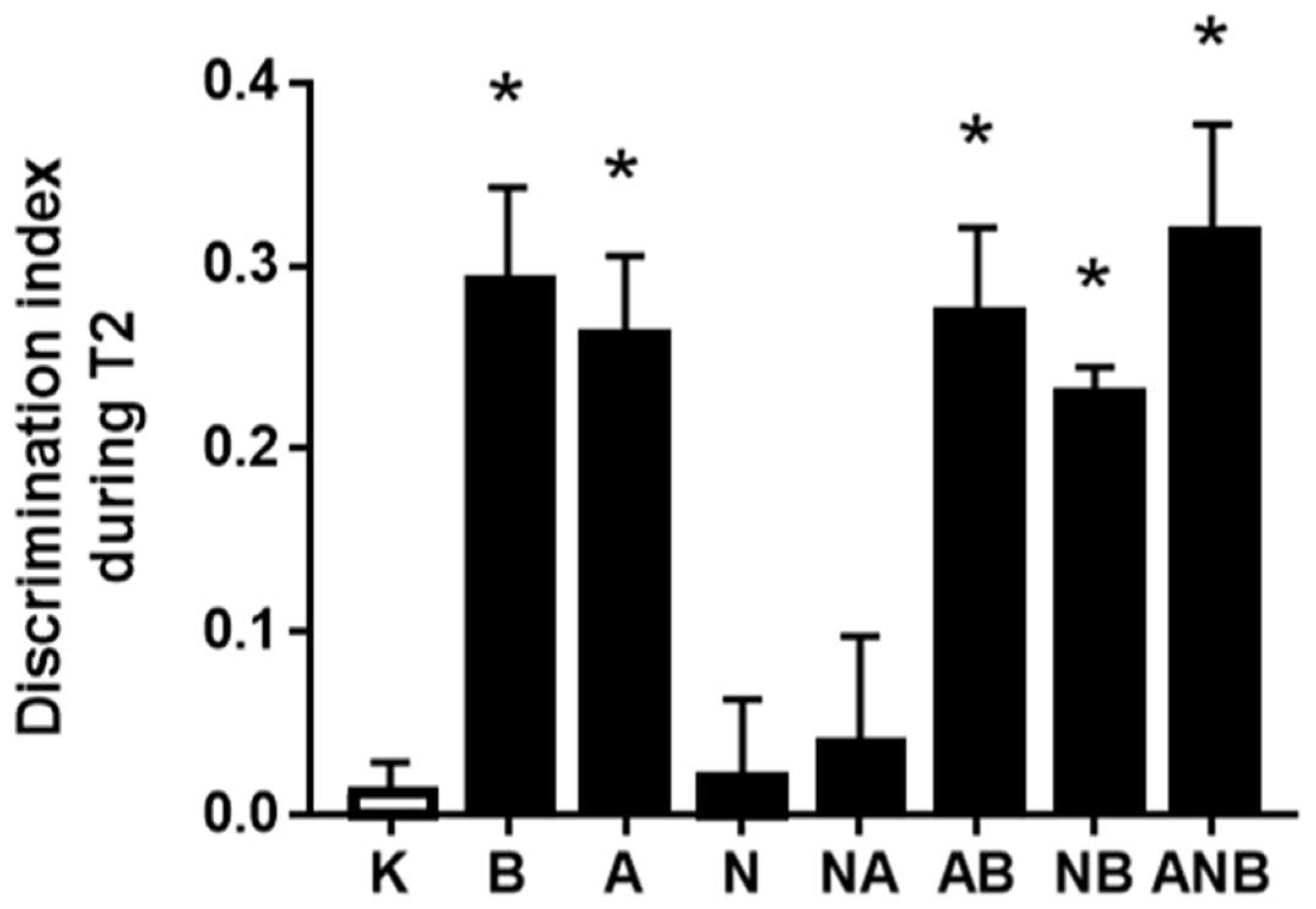
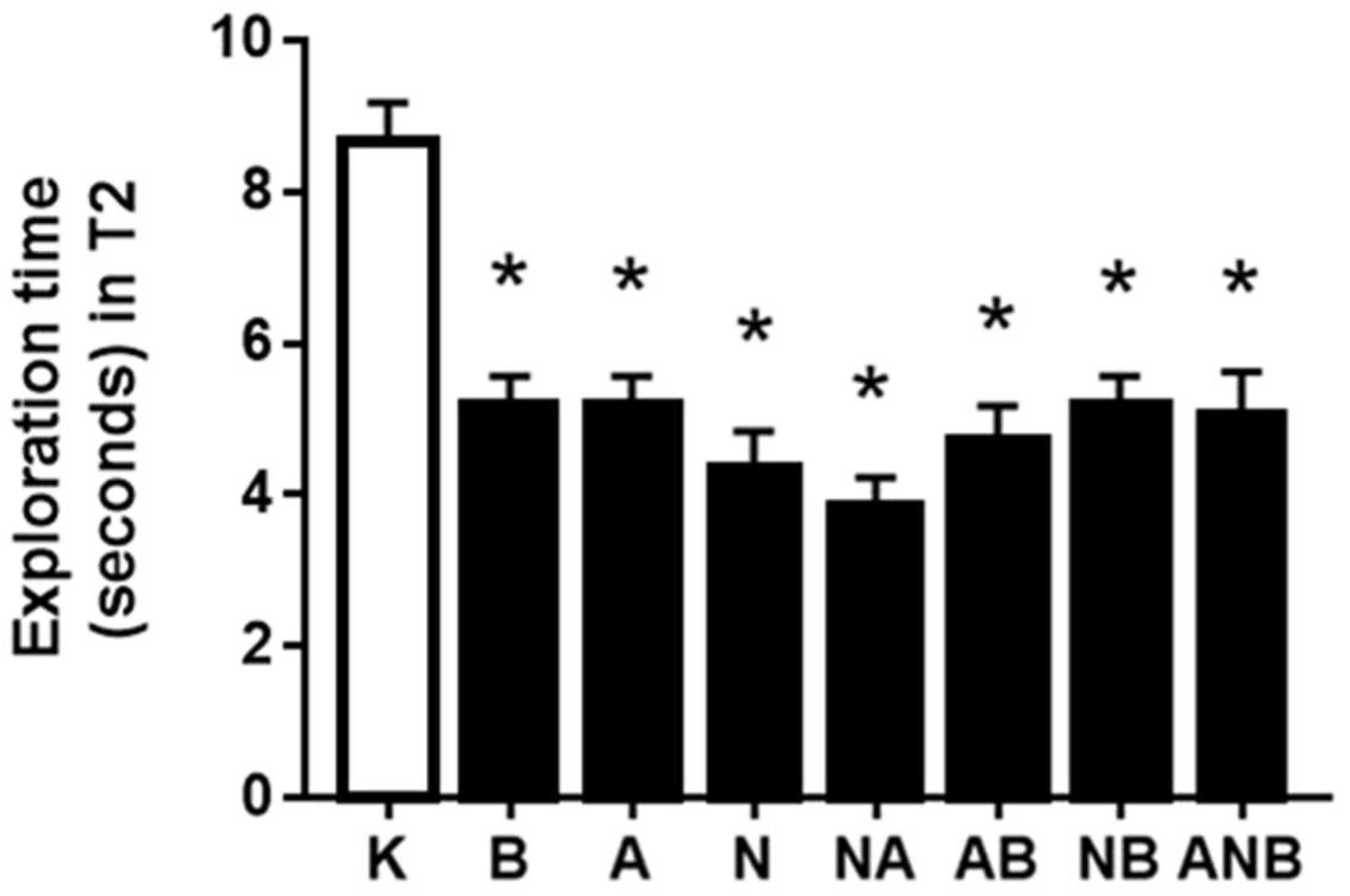
3.1. Novel Object Recognition Test, Cognitive Dysfunction
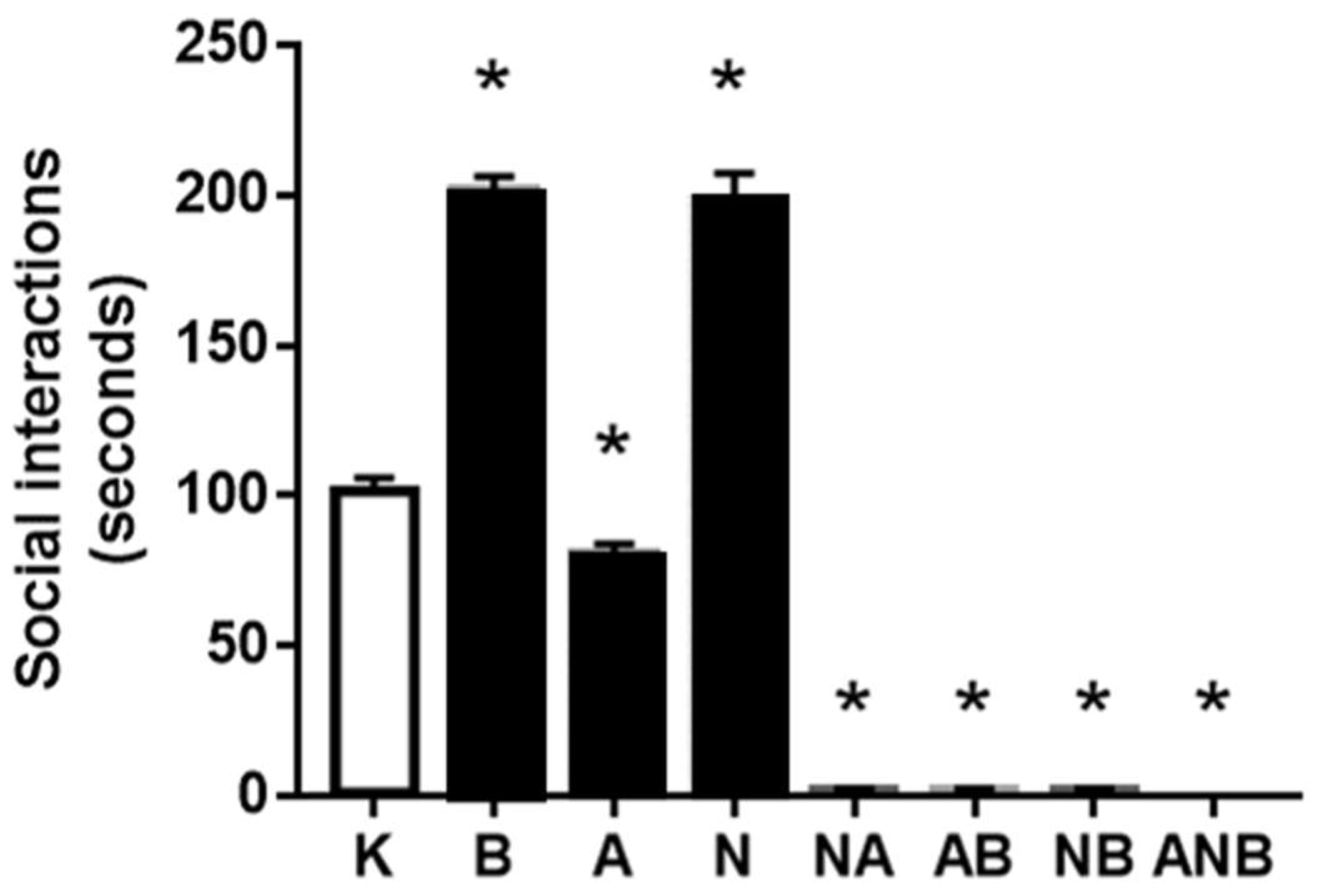
3.2. Social Interaction Test
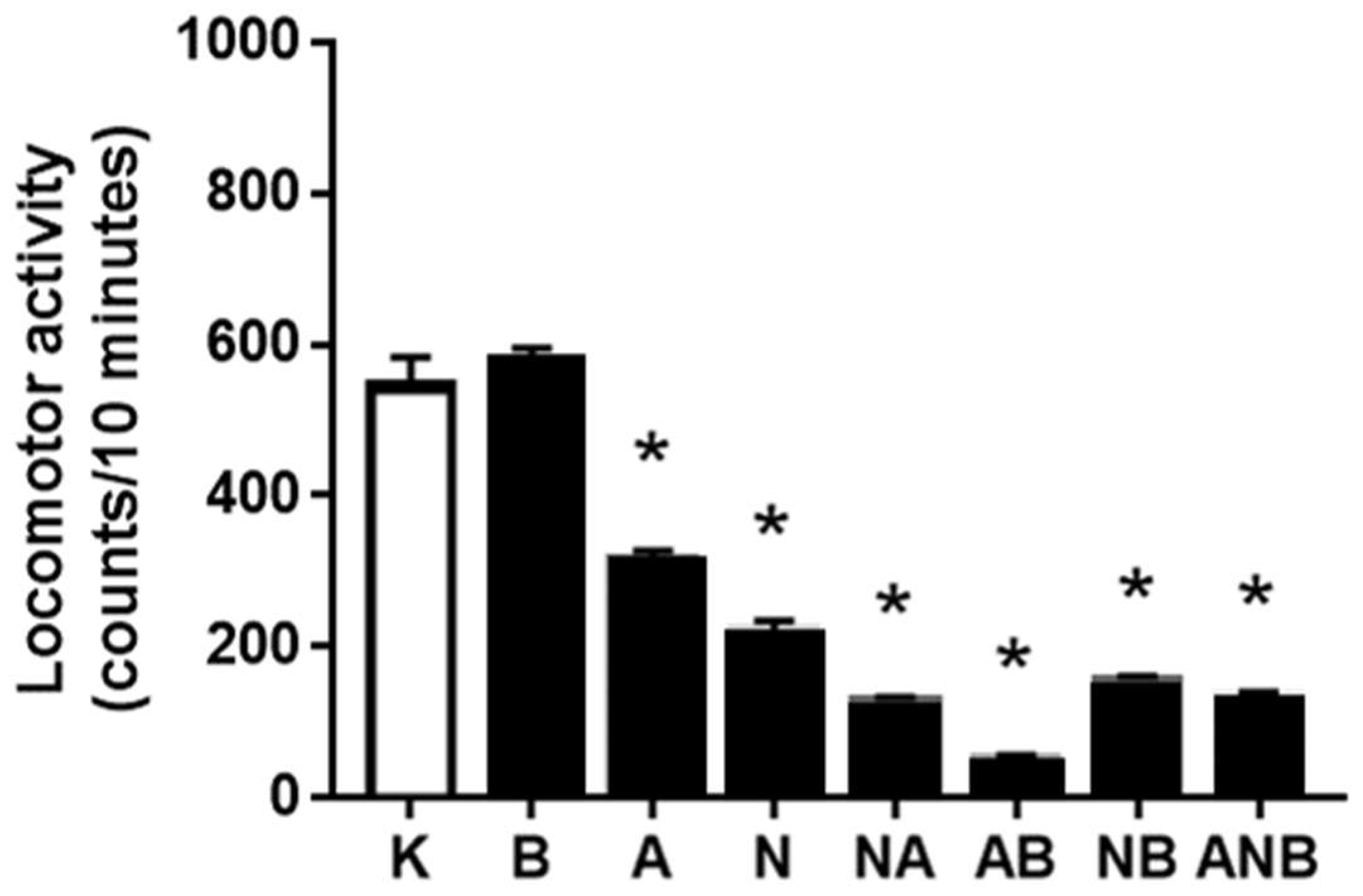
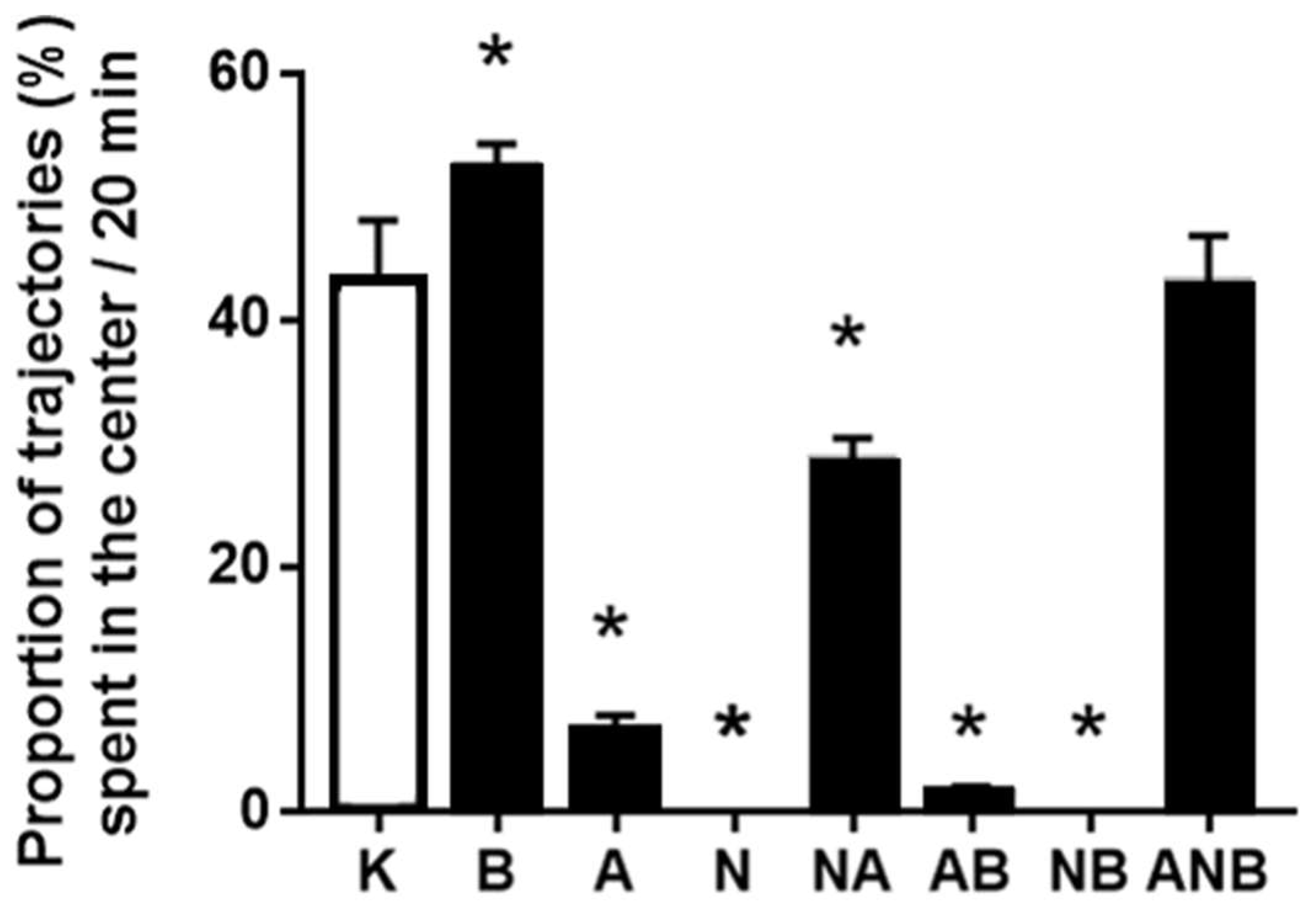
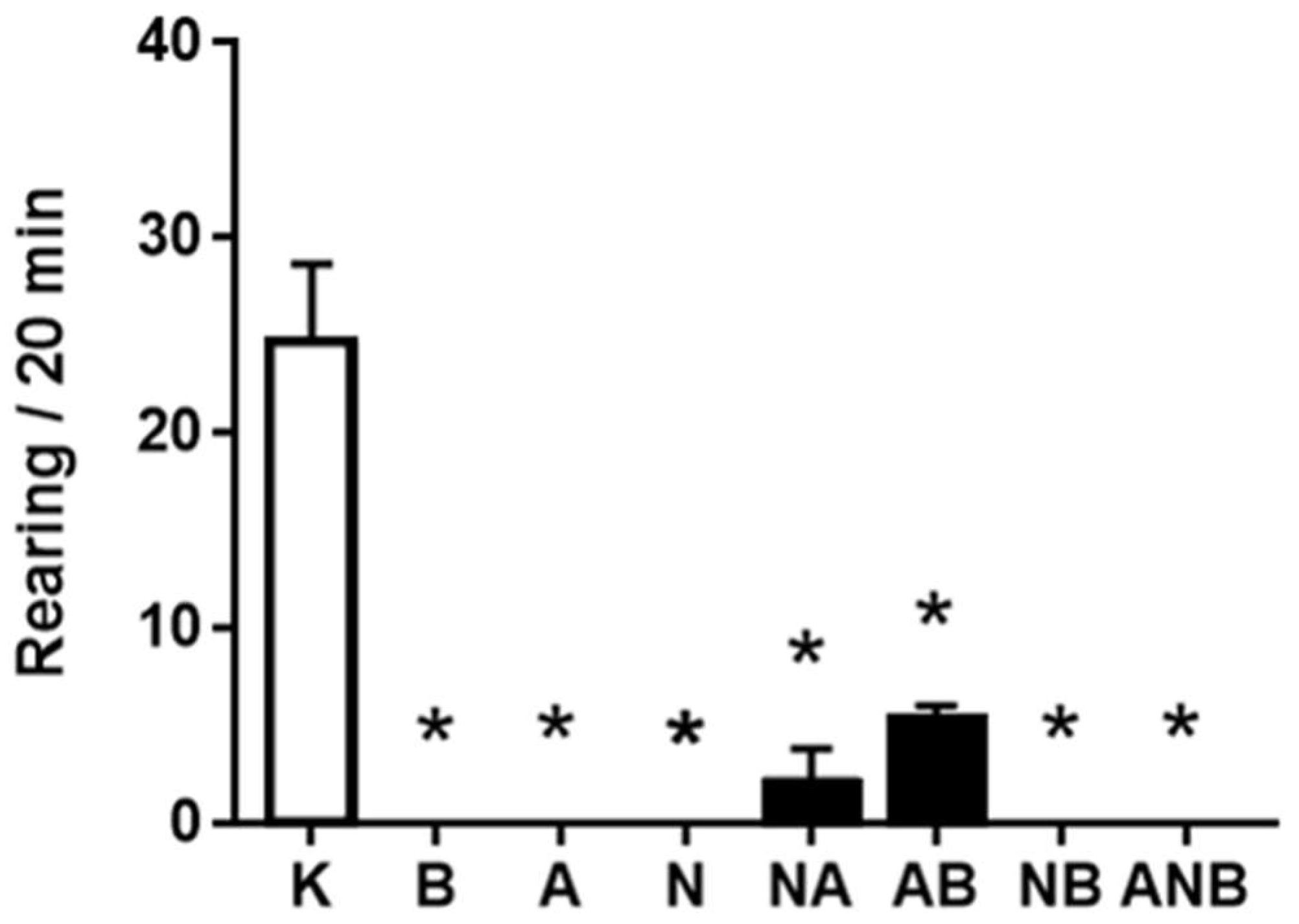
3.3. Open Field Test
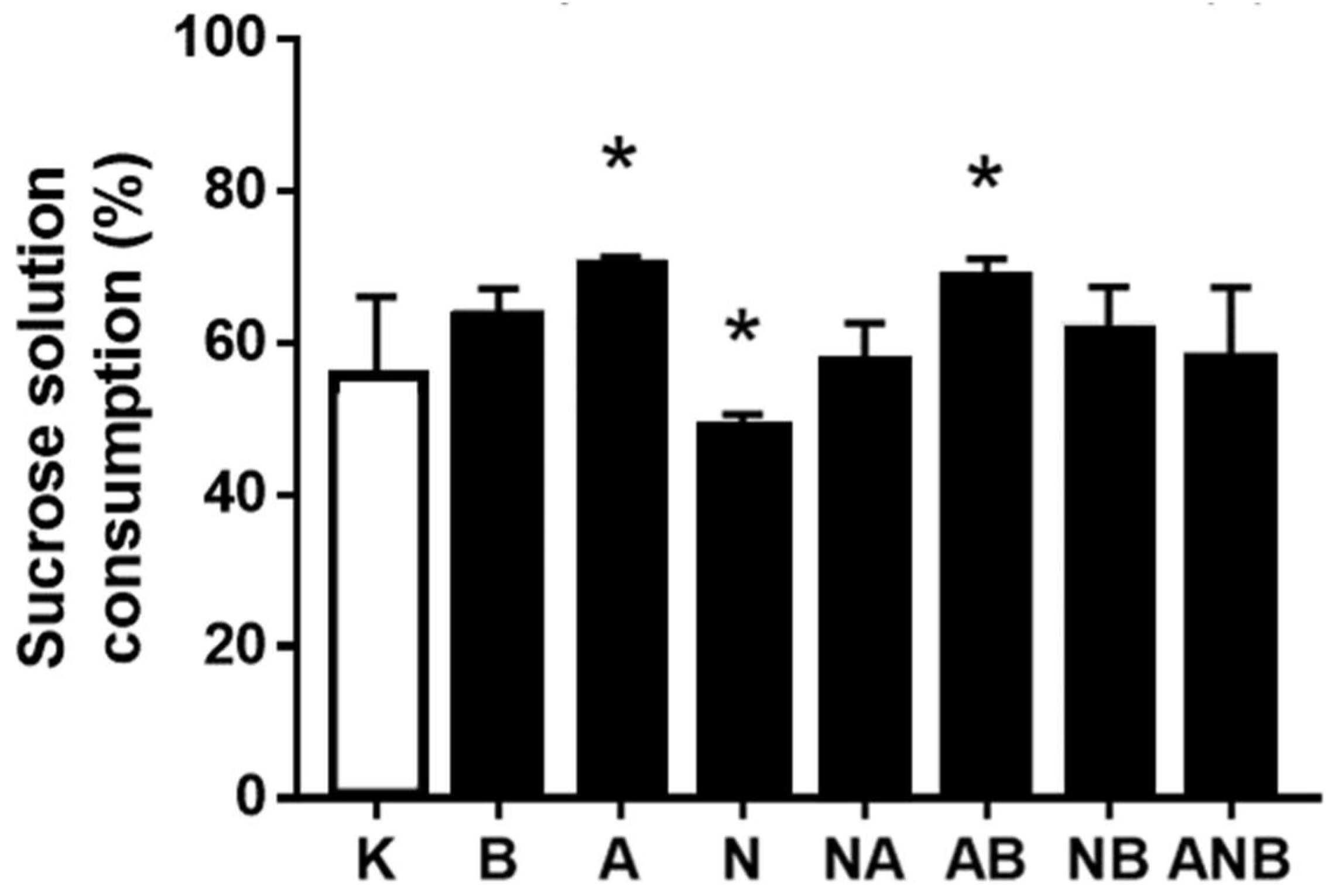
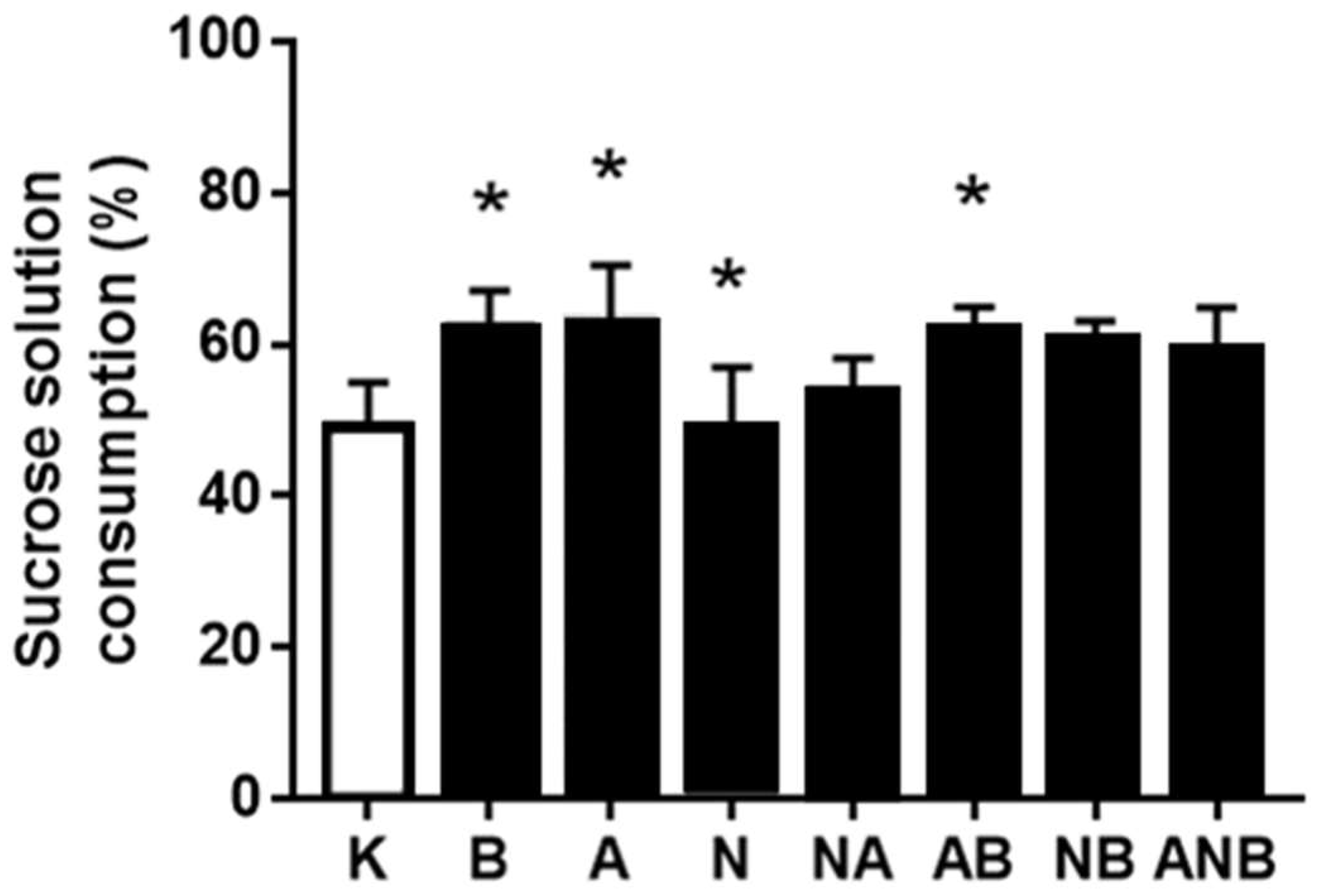
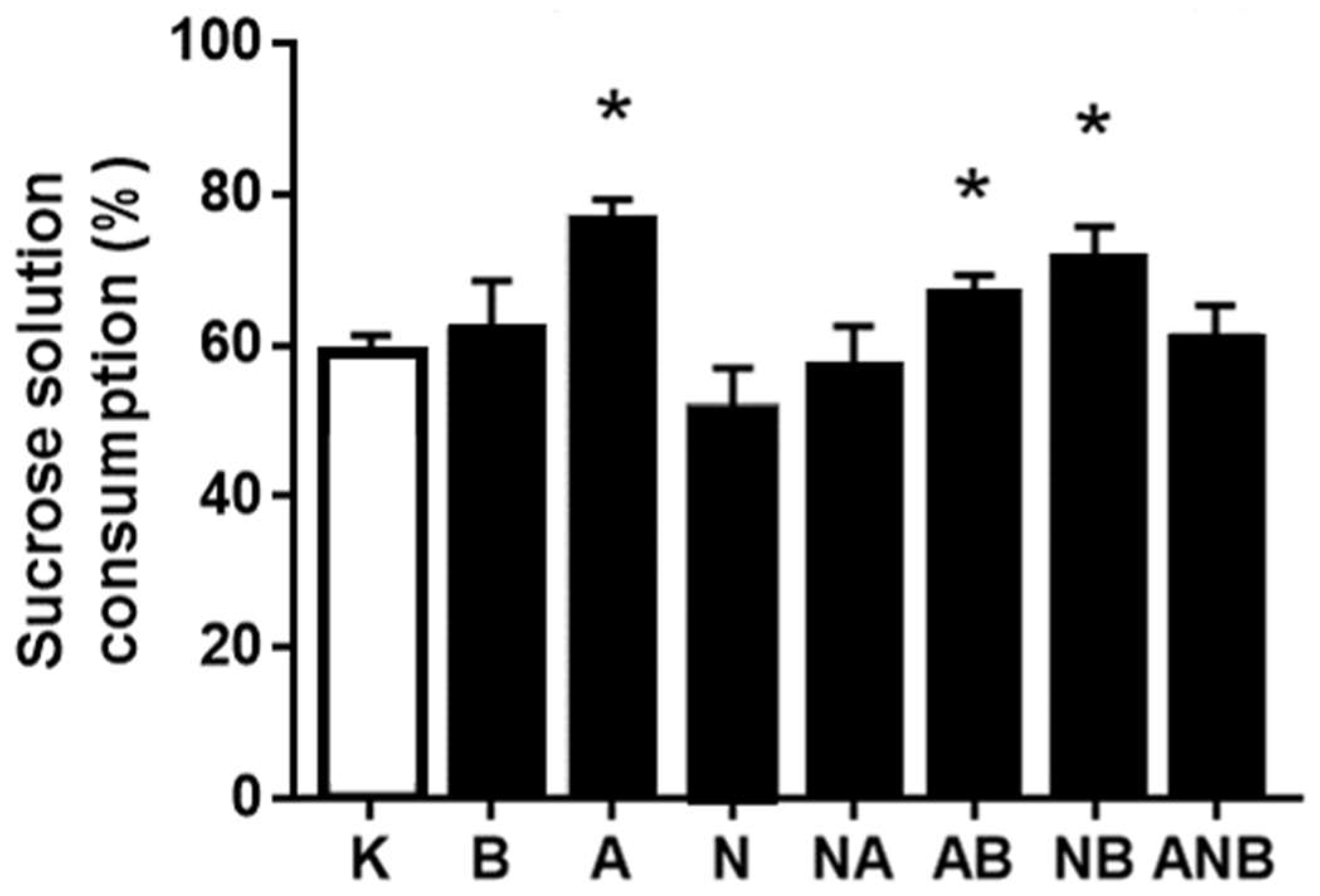
3.4. Anhedonia
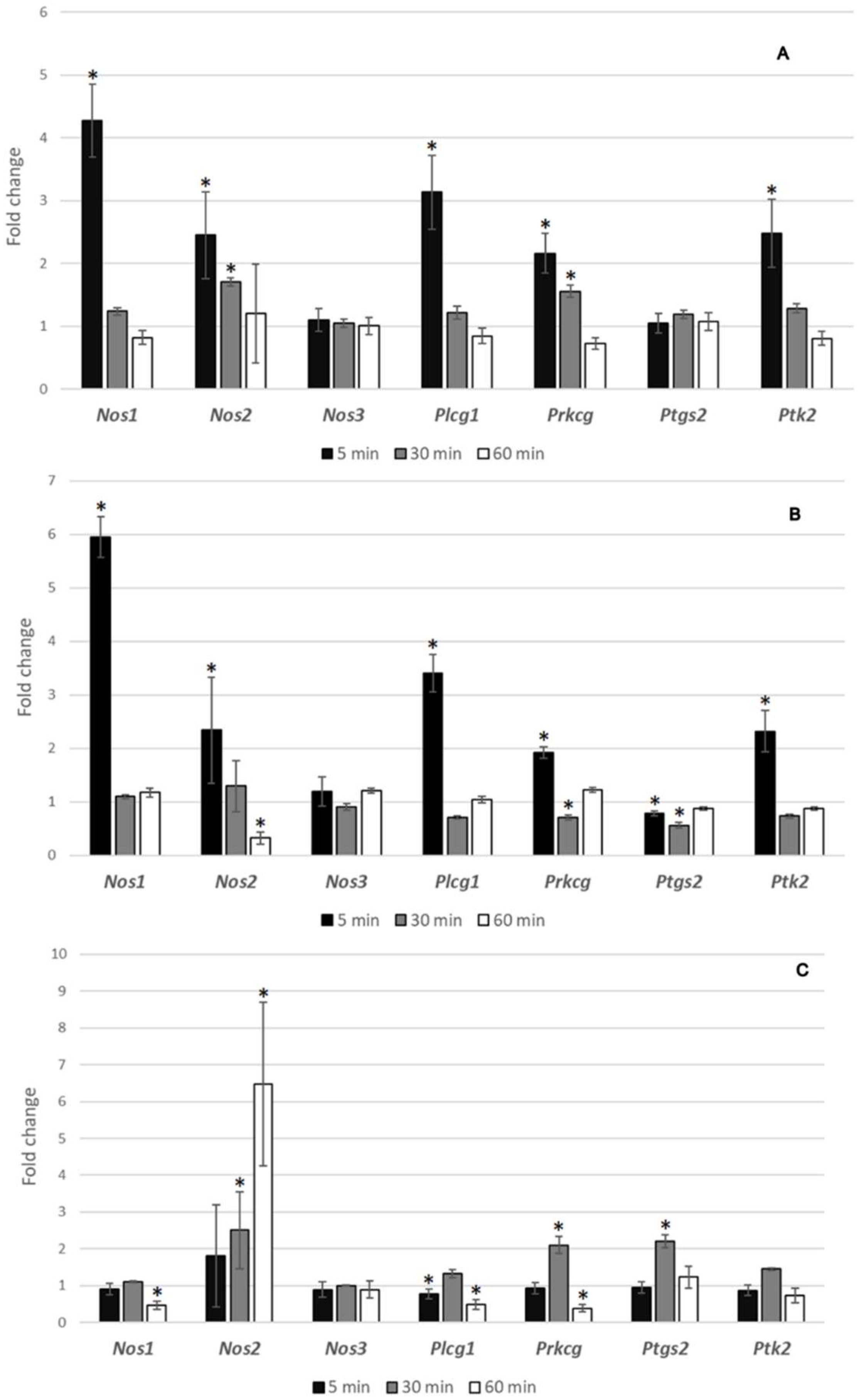
3.5. Gene Expression Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oh, S.J.; Fan, X. Current understanding on the role of nitric oxide and therapeutic potential of NO supplementation in schizophrenia. Schizophr. Res. 2020, 222, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, H.G.; Keilhoff, G.; Steiner, J.; Dobrowolny, H.; Bogerts, B. Nitric oxide and schizophrenia: Present knowledge and emerging concepts of therapy. CNS Neurol. Disord. Drug Targets 2011, 10, 792–807. [Google Scholar] [CrossRef] [PubMed]
- Pitsikas, N. The role of nitric oxide synthase inhibitors in schizophrenia. Curr. Med. Chem. 2016, 201, 2692–2705. [Google Scholar] [CrossRef] [PubMed]
- Pitsikas, N. The role of nitric oxide donors in schizophrenia: Basic studies and clinical applications. Eur. J. Pharmacol. 2015, 766, 106–113. [Google Scholar] [CrossRef]
- Correll, C.U.; Schooler, N.R. Negative symptoms in schizophrenia: A review and clinical guide for recognition, assessment, and treatment. Neuropsychiatr. Dis. Treat. 2020, 16, 519–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krystal, J.H.; Karper, L.P.; Seibyl, J.P.; Freeman, G.K.; Delaney, R.; Bremner, J.D.; Heninger, G.R.; Bowers, M.B., Jr.; Charney, D.S. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans: Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch. Gen. Psychiatry 1994, 51, 199–214. [Google Scholar] [CrossRef]
- Sikiric, P.; Skrtic, A.; Gojkovic, S.; Krezic, I.; Zizek, H.; Lovric, E.; Sikiric, S.; Knezevic, M.; Strbe, S.; Milavic, M.; et al. Gastric pentadecapeptide BPC 157 in cytoprotection to resolve major vessel occlusion disturbances, Pringle maneuver and Budd Chiari syndrome. World J. Gastroenterol. 2022, 28, 23–46. [Google Scholar] [CrossRef]
- Zemba Cilic, A.; Zemba, M.; Cilic, M.; Balenovic, I.; Strbe, S.; Ilic, S.; Vukojevic, J.; Zoricic, Z.; Filipcic, I.; Kokot, A.; et al. Pentadecapeptide BPC 157 counteracts L-NAME-induced catalepsy. BPC 157, L-NAME, L-arginine, NO-relation, in the suited rat acute and chronic models resembling ’positive-like’ symptoms of schizophrenia. Behav. Brain Res. 2021, 396, 112919. [Google Scholar] [CrossRef]
- Vukojevic, J.; Milavic, M.; Perovic, D.; Ilic, S.; Cilic, A.Z.; Duran, N.; Strbe, S.; Zoricic, Z.; Filipcic, I.; Brecic, P.; et al. Pentadecapeptide BPC 157 and the central nervous system. Neural Regen. Res. 2022, 17, 482–487. [Google Scholar] [CrossRef]
- Sikiric, P.; Seiwerth, S.; Rucman, R.; Kolenc, D.; Vuletic, L.B.; Drmic, D.; Grgic, T.; Strbe, S.; Zukanovic, G.; Crvenkovic, D.; et al. Brain-gut axis and pentadecapeptide BPC 157: Theoretical and practical implications. Curr. Neuropharmacol. 2016, 14, 857–865. [Google Scholar] [CrossRef]
- Sikiric, P.; Seiwerth, S.; Rucman, R.; Turkovic, B.; Rokotov, D.S.; Brcic, L.; Sever, M.; Klicek, R.; Radic, B.; Drmic, D.; et al. Stable gastric pentadecapeptide BPC 157-NO-system relation. Curr. Pharm. Des. 2014, 20, 1126–1135. [Google Scholar] [CrossRef] [PubMed]
- Boultadakis, A.; Georgiadou, G.; Pitsikas, N. Effects of the nitric oxide synthase inhibitor L-NAME on different memory components as assessed in the object recognition task in the rat. Behav. Brain Res. 2010, 20, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Pitsikas, N.; Boultadakis, A.; Sakellaridis, N. Effects of sub-anesthetic doses of ketamine on rats’ spatial and non-spatial recognition memory. Neuroscience 2008, 154, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Trevlopoulou, A.; Touzlatzi, N.; Pitsikas, N. The nitric oxide donor sodium nitroprusside attenuates recogniton memory deficits and social withdrawal produced by the NMDA receptor antagonist ketamine and induces anxiolytic-like behaviour in rats. Psychopharmacology 2016, 233, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Koros, E.; Rosenbrock, H.; Birk, G. The selective mGlu5 receptor antagonist MTEP, similar to NMDA receptor antagonists, induces social isolation in rats. Neuropsychopharmacology 2007, 32, 562–576. [Google Scholar] [CrossRef] [Green Version]
- Zoupa, E.; Gravanis, A.; Pitsikas, N. The novel dehydroepiandrosterone (DHEA) derivative BNN27 counteracts behavioural deficits induced by the NMDA receptor antagonist ketamine in rats. Neuropharmacology 2019, 151, 74–83. [Google Scholar] [CrossRef]
- Willner, P. Chronic mild stress (CMS) revisited: Consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology 2005, 52, 90–110. [Google Scholar] [CrossRef]
- Kandratavicius, L.; Balista, P.A.; Wolf, D.C.; Abrao, J.; Evora, P.R.; Rodrigues, A.J.; Chaves, C.; Maia-de-Oliveira, J.P.; Leite, J.P.; Dursun, S.M.; et al. Effects of nitric oxide-related compounds in the acute ketamine animal model of schizophrenia. BMC Neurosci. 2015, 16, 9. [Google Scholar] [CrossRef] [Green Version]
- Mazarati, A.; Shin, D.; Auvin, S. Kindling epileptogenesis in immature rats leads to persistent depressive behavior. Epilepsy Behav. 2007, 10, 377–383. [Google Scholar] [CrossRef] [Green Version]
- Grivas, V.; Markou, A.; Pitsikas, N. The metabotropic glutamate 2/3 receptor agonist LY379268 induces anxiety-like behavior at the highest dose tested in two rat models of anxiety. Eur. J. Pharmacol. 2013, 715, 105–110. [Google Scholar] [CrossRef] [Green Version]
- Bath, K.G.; Chuang, J.; Spencer-Segal, J.L.; Amso, D.; Altemus, M.; McEwen, B.S.; Lee, F.S. Variant brain-derived neurotrophic factor (Valine66Methionine) polymorphism contributes to developmental and estrous stage-specific expression of anxiety-like behavior in female mice. Biol. Psychiatry 2012, 72, 499–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, M.J.; Lee, C.H.; Chueh, H.Y.; Chang, G.J.; Huang, H.Y.; Lin, Y.; Pang, J.S. Modulatory effects of BPC 157 on vasomotor tone and the activation of Src-Caveolin-1-endothelial nitric oxide synthase pathway. Sci. Rep. 2020, 10, 17078. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.J.; Liu, H.T.; Wang, C.N.; Huang, H.Y.; Lin, Y.; Ko, Y.S.; Wang, J.S.; Chang, V.H.; Pang, J.S. Therapeutic potential of pro-angiogenic BPC157 is associated with VEGFR2 activation and up-regulation. J. Mol. Med. 2017, 95, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Tohyama, Y.; Sikirić, P.; Diksic, M. Effects of pentadecapeptide BPC157 on regional serotonin synthesis in the rat brain: Alpha-methyl-L-tryptophan autoradiographic measurements. Life Sci. 2004, 76, 345–357. [Google Scholar] [CrossRef]
- Joca, S.R.L.; Sartim, A.G.; Roncalho, A.L.; Diniz, C.F.A.; Wegener, G. Nitric oxide signalling and antidepressant action revisited. Cell Tissue Res. 2019, 377, 45–58. [Google Scholar] [CrossRef] [Green Version]
- Rahimi, N.; Modabberi, S.; Faghir-Ghanesefat, H.; Shayan, M.; Farzad Maroufi, S.; Asgari Dafe, E.; Reza Dehpour, A. The possible role of nitric oxide signaling and NMDA receptors in allopurinol effect on maximal electroshock- and pentylenetetrazol-induced seizures in mice. Neurosci. Lett. 2022, 778, 136620. [Google Scholar] [CrossRef]
- Baskin, V.; Eroglu, E.; Harmanci, N.; Erol, K. Antinociceptive, anxiolytic, and depression-like effects of hydrogen sulfide, nitric oxide, and carbon monoxide in rats and the role of opioidergic and serotonergic systems in antinociceptive activity. Fundam. Clin. Pharmacol. 2022. [Google Scholar] [CrossRef]
- Zarrindast, M.R.; Fazli-Tabaei, S.; Khakpai, F. Synergistic effect between quinpirole and L-NAME as well as sulpiride and L-arginine on the modulation of anxiety and memory processes in the 6-OHDA mouse model of Parkinson’s disease: An isobologram analysis. Neurobiol. Learn. Mem. 2021, 186, 107538. [Google Scholar] [CrossRef]
- Celik, T.; Zaglí, U.; Kayír, H.; Uzbay, I.T. Nitric oxide synthase inhibition blocks amphetamine-induced locomotor activity in mice. Drug Alcohol. Depend. 1999, 56, 109–113. [Google Scholar] [CrossRef]
- Przewlocka, B.; Turchan, J.; Machelska, H.; Labuz, D.; Lason, W. Nitric oxide synthase inhibitor L-NAME prevents amphetamine-induced prodynorphin gene expression in the rat. Prog. Neuropsychopharmacol. Biol. Psychiatry 1996, 20, 1229–1237. [Google Scholar] [CrossRef]
- Koçyigit, Y.; Yoca, G.; Karahan, S.; Ayhan, Y.; Yazıcı, M.R. L-arginine add-on treatment for schizophrenia: A randomized, double-blind, placebo-controlled, crossover study. Turk Psikiyatri Derg. 2018, 29, 147–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, M.; Balakrishnan, S.; Pandhi, P. Role of nitric oxide in experimental models of psychosis in rats. Methods Find. Exp. Clin. Pharmacol. 2001, 23, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Vukojevic, J.; Vrdoljak, B.; Malekinusic, D.; Siroglavic, M.; Milavic, M.; Kolenc, D.; Boban Blagaic, A.; Bateljam, L.; Drmic, D.; Seiwerth, S.; et al. The effect of pentadecapeptide BPC 157 on hippocampal ischemia/reperfusion injuries in rats. Brain Behav. 2020, 10, e01726. [Google Scholar] [CrossRef]
- McNeill, R.V.; Kerhwald, C.; Brum, M.; Knopf, K.; Brunkhorst-Kanaan, N.; Etyemez, S.; Koreny, C.; Bittner, R.A.; Freudenberg, F.; Herterich, S.; et al. Uncovering associations between mental illness diagnosis, nitric oxide synthase gene variation, and peripheral nitric oxide concentration. Brain Behav. Immun. 2022, 101, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Freudenberg, F.; Alttoa, A.; Reif, A. Neuronal nitric oxide synthase (NOS1) and its adaptor, NOS1AP, as a genetic risk factors for psychiatric disorders. Genes Brain Behav. 2015, 14, 46–63. [Google Scholar] [CrossRef] [PubMed]
- Esshili, A.; Manitz, M.P.; Freund, N.; Juckel, G. Induction of inducible nitric oxide synthase expression in activated microglia and astrocytes following pre- and postnatal immune challenge in an animal model of schizophrenia. Eur. Neuropsychopharmacol. 2020, 35, 100–110. [Google Scholar] [CrossRef]
- Ribeiro, B.M.; do Carmo, M.R.; Freire, R.S.; Rocha, N.F.; Borella, V.C.; de Menezes, A.T.; Monte, A.S.; Gomes, P.X.; de Sousa, F.C.; Vale, M.L.; et al. Evidences for a progressive microglial activation and increase in iNOS expression in rats submitted to a neurodevelopmental model of schizophrenia: Reversal by clozapine. Schizophr. Res. 2013, 151, 12–19. [Google Scholar] [CrossRef]
- Liou, Y.J.; Lai, I.C.; Lin, M.W.; Bai, Y.M.; Lin, C.C.; Liao, D.L.; Chen, J.Y.; Lin, C.Y.; Wang, Y.C. Haplotype analysis of endothelial nitric oxide synthase (NOS3) genetic variants and tardive dyskinesia in patients with schizophrenia. Pharmacogenet. Genom. 2006, 16, 151–157. [Google Scholar] [CrossRef]
- Watanave, M.; Takahashi, N.; Hosoi, N.; Konno, A.; Yamamoto, H.; Yasui, H.; Kawachi, M.; Horii, T.; Matsuzaki, Y.; Hatada, I.; et al. Protein kinase Cgamma in cerebellar Purkinje cells regulates Ca2+-activated large-conductance K+ channels and motor coordination. Proc. Natl. Acad. Sci. USA 2022, 119, e2113336119. [Google Scholar] [CrossRef]
- Gomis-González, M.; Galera-López, L.; Ten-Blanco, M.; Busquets-Garcia, A.; Cox, T.; Maldonado, R.; Ozaita, A. Protein kinase C-gamma knockout mice show impaired hippocampal short-term memory while preserved long-term memory. Mol. Neurobiol. 2021, 58, 617–630. [Google Scholar] [CrossRef]
- Zhang, F.; Fang, Z.; Wang, J.B. Hint1 knockout results in a compromised activation of protein kinase C gamma in the brain. Brain Res. 2015, 1622, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Martins-de-Souza, D.; Maccarrone, G.; Wobrock, T.; Zerr, I.; Gormanns, P.; Reckow, S.; Falkai, P.; Schmitt, A.; Turck, C.W. Proteome analysis of the thalamus and cerebrospinal fluid reveals glycolysis dysfunction and potential biomarkers candidates for schizophrenia. J. Psychiatr. Res. 2010, 44, 1176–1189. [Google Scholar] [CrossRef] [PubMed]
- Strekalova, T.; Pavlov, D.; Trofimov, A.; Anthony, D.C.; Svistunov, A.; Proshin, A.; Umriukhin, A.; Lyundup, A.; Lesch, K.P.; Cespuglio, R. Hippocampal over-expression of cyclooxygenase-2 (COX-2) is associated with susceptibility to stress-induced anhedonia in mice. Int. J. Mol. Sci. 2022, 23, 2061. [Google Scholar] [CrossRef]
- Muntané, G.; Chillida, M.; Aranda, S.; Navarro, A.; Vilella, E. Coexpression of the discoidin domain receptor 1 gene with oligodendrocyte-related and schizophrenia risk genes in the developing and adult human brain. Brain Behav. 2021, 11, e2309. [Google Scholar] [CrossRef]
- Qin, Z.; Zhang, L.; Cruz, S.A.; Stewart, A.F.R.; Chen, H.H. Activation of tyrosine phosphatase PTP1B in pyramidal neurons impairs endocannabinoid signaling by tyrosine receptor kinase trkB and causes schizophrenia-like behaviors in mice. Neuropsychopharmacology 2020, 45, 1884–1895. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.J.; Machin, D. Medical Statistics, Summary Approach, 3rd ed.; Wiley: Chichester, UK, 1999. [Google Scholar]
- Garthwaite, J. Concepts of neural nitric oxide-mediated transmission. Eur. J. Neurosci. 2008, 27, 2783–2802. [Google Scholar] [CrossRef] [Green Version]
- Amaghnouje, A.; Bohza, S.; Bohdan, N.; Es-Safi, I.; Kyrylchuk, A.; Achour, S.; El Fatemi, H.; Bousta, D.; Grafov, A. New 2,3-benzodiazepine derivative: Synthesis, activity on central nervous system, and toxicity study in mice. Pharmaceuticals 2021, 14, 814. [Google Scholar] [CrossRef] [PubMed]
- Matveychuk, D.; Thomas, R.K.; Swainson, J.; Khullar, A.; MacKay, M.A.; Baker, G.B.; Dursun, S.M. Ketamine as an antidepressant: Overview of its mechanisms of action and potential predictive biomarkers. Ther. Adv. Psychopharmacol. 2020, 10, 2045125320916657. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, J.A.; Stroup, T.S.; McEvoy, J.P.; Swartz, M.S.; Rosenheck, R.A.; Perkins, D.O.; Keefe, R.S.; Davis, S.M.; Davis, C.E.; Lebowitz, B.D.; et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. N. Engl. J. Med. 2005, 353, 1209–1223. [Google Scholar] [CrossRef] [Green Version]
- Németh, G.; Laszlovszky, I.; Czobor, P.; Szalai, E.; Szatmári, B.; Harsányi, J.; Barabássy, A.; Debelle, M.; Durgam, S.; Bitter, I.; et al. Cariprazine versus risperidone monotherapy for treatment of predominant negative symptoms in patients with schizophrenia: A randomised, double-blind, controlled trial. Lancet 2017, 389, 1103–1113. [Google Scholar] [CrossRef]
- Krause, M.; Zhu, Y.; Huhn, M.; Schneider-Thoma, J.; Bighelli, I.; Nikolakopoulou, A.; Leucht, S. Antipsychotic drugs for patients with schizophrenia and predominant or prominent negative symptoms: A systematic review and meta-analysis. Eur. Arch. Psychiatry Clin. Neurosci. 2018, 268, 625–639. [Google Scholar] [CrossRef] [PubMed]
- Oyamada, Y.; Horiguchi, M.; Rajagopal, L.; Miyauchi, M.; Meltzer, H.Y. Combined serotonin (5-HT)1A agonism, 5-HT(2A) and dopamine D2 receptor antagonism reproduces atypical antipsychotic drug effects on phencyclidine-impaired novel object recognition in rats. Behav. Brain Res. 2015, 285, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Meftah, A.; Hasegawa, H.; Kantrowitz, J.T. D-Serine: A cross species review of safety. Front. Psychiatry 2021, 12, 726365. [Google Scholar] [CrossRef] [PubMed]
- Galling, B.; Vernon, J.A.; Pagsberg, A.K.; Wadhwa, A.; Grudnikoff, E.; Seidman, A.J.; Tsoy-Podosenin, M.; Poyurovsky, M.; Kane, J.M.; Correll, C.U. Efficacy and safety of antidepressant augmentation of continued antipsychotic treatment in patients with schizophrenia. Acta Psychiatr. Scand. 2018, 137, 187–205. [Google Scholar] [CrossRef]
- Lasser, R.A.; Dirks, B.; Nasrallah, H.; Kirsch, C.; Gao, J.; Pucci, M.L.; Knesevich, M.A.; Lindenmayer, J.P. Adjunctive lisdexamfetamine dimesylate therapy in adult outpatients with predominant negative symptoms of schizophrenia: Open-label and randomized-withdrawal phases. Neuropsychopharmacology 2013, 38, 2140–2149. [Google Scholar] [CrossRef] [Green Version]
- Millan, M.J.; Fone, K.; Steckler, T.; Horan, W.P. Negative symptoms of schizophrenia: Clinical characteristics, pathophysiological substrates, experimental models and prospects for improved treatment. Eur. Neuropsychopharmacol. 2014, 24, 645–692. [Google Scholar] [CrossRef] [Green Version]
- Tsapakis, E.M.; Dimopoulou, T.; Tarazi, F.I. Clinical management of negative symptoms of schizophrenia: An update. Pharmacol. Ther. 2015, 153, 135–147. [Google Scholar] [CrossRef]
- Brannan, S.K.; Sawchak, S.; Miller, A.C.; Lieberman, J.A.; Paul, S.M.; Breier, A. Muscarinic cholinergic receptor agonist and peripheral antagonist for schizophrenia. N. Engl. J. Med. 2021, 384, 717–726. [Google Scholar] [CrossRef]
- Rezaei, F.; Mesgarpour, B.; Jeddian, A.; Zeionoddini, A.; Mohammadinejad, P.; Salardini, E.; Shahriari, M.; Zeinoddini, A.; Akhondzadeh, S. Cilostazol adjunctive therapy in treatment of negative symptoms in chronic schizophrenia: Randomized, double-blind, placebo-controlled study. Hum. Psychopharmacol. 2017, 32, e2583. [Google Scholar] [CrossRef]
- Tajik-Esmaeeli, S.; Moazen-Zadeh, E.; Abbasi, N.; Shariat, S.V.; Rezaei, F.; Salehi, B.; Akhondzadeh, S. Simvastatin adjunct therapy for negative symptoms of schizophrenia: A randomized double-blind placebo-controlled trial. Int. Clin. Psychopharmacol. 2017, 32, 87–94. [Google Scholar] [CrossRef]
- Akhtar, A. The flaws and human harms of animal experimentation. Camb. Q. Healthc. Ethics 2015, 24, 407–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.M.; Lee, H.J.; Sikiric, P.; Hahm, K.B. BPC 157 rescued NSAID-cytotoxicity via stabilizing intestinal permeability and enhancing cytoprotection. Curr. Pharm. Des. 2020, 26, 2971–2981. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Tsai, W.C.; Lin, M.S.; Hsu, Y.H.; Pang, J.H.S. The promoting effect of pentadecapeptide BPC 157 on tendon healing involves tendon outgrowth, cell survival, and cell migration. J. Appl. Physiol. 2011, 110, 774–780. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.H.; Tsai, W.C.; Hsu, Y.H.; Pang, J.H.S. Pentadecapeptide BPC 157 enhances the growth hormone receptor expression in tendon fibroblasts. Molecules 2014, 19, 19066–19077. [Google Scholar] [CrossRef] [Green Version]
- Huang, T.; Zhang, K.; Sun, L.; Xue, X.; Zhang, C.; Shu, Z.; Mu, N.; Zhang, W.; Wang, Y. Body protective compound-157 enhances alkali-burn wound healing in vivo and promotes proliferation, migration, and angiogenesis in vitro. Drug Des. Devel. Ther. 2017, 9, 2485–2499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tkalcevic, V.I.; Cuzic, S.; Brajsa, K.; Mildner, B.; Bokulic, A.; Situm, K.; Perovic, D.; Glojnaric, I.; Parnham, M. Enhancement by PL 14736 of granulation and collagen organization in healing wounds and the potential role of egr-1 expression. Eur. J. Pharmacol. 2007, 570, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Qu, M.; Duan, R.; Shi, D.; Jin, L.; Gao, J.; Wood, J.D.; Li, J.; Wang, G.D. Cytoprotective mechanism of the novel gastric peptide BPC157 in gastrointestinal tract and cultured enteric neurons and glial cells. Neurosci. Bull. 2019, 35, 167–170. [Google Scholar] [CrossRef]
- Wu, H.; Wei, M.; Li, N.; Lu, Q.; Shrestha, S.M.; Tan, J.; Zhang, Z.; Wu, G.; Shi, R. Clopidogrel-induced gastric injury in rats is attenuated by stable gastric pentadecapeptide BPC 157. Drug Des. Devel. Ther. 2020, 14, 5599–5610. [Google Scholar] [CrossRef]
- Maes, M.; Kubera, M.; Leunis, J.C. The gut-brain barrier in major depression: Intestinal mucosal dysfunction with an increased translocation of LPS from gram negative enterobacteria (leaky gut) plays a role in the inflammatory pathophysiology of depression. Neuro. Endocrinol. Lett. 2008, 29, 117–124. [Google Scholar]
- Ishida, I.; Ogura, J.; Aizawa, E.; Ota, M.; Hidese, S.; Yomogida, Y.; Matsuo, J.; Yoshida, S.; Kunugi, H. Gut permeability and its clinical relevance in schizophrenia. Neuropsychopharmacol. Rep. 2022, 42, 70–76. [Google Scholar] [CrossRef]
- Maes, M.; Vojdani, A.; Sirivichayakul, S.; Barbosa, D.S.; Kanchanatawan, B. Inflammatory and oxidative pathways are new drug targets in multiple episode schizophrenia and leaky gut, Klebsiella pneumoniae, and C1q immune complexes are additional drug targets in first episode schizophrenia. Mol. Neurobiol. 2021, 58, 3319–3334. [Google Scholar] [CrossRef] [PubMed]
- Strbe, S.; Gojkovic, S.; Krezic, I.; Zizek, H.; Vranes, H.; Barisic, I.; Strinic, D.; Orct, T.; Vukojevic, J.; Ilic, S.; et al. . Over-dose lithium toxicity as an occlusive-like syndrome in rats and gastric pentadecapeptide BPC 157. Biomedicines 2021, 9, 1506. [Google Scholar] [CrossRef] [PubMed]
- Seiwerth, S.; Rucman, R.; Turkovic, B.; Sever, M.; Klicek, R.; Radic, B.; Drmic, D.; Stupnisek, M.; Misic, M.; Vuletic, L.B.; et al. BPC 157 and standard angiogenic growth factors. Gastrointestinal tract healing, lessons from tendon, ligament, muscle and bone healing. Curr. Pharm. Des. 2018, 24, 1972–1989. [Google Scholar] [CrossRef]
- Xu, C.; Sun, L.; Ren, F.; Huang, P.; Tian, Z.; Cui, J.; Zhang, W.; Wang, S.; Zhang, K.; He, L.; et al. Preclinical safety evaluation of body protective compound-157, a potential drug for treating various wounds. Regul. Toxicol. Pharmacol. 2020, 114, 104665. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zemba Cilic, A.; Zemba, M.; Cilic, M.; Strbe, S.; Ilic, S.; Vukojevic, J.; Zoricic, Z.; Filipcic, I.; Kokot, A.; Smoday, I.M.; et al. BPC 157, L-NAME, L-Arginine, NO-Relation, in the Suited Rat Ketamine Models Resembling “Negative-Like” Symptoms of Schizophrenia. Biomedicines 2022, 10, 1462. https://doi.org/10.3390/biomedicines10071462
Zemba Cilic A, Zemba M, Cilic M, Strbe S, Ilic S, Vukojevic J, Zoricic Z, Filipcic I, Kokot A, Smoday IM, et al. BPC 157, L-NAME, L-Arginine, NO-Relation, in the Suited Rat Ketamine Models Resembling “Negative-Like” Symptoms of Schizophrenia. Biomedicines. 2022; 10(7):1462. https://doi.org/10.3390/biomedicines10071462
Chicago/Turabian StyleZemba Cilic, Andrea, Mladen Zemba, Matija Cilic, Sanja Strbe, Spomenko Ilic, Jaksa Vukojevic, Zoran Zoricic, Igor Filipcic, Antonio Kokot, Ivan Maria Smoday, and et al. 2022. "BPC 157, L-NAME, L-Arginine, NO-Relation, in the Suited Rat Ketamine Models Resembling “Negative-Like” Symptoms of Schizophrenia" Biomedicines 10, no. 7: 1462. https://doi.org/10.3390/biomedicines10071462
APA StyleZemba Cilic, A., Zemba, M., Cilic, M., Strbe, S., Ilic, S., Vukojevic, J., Zoricic, Z., Filipcic, I., Kokot, A., Smoday, I. M., Rukavina, I., Boban Blagaic, A., Tvrdeic, A., Duplancic, B., Stambolija, V., Marcinko, D., Skrtic, A., Seiwerth, S., & Sikiric, P. (2022). BPC 157, L-NAME, L-Arginine, NO-Relation, in the Suited Rat Ketamine Models Resembling “Negative-Like” Symptoms of Schizophrenia. Biomedicines, 10(7), 1462. https://doi.org/10.3390/biomedicines10071462






