The Current Status of Molecular Biomarkers for Inflammatory Bowel Disease
Abstract
1. Introduction
2. Non-Invasive Molecular Biomarkers of IBD: Serum Proteins, Serological Antibodies, and Fecal Proteins
2.1. Serum Biomarkers
2.2. Serological Antibodies
2.3. Fecal Biomarkers
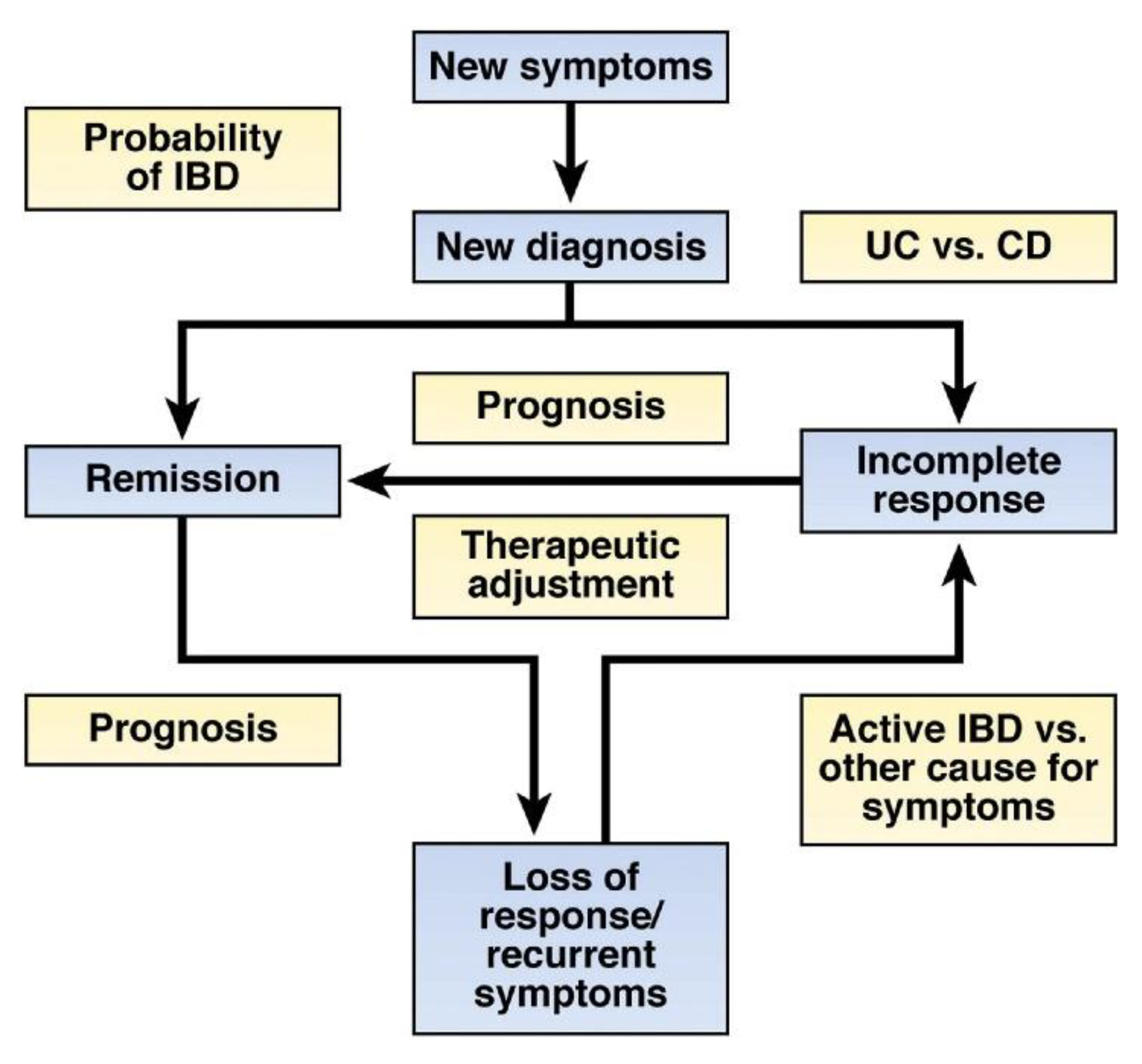
2.4. Diagnostic/Prognostic Accuracy
3. Trends in IBD Biomarker Discovery
3.1. Proteomics
3.2. Genetics
3.3. Epigenetics
4. Challenges and Future Directions
4.1. Proteomic Biomarker Discovery
4.2. Epigenetics in Diagnostic Biomarkers
5. Conclusions
Author Contributions
Funding
Data Availability Statements
Acknowledgments
Conflicts of Interest
References
- Baumgart, D.C.; Sandborn, W.J. Crohn’s disease. Lancet 2012, 380, 1590–1605. [Google Scholar] [CrossRef]
- Ordas, I.; Eckmann, L.; Talamini, M.; Baumgart, D.C.; Sandborn, W.J. Ulcerative colitis. Lancet 2012, 380, 1606–1619. [Google Scholar] [CrossRef]
- Ekbom, A.; Helmick, C.; Zack, M.; Adami, H.O. Ulcerative colitis and colorectal cancer. A population-based study. N. Engl. J. Med. 1990, 323, 1228–1233. [Google Scholar] [CrossRef] [PubMed]
- Mattar, M.C.; Lough, D.; Pishvaian, M.J.; Charabaty, A. Current management of inflammatory bowel disease and colorectal cancer. Gastrointest Cancer Res. 2011, 4, 53–61. [Google Scholar]
- Keller, D.S.; Windsor, A.; Cohen, R.; Chand, M. Colorectal cancer in inflammatory bowel disease: Review of the evidence. Tech. Coloproctol. 2019, 23, 3–13. [Google Scholar] [CrossRef]
- Khor, B.; Gardet, A.; Xavier, R.J. Genetics and pathogenesis of inflammatory bowel disease. Nature 2011, 474, 307–317. [Google Scholar] [CrossRef]
- Gomollon, F.; Dignass, A.; Annese, V.; Tilg, H.; Van Assche, G.; Lindsay, J.O.; Peyrin-Biroulet, L.; Cullen, G.J.; Daperno, M.; Kucharzik, T.; et al. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn’s Disease 2016: Part 1: Diagnosis and Medical Management. J. Crohn’s Colitis 2017, 11, 3–25. [Google Scholar] [CrossRef]
- Rubin, D.T.; Ananthakrishnan, A.N.; Siegel, C.A.; Sauer, B.G.; Long, M.D. ACG Clinical Guideline: Ulcerative Colitis in Adults. Am. J. Gastroenterol. 2019, 114, 384–413. [Google Scholar] [CrossRef]
- Gecse, K.B.; Vermeire, S. Differential diagnosis of inflammatory bowel disease: Imitations and complications. Lancet Gastroenterol. Hepatol. 2018, 3, 644–653. [Google Scholar] [CrossRef]
- Simpson, P.; Papadakis, K.A. Endoscopic evaluation of patients with inflammatory bowel disease. Inflamm. Bowel Dis. 2008, 14, 1287–1297. [Google Scholar] [CrossRef]
- Shergill, A.K.; Lightdale, J.R.; Bruining, D.H.; Acosta, R.D.; Chandrasekhara, V.; Chathadi, K.V.; Decker, G.A.; Early, D.S.; Evans, J.A.; Fanelli, R.D.; et al. The role of endoscopy in inflammatory bowel disease. Gastrointest. Endosc. 2015, 81, 1101–1121.e1113. [Google Scholar] [CrossRef] [PubMed]
- Lamb, C.A.; Kennedy, N.A.; Raine, T.; Hendy, P.A.; Smith, P.J.; Limdi, J.K.; Hayee, B.H.; Lomer, M.C.E.; Parkes, G.C.; Selinger, C.; et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019, 68 (Suppl. 3), s1–s106. [Google Scholar] [CrossRef] [PubMed]
- Iddan, G.; Meron, G.; Glukhovsky, A.; Swain, P. Wireless capsule endoscopy. Nature 2000, 405, 417. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, B.F.; Samaan, M.; Langmead, L.; Khasraw, M. Small bowel video capsule endoscopy: An overview. Expert Rev. Gastroenterol. Hepatol. 2013, 7, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Rezapour, M.; Amadi, C.; Gerson, L.B. Retention associated with video capsule endoscopy: Systematic review and meta-analysis. Gastrointest. Endosc. 2017, 85, 1157–1168.e1152. [Google Scholar] [CrossRef]
- Kiesslich, R.; Burg, J.; Vieth, M.; Gnaendiger, J.; Enders, M.; Delaney, P.; Polglase, A.; McLaren, W.; Janell, D.; Thomas, S.; et al. Confocal laser endoscopy for diagnosing intraepithelial neoplasias and colorectal cancer in vivo. Gastroenterology 2004, 127, 706–713. [Google Scholar] [CrossRef]
- Bharadwaj, S.; Narula, N.; Tandon, P.; Yaghoobi, M. Role of endoscopy in inflammatory bowel disease. Gastroenterol. Rep. 2018, 6, 75–82. [Google Scholar] [CrossRef]
- Maaser, C.; Sturm, A.; Vavricka, S.R.; Kucharzik, T.; Fiorino, G.; Annese, V.; Calabrese, E.; Baumgart, D.C.; Bettenworth, D.; Borralho Nunes, P.; et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J. Crohn’s Colitis 2018, 13, 144–164. [Google Scholar] [CrossRef]
- Panes, J.; Jairath, V.; Levesque, B.G. Advances in Use of Endoscopy, Radiology, and Biomarkers to Monitor Inflammatory Bowel Diseases. Gastroenterology 2017, 152, 362–373.e363. [Google Scholar] [CrossRef]
- Walsh, A.J.; Bryant, R.V.; Travis, S.P.L. Current best practice for disease activity assessment in IBD. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 567–579. [Google Scholar] [CrossRef]
- Biomarkers Definitions Working Group; Atkinson, A.J., Jr.; Colburn, W.A.; DeGruttola, V.G.; DeMets, D.L.; Downing, G.J.; Hoth, D.F.; Oates, J.A.; Peck, C.C.; Spilker, B.A.; et al. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001, 69, 89–95. [Google Scholar] [CrossRef]
- Sands, B.E. Biomarkers of Inflammation in Inflammatory Bowel Disease. Gastroenterology 2015, 149, 1275–1285.e1272. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.D. The utility of biomarkers in the diagnosis and therapy of inflammatory bowel disease. Gastroenterology 2011, 140, 1817–1826.e1812. [Google Scholar] [CrossRef]
- Viennois, E.; Zhao, Y.; Merlin, D. Biomarkers of Inflammatory Bowel Disease: From Classical Laboratory Tools to Personalized Medicine. Inflamm. Bowel Dis. 2015, 21, 2467–2474. [Google Scholar] [CrossRef]
- Pepys, M.B.; Hirschfield, G.M. C-reactive protein: A critical update. J. Clin. Investig. 2003, 111, 1805–1812. [Google Scholar] [CrossRef]
- Darlington, G.J.; Wilson, D.R.; Lachman, L.B. Monocyte-conditioned medium, interleukin-1, and tumor necrosis factor stimulate the acute phase response in human hepatoma cells in vitro. J. Cell Biol. 1986, 103, 787–793. [Google Scholar] [CrossRef]
- Vermeire, S.; Van Assche, G.; Rutgeerts, P. C-Reactive Protein as a Marker for Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2004, 10, 661–665. [Google Scholar] [CrossRef]
- Vermeire, S.; Van Assche, G.; Rutgeerts, P. Laboratory markers in IBD: Useful, magic, or unnecessary toys? Gut 2006, 55, 426. [Google Scholar] [CrossRef]
- Saverymuttu, S.H.; Hodgson, H.J.; Chadwick, V.S.; Pepys, M.B. Differing acute phase responses in Crohn’s disease and ulcerative colitis. Gut 1986, 27, 809–813. [Google Scholar] [CrossRef]
- Peyrin-Biroulet, L.; Gonzalez, F.; Dubuquoy, L.; Rousseaux, C.; Dubuquoy, C.; Decourcelle, C.; Saudemont, A.; Tachon, M.; Béclin, E.; Odou, M.-F.; et al. Mesenteric fat as a source of C reactive protein and as a target for bacterial translocation in Crohn’s disease. Gut 2012, 61, 78–85. [Google Scholar] [CrossRef]
- Mendoza, J.L.; Abreu, M.T. Biological markers in inflammatory bowel disease: Practical consideration for clinicians. Gastroentérologie Clin. Biol. 2009, 33, S158–S173. [Google Scholar] [CrossRef]
- Barnes, B.H.; Borowitz, S.M.; Saulsbury, F.T.; Hellems, M.; Sutphen, J.L. Discordant Erythrocyte Sedimentation Rate and C-reactive Protein in Children with Inflammatory Bowel Disease Taking Azathioprine or 6-Mercaptopurine. J. Pediatric Gastroenterol. Nutr. 2004, 38, 509–512. [Google Scholar] [CrossRef]
- Gabay, C.; Kushner, I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 1999, 340, 448–454. [Google Scholar] [CrossRef]
- O’Donnell, L.C.; Druhan, L.J.; Avalos, B.R. Molecular characterization and expression analysis of leucine-rich alpha2-glycoprotein, a novel marker of granulocytic differentiation. J. Leukoc. Biol. 2002, 72, 478–485. [Google Scholar]
- Shirai, R.; Hirano, F.; Ohkura, N.; Ikeda, K.; Inoue, S. Up-regulation of the expression of leucine-rich alpha(2)-glycoprotein in hepatocytes by the mediators of acute-phase response. Biochem. Biophys. Res. Commun. 2009, 382, 776–779. [Google Scholar] [CrossRef]
- Naka, T.; Fujimoto, M. LRG is a novel inflammatory marker clinically useful for the evaluation of disease activity in rheumatoid arthritis and inflammatory bowel disease. Immunol. Med. 2018, 41, 62–67. [Google Scholar] [CrossRef]
- Serada, S.; Fujimoto, M.; Terabe, F.; Iijima, H.; Shinzaki, S.; Matsuzaki, S.; Ohkawara, T.; Nezu, R.; Nakajima, S.; Kobayashi, T.; et al. Serum leucine-rich alpha-2 glycoprotein is a disease activity biomarker in ulcerative colitis. Inflamm. Bowel Dis. 2012, 18, 2169–2179. [Google Scholar] [CrossRef]
- Shinzaki, S.; Matsuoka, K.; Iijima, H.; Mizuno, S.; Serada, S.; Fujimoto, M.; Arai, N.; Koyama, N.; Morii, E.; Watanabe, M.; et al. Leucine-rich Alpha-2 Glycoprotein is a Serum Biomarker of Mucosal Healing in Ulcerative Colitis. J. Crohn’s Colitis 2017, 11, 84–91. [Google Scholar] [CrossRef]
- Kawamoto, A.; Takenaka, K.; Hibiya, S.; Ohtsuka, K.; Okamoto, R.; Watanabe, M. Serum Leucine-Rich alpha2 Glycoprotein: A Novel Biomarker For Small Bowel Mucosal Activity in Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2022, 20, e1196–e1200. [Google Scholar] [CrossRef]
- Kawamura, T.; Yamamura, T.; Nakamura, M.; Maeda, K.; Sawada, T.; Ishikawa, E.; Iida, T.; Mizutani, Y.; Ishikawa, T.; Kakushima, N.; et al. Accuracy of Serum Leucine-Rich Alpha-2 Glycoprotein in Evaluating Endoscopic Disease Activity in Crohn’s Disease. Inflamm. Bowel Dis. 2022; Epub Ahead of Print. [Google Scholar] [CrossRef]
- Yasutomi, E.; Inokuchi, T.; Hiraoka, S.; Takei, K.; Igawa, S.; Yamamoto, S.; Ohmori, M.; Oka, S.; Yamasaki, Y.; Kinugasa, H.; et al. Leucine-rich alpha-2 glycoprotein as a marker of mucosal healing in inflammatory bowel disease. Sci. Rep. 2021, 11, 11086. [Google Scholar] [CrossRef]
- Sellin, J.H.; Shah, R.R. The Promise and Pitfalls of Serologic Testing in Inflammatory Bowel Disease. Gastroenterol. Clin. N. Am. 2012, 41, 463–482. [Google Scholar] [CrossRef] [PubMed]
- Iskandar, H.N.; Ciorba, M.A. Biomarkers in inflammatory bowel disease: Current practices and recent advances. Transl. Res. 2012, 159, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Lopez, R.N.; Leach, S.T.; Lemberg, D.A.; Duvoisin, G.; Gearry, R.B.; Day, A.S. Fecal biomarkers in inflammatory bowel disease. J. Gastroenterol. Hepatol. 2017, 32, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Johne, B.; Fagerhol, M.K.; Lyberg, T.; Prydz, H.; Brandtzaeg, P.; Naess-Andresen, C.F.; Dale, I. Functional and clinical aspects of the myelomonocyte protein calprotectin. Mol. Pathol. 1997, 50, 113–123. [Google Scholar] [CrossRef]
- RØseth, A.G.; Fagerhol, M.K.; Aadland, E.; Schjønsby, H. Assessment of the Neutrophil Dominating Protein Calprotectin in Feces: A Methodologic Study. Scand. J. Gastroenterol. 1992, 27, 793–798. [Google Scholar] [CrossRef]
- Poullis, A.; Foster, R.; Mendall, M.A.; Fagerhol, M.K. Emerging role of calprotectin in gastroenterology. J. Gastroenterol. Hepatol. 2003, 18, 756–762. [Google Scholar] [CrossRef]
- Foell, D.; Kucharzik, T.; Kraft, M.; Vogl, T.; Sorg, C.; Domschke, W.; Roth, J. Neutrophil derived human S100A12 (EN-RAGE) is strongly expressed during chronic active inflammatory bowel disease. Gut 2003, 52, 847–853. [Google Scholar] [CrossRef]
- Perera, C.; McNeil, H.P.; Geczy, C.L. S100 Calgranulins in inflammatory arthritis. Immunol. Cell Biol. 2010, 88, 41–49. [Google Scholar] [CrossRef]
- Kane, S.V.; Sandborn, W.J.; Rufo, P.A.; Zholudev, A.; Boone, J.; Lyerly, D.; Camilleri, M.; Hanauer, S.B. Fecal lactoferrin is a sensitive and specific marker in identifying intestinal inflammation. Am. J. Gastroenterol. 2003, 98, 1309–1314. [Google Scholar] [CrossRef]
- Krzystek-Korpacka, M.; Kempiński, R.; Bromke, M.; Neubauer, K. Biochemical Biomarkers of Mucosal Healing for Inflammatory Bowel Disease in Adults. Diagnostics 2020, 10, 367. [Google Scholar] [CrossRef]
- Buisson, A.; Vazeille, E.; Minet-Quinard, R.; Goutte, M.; Bouvier, D.; Goutorbe, F.; Pereira, B.; Barnich, N.; Bommelaer, G. Fecal Matrix Metalloprotease-9 and Lipocalin-2 as Biomarkers in Detecting Endoscopic Activity in Patients With Inflammatory Bowel Diseases. J. Clin. Gastroenterol. 2018, 52, e53–e62. [Google Scholar] [CrossRef]
- Stallhofer, J.; Friedrich, M.; Konrad-Zerna, A.; Wetzke, M.; Lohse, P.; Glas, J.; Tillack-Schreiber, C.; Schnitzler, F.; Beigel, F.; Brand, S. Lipocalin-2 Is a Disease Activity Marker in Inflammatory Bowel Disease Regulated by IL-17A, IL-22, and TNF-alpha and Modulated by IL23R Genotype Status. Inflamm. Bowel Dis. 2015, 21, 2327–2340. [Google Scholar] [CrossRef]
- West, N.R.; Hegazy, A.N.; Owens, B.M.J.; Bullers, S.J.; Linggi, B.; Buonocore, S.; Coccia, M.; Gortz, D.; This, S.; Stockenhuber, K.; et al. Oncostatin M drives intestinal inflammation and predicts response to tumor necrosis factor-neutralizing therapy in patients with inflammatory bowel disease. Nat. Med. 2017, 23, 579–589. [Google Scholar] [CrossRef]
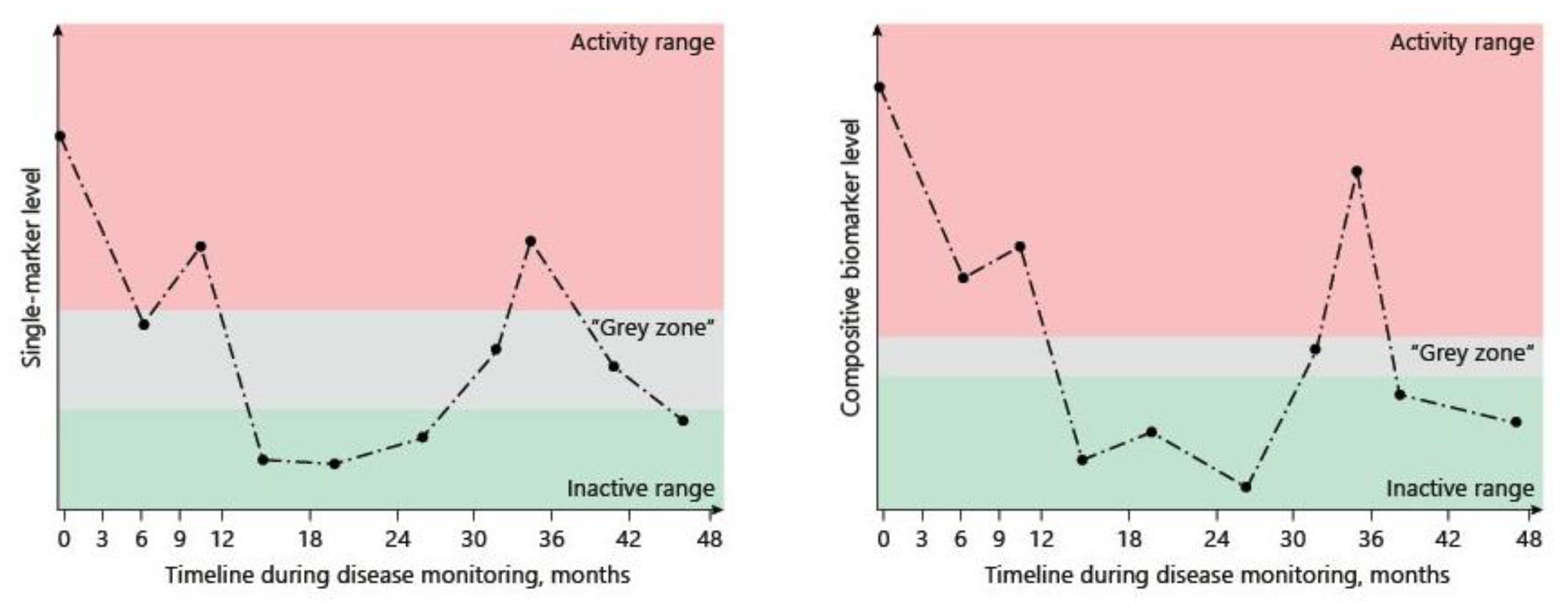
- Dragoni, G.; Innocenti, T.; Galli, A. Biomarkers of Inflammation in Inflammatory Bowel Disease: How Long before Abandoning Single-Marker Approaches? Dig. Dis. 2020, 39, 190–203. [Google Scholar] [CrossRef]
- Tyers, M.; Mann, M. From genomics to proteomics. Nature 2003, 422, 193–197. [Google Scholar] [CrossRef]
- Wright, I.; Van Eyk, J.E. A Roadmap to Successful Clinical Proteomics. Clin. Chem. 2017, 63, 245–247. [Google Scholar] [CrossRef]
- Shillingford, J.M.; Miyoshi, K.; Robinson, G.W.; Bierie, B.; Cao, Y.; Karin, M.; Hennighausen, L. Proteotyping of Mammary Tissue from Transgenic and Gene Knockout Mice with Immunohistochemical Markers: A Tool To Define Developmental Lesions. J. Histochem. Cytochem. 2003, 51, 555–565. [Google Scholar] [CrossRef]
- Ellis, M.J.; Gillette, M.; Carr, S.A.; Paulovich, A.G.; Smith, R.D.; Rodland, K.K.; Townsend, R.R.; Kinsinger, C.; Mesri, M.; Rodriguez, H.; et al. Connecting Genomic Alterations to Cancer Biology with Proteomics: The NCI Clinical Proteomic Tumor Analysis Consortium. Cancer Discov. 2013, 3, 1108–1112. [Google Scholar] [CrossRef]
- Zhang, B.; Whiteaker, J.R.; Hoofnagle, A.N.; Baird, G.S.; Rodland, K.D.; Paulovich, A.G. Clinical potential of mass spectrometry-based proteogenomics. Nat. Rev. Clin. Oncol. 2019, 16, 256–268. [Google Scholar] [CrossRef]
- Trenchevska, O.; Nelson, R.W.; Nedelkov, D. Mass Spectrometric Immunoassays in Characterization of Clinically Significant Proteoforms. Proteomes 2016, 4, 13. [Google Scholar] [CrossRef]
- Smith, L.M.; Kelleher, N.L.; Linial, M.; Goodlett, D.; Langridge-Smith, P.; Ah Goo, Y.; Safford, G.; Bonilla, L.; Kruppa, G.; Zubarev, R.; et al. Proteoform: A single term describing protein complexity. Nat. Methods 2013, 10, 186–187. [Google Scholar] [CrossRef]
- Berndt, U.; Bartsch, S.; Philipsen, L.; Danese, S.; Wiedenmann, B.; Dignass, A.U.; Hämmerle, M.; Sturm, A. Proteomic Analysis of the Inflamed Intestinal Mucosa Reveals Distinctive Immune Response Profiles in Crohn’s Disease and Ulcerative Colitis. J. Immunol. 2007, 179, 295–304. [Google Scholar] [CrossRef]
- Yau, Y.Y.; Leong, R.W.L.; Pudipeddi, A.; Redmond, D.; Wasinger, V.C. Serological Epithelial Component Proteins Identify Intestinal Complications in Crohn’s Disease. Mol. Cell. Proteom. 2017, 16, 1244–1257. [Google Scholar] [CrossRef]
- Deeke, S.A.; Starr, A.E.; Ning, Z.; Ahmadi, S.; Zhang, X.; Mayne, J.; Chiang, C.-K.; Singleton, R.; Benchimol, E.I.; Mack, D.R.; et al. Open:Mucosal-luminal interface proteomics reveals biomarkers of pediatric inflammatory bowel disease-associated colitis. Am. J. Gastroenterol. 2018, 113, 713–724. [Google Scholar] [CrossRef]
- M’Koma, A.E.; Seeley, E.H.; Washington, M.K.; Schwartz, D.A.; Muldoon, R.L.; Herline, A.J.; Wise, P.E.; Caprioli, R.M. Proteomic profiling of mucosal and submucosal colonic tissues yields protein signatures that differentiate the inflammatory colitides. Inflamm. Bowel Dis. 2010, 17, 875–883. [Google Scholar] [CrossRef]
- Seeley, E.H.; Washington, M.K.; Caprioli, R.M.; M’Koma, A.E. Proteomic patterns of colonic mucosal tissues delineate Crohn’s colitis and ulcerative colitis. Proteom. Clin. Appl. 2013, 7, 541–549. [Google Scholar] [CrossRef]
- Starr, A.E.; Deeke, S.A.; Ning, Z.; Chiang, C.-K.; Zhang, X.; Mottawea, W.; Singleton, R.; Benchimol, E.I.; Wen, M.; Mack, D.R.; et al. Proteomic analysis of ascending colon biopsies from a paediatric inflammatory bowel disease inception cohort identifies protein biomarkers that differentiate Crohn’s disease from UC. Gut 2017, 66, 1573–1583. [Google Scholar] [CrossRef]
- Meuwis, M.-A.; Fillet, M.; Geurts, P.; de Seny, D.; Lutteri, L.; Chapelle, J.-P.; Bours, V.; Wehenkel, L.; Belaiche, J.; Malaise, M.; et al. Biomarker discovery for inflammatory bowel disease, using proteomic serum profiling. Biochem. Pharmacol. 2007, 73, 1422–1433. [Google Scholar] [CrossRef]
- Townsend, P.; Zhang, Q.; Shapiro, J.; Webb-Robertson, B.-J.; Bramer, L.; Schepmoes, A.A.; Weitz, K.K.; Mallette, M.; Moniz, H.; Bright, R.; et al. Serum Proteome Profiles in Stricturing Crohn’s Disease: A Pilot Study. Inflamm. Bowel Dis. 2015, 21, 1935–1941. [Google Scholar] [CrossRef]
- Magnusson, M.K.; Strid, H.; Isaksson, S.; Bajor, A.; Lasson, A.; Ung, K.-A.; Öhman, L. Response to Infliximab Therapy in Ulcerative Colitis is Associated with Decreased Monocyte Activation, Reduced CCL2 Expression and Downregulation of Tenascin C. J. Crohn’s Colitis 2014, 9, 56–65. [Google Scholar] [CrossRef]
- Heier, C.R.; Fiorillo, A.A.; Chaisson, E.; Gordish-Dressman, H.; Hathout, Y.; Damsker, J.M.; Hoffman, E.P.; Conklin, L.S. Identification of Pathway-Specific Serum Biomarkers of Response to Glucocorticoid and Infliximab Treatment in Children with Inflammatory Bowel Disease. Clin. Transl. Gastroenterol. 2016, 7, e192. [Google Scholar] [CrossRef]
- de Lange, K.M.; Moutsianas, L.; Lee, J.C.; Lamb, C.A.; Luo, Y.; Kennedy, N.A.; Jostins, L.; Rice, D.L.; Gutierrez-Achury, J.; Ji, S.-G.; et al. Genome-wide association study implicates immune activation of multiple integrin genes in inflammatory bowel disease. Nat. Genet. 2017, 49, 256–261. [Google Scholar] [CrossRef]
- Mesko, B.; Poliskal, S.; Szegedi, A.; Szekanecz, Z.; Palatka, K.; Papp, M.; Nagy, L. Peripheral blood gene expression patterns discriminate among chronic inflammatory diseases and healthy controls and identify novel targets. BMC Med. Genom. 2010, 3, 15. [Google Scholar] [CrossRef]
- Burakoff, R.; Hande, S.; Ma, J.; Banks, P.A.; Friedman, S.; Makrauer, F.; Liew, C.-C. Differential Regulation of Peripheral Leukocyte Genes in Patients with Active Crohn’s Disease and Crohn’s Disease in Remission. J. Clin. Gastroenterol. 2010, 44, 120–126. [Google Scholar] [CrossRef]
- Burakoff, R.; Chao, S.; Perencevich, M.; Ying, J.; Friedman, S.; Makrauer, F.; Odze, R.; Khurana, H.; Liew, C.-C. Blood-based biomarkers can differentiate ulcerative colitis from crohn’s disease and noninflammatory diarrhea. Inflamm. Bowel Dis. 2011, 17, 1719–1725. [Google Scholar] [CrossRef]
- Burakoff, R.; Pabby, V.; Onyewadume, L.; Odze, R.; Adackapara, C.; Wang, W.; Friedman, S.; Hamilton, M.; Korzenik, J.; Levine, J.; et al. Blood-based Biomarkers Used to Predict Disease Activity in Crohn’s Disease and Ulcerative Colitis. Inflamm. Bowel Dis. 2015, 21, 1132–1140. [Google Scholar] [CrossRef]
- Burczynski, M.E.; Peterson, R.L.; Twine, N.C.; Zuberek, K.A.; Brodeur, B.J.; Casciotti, L.; Maganti, V.; Reddy, P.S.; Strahs, A.; Immermann, F.; et al. Molecular Classification of Crohn’s Disease and Ulcerative Colitis Patients Using Transcriptional Profiles in Peripheral Blood Mononuclear Cells. J. Mol. Diagn. 2006, 8, 51–61. [Google Scholar] [CrossRef]
- van Lierop, P.P.E.; Swagemakers, S.M.; de Bie, C.I.; Middendorp, S.; van Baarlen, P.; Samsom, J.N.; van Ijcken, W.F.J.; Escher, J.C.; van der Spek, P.J.; Nieuwenhuis, E.E.S. Gene Expression Analysis of Peripheral Cells for Subclassification of Pediatric Inflammatory Bowel Disease in Remission. PLoS ONE 2013, 8, e79549. [Google Scholar] [CrossRef]
- Wu, F.; Dassopoulos, T.; Cope, L.; Maitra, A.; Brant, S.R.; Harris, M.L.; Bayless, T.M.; Parmigiani, G.; Chakravarti, S. Genome-wide gene expression differences in Crohn’s disease and ulcerative colitis from endoscopic pinch biopsies: Insights into distinctive pathogenesis. Inflamm. Bowel Dis. 2007, 13, 807–821. [Google Scholar] [CrossRef]
- Dieckgraefe, B.K.; Stenson, W.F.; Korzenik, J.R.; Swanson, P.E.; Harrington, C.A. Analysis of mucosal gene expression in inflammatory bowel disease by parallel oligonucleotide arrays. Physiol. Genom. 2000, 4, 1–11. [Google Scholar] [CrossRef]
- Graham, D.B.; Xavier, R.J. Pathway paradigms revealed from the genetics of inflammatory bowel disease. Nature 2020, 578, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Ventham, N.T.; Kennedy, N.A.; Nimmo, E.R.; Satsangi, J. Beyond Gene Discovery in Inflammatory Bowel Disease: The Emerging Role of Epigenetics. Gastroenterology 2013, 145, 293–308. [Google Scholar] [CrossRef] [PubMed]
- Jenke, A.C.; Zilbauer, M. Epigenetics in inflammatory bowel disease. Curr. Opin. Gastroenterol. 2012, 28, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.A. Functions of DNA methylation: Islands, start sites, gene bodies and beyond. Nat. Rev. Genet. 2012, 13, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Issa, J.-P.J.; Ahuja, N.; Toyota, M.; Bronner, M.P.; Brentnall, T.A. Accelerated Age-related CpG Island Methylation in Ulcerative Colitis. Cancer Res. 2001, 61, 3573–3577. [Google Scholar]
- Nimmo, E.R.; Prendergast, J.G.; Aldhous, M.C.; Kennedy, N.A.; Henderson, P.; Drummond, H.E.; Ramsahoye, B.H.; Wilson, D.C.; Semple, C.A.; Satsangi, J. Genome-wide Methylation Profiling in Crohn’s Disease Identifies Altered Epigenetic Regulation of Key Host Defense Mechanisms Including the Th17 Pathway. Inflamm. Bowel Dis. 2011, 18, 889–899. [Google Scholar] [CrossRef]
- Cooke, J.; Zhang, H.; Greger, L.; Silva, A.-L.; Massey, D.; Dawson, C.; Metz, A.; Ibrahim, A.; Parkes, M. Mucosal Genome-wide Methylation Changes in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2012, 18, 2128–2137. [Google Scholar] [CrossRef]
- Lin, Z.; Hegarty, J.; Cappel, J.; Yu, W.; Chen, X.; Faber, P.; Wang, Y.; Kelly, A.; Poritz, L.; Peterson, B.; et al. Identification of disease-associated DNA methylation in intestinal tissues from patients with inflammatory bowel disease. Clin. Genet. 2011, 80, 59–67. [Google Scholar] [CrossRef]
- McDermott, E.; Ryan, E.J.; Tosetto, M.; Gibson, D.; Burrage, J.; Keegan, D.; Byrne, K.; Crowe, E.; Sexton, G.; Malone, K.; et al. DNA Methylation Profiling in Inflammatory Bowel Disease Provides New Insights into Disease Pathogenesis. J. Crohn’s Colitis 2015, 10, 77–86. [Google Scholar] [CrossRef]
- Esteller, M. Non-coding RNAs in human disease. Nat. Rev. Genet. 2011, 12, 861–874. [Google Scholar] [CrossRef]
- James, J.P.; Riis, L.B.; Malham, M.; Hogdall, E.; Langholz, E.; Nielsen, B.S. MicroRNA Biomarkers in IBD-Differential Diagnosis and Prediction of Colitis-Associated Cancer. Int. J. Mol. Sci. 2020, 21, 7893. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zikusoka, M.; Trindade, A.; Dassopoulos, T.; Harris, M.L.; Bayless, T.M.; Brant, S.R.; Chakravarti, S.; Kwon, J.H. MicroRNAs Are Differentially Expressed in Ulcerative Colitis and Alter Expression of Macrophage Inflammatory Peptide-2α. Gastroenterology 2008, 135, 1624–1635.e1624. [Google Scholar] [CrossRef] [PubMed]
- Takagi, T.; Naito, Y.; Mizushima, K.; Hirata, I.; Yagi, N.; Tomatsuri, N.; Ando, T.; Oyamada, Y.; Isozaki, Y.; Hongo, H.; et al. Increased expression of microRNA in the inflamed colonic mucosa of patients with active ulcerative colitis. J. Gastroenterol. Hepatol. 2010, 25, S129–S133. [Google Scholar] [CrossRef] [PubMed]
- Bian, Z.; Li, L.; Cui, J.; Zhang, H.; Liu, Y.; Zhang, C.-Y.; Zen, K. Role of miR-150-targeting c-Myb in colonic epithelial disruption during dextran sulphate sodium-induced murine experimental colitis and human ulcerative colitis. J. Pathol. 2011, 225, 544–553. [Google Scholar] [CrossRef]
- Pekow, J.R.; Dougherty, U.; Mustafi, R.; Zhu, H.; Kocherginsky, M.; Rubin, D.T.; Hanauer, S.B.; Hart, J.; Chang, E.B.; Fichera, A.; et al. miR-143 and miR-145 are Downregulated in Ulcerative Colitis: Putative Regulators of Inflammation and Protooncogenes. Inflamm. Bowel Dis. 2011, 18, 94–100. [Google Scholar] [CrossRef]
- Brest, P.; Lapaquette, P.; Souidi, M.; Lebrigand, K.; Cesaro, A.; Vouret-Craviari, V.; Mari, B.; Barbry, P.; Mosnier, J.-F.; Hébuterne, X.; et al. A synonymous variant in IRGM alters a binding site for miR-196 and causes deregulation of IRGM-dependent xenophagy in Crohn’s disease. Nat. Genet. 2011, 43, 242–245. [Google Scholar] [CrossRef]
- Nguyen, H.T.T.; Dalmasso, G.; Yan, Y.; Laroui, H.; Dahan, S.; Mayer, L.; Sitaraman, S.V.; Merlin, D. MicroRNA-7 Modulates CD98 Expression during Intestinal Epithelial Cell Differentiation. J. Biol. Chem. 2010, 285, 1479–1489. [Google Scholar] [CrossRef]
- Wu, F.; Zhang, S.; Dassopoulos, T.; Harris, M.L.; Bayless, T.M.; Meltzer, S.J.; Brant, S.R.; Kwon, J.H. Identification of microRNAs associated with ileal and colonic Crohn’s disease. Inflamm. Bowel Dis. 2010, 16, 1729–1738. [Google Scholar] [CrossRef]
- Fasseu, M.; Tréton, X.; Guichard, C.; Pedruzzi, E.; Cazals-Hatem, D.; Richard, C.; Aparicio, T.; Daniel, F.; Soulé, J.-C.; Moreau, R.; et al. Identification of Restricted Subsets of Mature microRNA Abnormally Expressed in Inactive Colonic Mucosa of Patients with Inflammatory Bowel Disease. PLoS ONE 2010, 5, e13160. [Google Scholar] [CrossRef]
- Iborra, M.; Bernuzzi, F.; Correale, C.; Vetrano, S.; Fiorino, G.; Beltrán, B.; Marabita, F.; Locati, M.; Spinelli, A.; Nos, P.; et al. Identification of serum and tissue micro-RNA expression profiles in different stages of inflammatory bowel disease. Clin. Exp. Immunol. 2013, 173, 250–258. [Google Scholar] [CrossRef]
- Duttagupta, R.; DiRienzo, S.; Jiang, R.; Bowers, J.; Gollub, J.; Kao, J.; Kearney, K.; Rudolph, D.; Dawany, N.B.; Showe, M.K.; et al. Genome-Wide Maps of Circulating miRNA Biomarkers for Ulcerative Colitis. PLoS ONE 2012, 7, e31241. [Google Scholar] [CrossRef] [PubMed]
- Paraskevi, A.; Theodoropoulos, G.; Papaconstantinou, I.; Mantzaris, G.; Nikiteas, N.; Gazouli, M. Circulating MicroRNA in inflammatory bowel disease. J. Crohn’s Colitis 2012, 6, 900–904. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Guo, N.J.; Tian, H.; Marohn, M.; Gearhart, S.; Bayless, T.M.; Brant, S.R.; Kwon, J.H. Peripheral blood MicroRNAs distinguish active ulcerative colitis and Crohn’s disease. Inflamm. Bowel Dis. 2010, 17, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Zahm, A.M.; Thayu, M.; Hand, N.J.; Horner, A.; Leonard, M.B.; Friedman, J.R. Circulating microRNA is a biomarker of pediatric Crohn disease. J. Pediatric Gastroenterol. Nutr. 2011, 53, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Verdier, J.; Breunig, I.R.; Ohse, M.C.; Roubrocks, S.; Kleinfeld, S.; Roy, S.; Streetz, K.; Trautwein, C.; Roderburg, C.; Sellge, G. Faecal Micro-RNAs in Inflammatory Bowel Diseases. J. Crohn’s Colitis 2019, 14, 110–117. [Google Scholar] [CrossRef]
- Schönauen, K.; Le, N.; von Arnim, U.; Schulz, C.; Malfertheiner, P.; Link, A. Circulating and Fecal microRNAs as Biomarkers for Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2018, 24, 1547–1557. [Google Scholar] [CrossRef] [PubMed]
- Wohnhaas, C.T.; Schmid, R.; Rolser, M.; Kaaru, E.; Langgartner, D.; Rieber, K.; Strobel, B.; Eisele, C.; Wiech, F.; Jakob, I.; et al. Fecal MicroRNAs Show Promise as Noninvasive Crohn’s Disease Biomarkers. Crohn’s Colitis 360 2020, 2, otaa003. [Google Scholar] [CrossRef]
- Kanaan, Z.; Rai, S.N.; Eichenberger, M.R.; Roberts, H.; Keskey, B.; Pan, J.; Galandiuk, S. Plasma MiR-21: A Potential Diagnostic Marker of Colorectal Cancer. Ann. Surg. 2012, 256, 544–551. [Google Scholar] [CrossRef]
- Schaefer, J.S.; Attumi, T.; Opekun, A.R.; Abraham, B.; Hou, J.; Shelby, H.; Graham, D.Y.; Streckfus, C.; Klein, J.R. MicroRNA signatures differentiate Crohn’s disease from ulcerative colitis. BMC Immunol. 2015, 16, 5. [Google Scholar] [CrossRef]
- Rifai, N.; Gillette, M.A.; Carr, S.A. Protein biomarker discovery and validation: The long and uncertain path to clinical utility. Nat. Biotechnol. 2006, 24, 971–983. [Google Scholar] [CrossRef]
- Anderson, N.L. The Clinical Plasma Proteome: A Survey of Clinical Assays for Proteins in Plasma and Serum. Clin. Chem. 2010, 56, 177–185. [Google Scholar] [CrossRef] [PubMed]
- van der Burgt, Y.E.M. Protein biomarker discovery is still relevant and has entered a new phase. EBioMedicine 2019, 43, 15. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.L.; Anderson, N.G. The Human Plasma Proteome: History, Character, and Diagnostic Prospects. Mol. Cell. Proteom. 2002, 1, 845–867. [Google Scholar] [CrossRef]
- Alex, P.; Gucek, M.; Li, X. Applications of proteomics in the study of inflammatory bowel diseases: Current status and future directions with available technologies. Inflamm. Bowel Dis. 2009, 15, 616–629. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pisanu, S.; Biosa, G.; Carcangiu, L.; Uzzau, S.; Pagnozzi, D. Comparative evaluation of seven commercial products for human serum enrichment/depletion by shotgun proteomics. Talanta 2018, 185, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Skates, S.J.; Gillette, M.A.; LaBaer, J.; Carr, S.A.; Anderson, L.; Liebler, D.C.; Ransohoff, D.; Rifai, N.; Kondratovich, M.; Težak, Ž.; et al. Statistical Design for Biospecimen Cohort Size in Proteomics-based Biomarker Discovery and Verification Studies. J. Proteome Res. 2013, 12, 5383–5394. [Google Scholar] [CrossRef]
- Peck, B.C.; Weiser, M.; Lee, S.E.; Gipson, G.R.; Iyer, V.B.; Sartor, R.B.; Herfarth, H.H.; Long, M.D.; Hansen, J.J.; Isaacs, K.L.; et al. MicroRNAs Classify Different Disease Behavior Phenotypes of Crohn’s Disease and May Have Prognostic Utility. Inflamm. Bowel Dis. 2015, 21, 2178–2187. [Google Scholar] [CrossRef]
- Kalla, R.; Ventham, N.T.; Kennedy, N.A.; Quintana, J.F.; Nimmo, E.R.; Buck, A.H.; Satsangi, J. MicroRNAs: New players in IBD. Gut 2015, 64, 504–517. [Google Scholar] [CrossRef]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.-F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef]
- Torres, J.; Mehandru, S.; Colombel, J.F.; Peyrin-Biroulet, L. Crohn’s disease. Lancet 2017, 389, 1741–1755. [Google Scholar] [CrossRef]
- van der Sloot, K.W.J.; Amini, M.; Peters, V.; Dijkstra, G.; Alizadeh, B.Z. Inflammatory Bowel Diseases: Review of Known Environmental Protective and Risk Factors Involved. Inflamm. Bowel Dis. 2017, 23, 1499–1509. [Google Scholar] [CrossRef] [PubMed]
- Xavier, R.J.; Podolsky, D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007, 448, 427–434. [Google Scholar] [CrossRef] [PubMed]
| Patient Population | Assessment of Endoscopic Disease Activity | Lactoferrin (Correlation Coefficient) | Calprotectin (Correlation Coefficient) | CRP (Correlation Coefficient) |
|---|---|---|---|---|
| CD | CDEIS * | 0.77 | 0.73 | 0.55 |
| CD | SES-CD ** | 0.19 | 0.48 | |
| UC | Mayo score | 0.35 | 0.51 | |
| UC | Matt’s index | 0.81 | ||
| CD | SES-CD | 0.63 | 0.64 | 0.52 |
| IBD | 0.52 | |||
| UC | Mayo score | 0.57 | ||
| CD | SES-CD | 0.76 | 0.72 | 0.46 |
| CD | CDEIS | 0.87 | 0.83 | 0.61 |
| UC | Rachmilewitz index | 0.83 | 0.50 | |
| CD | CDEIS | 0.75 | 0.53 |
| # | MiRNAs | Disease Subtype | Sample Type | Techniques Used | Outcome |
|---|---|---|---|---|---|
| 1 | miR-19a | UC, HC | Biopsy, murine tissue | RT-qPCR | Reduced expression of miR-19a in human colon tissue with UC and DSS-treated murine colitis. |
| 2 | miR-21 | UC, HC | Biopsy | RT-qPCR, ISH | Overexpression of miR-21 in UC. |
| 3 | miR-21-5p | UC, HC | Sera, rat tissue | RT-qPCR, Transfection | MiR-21-5p was downregulated in the sera and colon tissue of UC compared with healthy people and the control group. |
| 4 | miR-124 | UC, HC | Biopsy | RT-qPCR | MiR-124 regulated the expression of STAT3. Reduced levels of miR-124 in colon tissues of children with active UC appeared to increase the expression and activity of STAT3. |
| 5 | miR-141 | UC, HC | Biopsy | Microarray, RT-qPCR | MiR-141 played a role in the bowel inflammation of individuals with active UC via downregulation of CXCL5 expression. |
| 6 | miR-150 | UC, HC | Murine model | RT-qPCR | MiR-150 was elevated and c-Myb was downregulated in the human colon with active UC compared to HC. |
| 7 | miR-155 | Colitis | Murine tissue, cell culture | RT-qPCR, transfection | MiR-155 promoted the pathogenesis of experimental colitis by repressing SHIP-1 expression. |
| 8 | miR-193a-3p | UC, HC | Cell culture, biopsy | RT-qPCR, ISH | MiR-193a-3p reduced intestinal inflammation in response to microbiota. |
| 9 | miR-206 | UC, HC | Cell culture, biopsy | RT-qPCR, | MiR-206 as a biomarker for response to mesalamine treatment in UC. |
| 10 | miR-21, miR-155 | UC, HC | Biopsy | RT-qPCR | MiR-21 and miR-155 were highly expressed in UC. |
| 11 | miR-143, miR-145 | UC, HC | Biopsy | RT-qPCR, ISH | MiR-143 and miR-145 were downregulated in UC. |
| 12 | miR-125b, miR-155, miR-223 and miR-138 | UC | Biopsy | RT-qPCR, microarray | Differential expression of miR-223, miR-125b, miR-138, and miR-155 in the inflamed mucosa compared to non-inflamed mucosa and controls. |
| 13 | miR-7 | CD, HC | Cell culture, biopsy | Transfection, RT-qPCR | MiR-7 modulated CD98 expression during intestinal epithelial cell differentiation. |
| 14 | miR-19b | CD, HC | Biopsy, cell culture | RT-qPCR, ISH | MiR-19b suppressed the inflammation and prevented the pathogenesis of CD. |
| 15 | miR-29b | CD | Fibroblasts | RT-qPCR | MCL-1 was modulated in CD fibrosis by miR-29b via IL-6 and IL-8 |
| 16 | miR-122 | CD, HC | Cell culture, biopsy | RT-qPCR, Transfection | MiR-122 reduced the expression of pro-inflammatory cytokines (TNF and IFN-γ) and promoted the release of anti-inflammatory cytokines (e.g., IL-4 and IL-10). Significant increase in miR-122 expression in cells treated with 5′-AZA. |
| 17 | miR-141 | CD | Murine models, biopsy | Microarray, RT-qPCR | MiR-141 regulated colonic leukocytic trafficking by targeting CXCL12β during murine colitis and human CD. |
| 18 | miR-155 | CD, HC | PBMC | RT-qPCR, transfection | MiR-155 regulated IL-10-producing CD24 CD27+ B Cells. |
| 19 | miR-200b | CD, HC | Biopsy, serum. cell culture | RT-qPCR | MiR-200b was involved in intestinal fibrosis of CD. |
| 20 | miR-590-5p | CD, HC | Human and murine tissues | RT-qPCR | Decreased miR-590-5p levels in CD. |
| 21 | miR-146a, miR-155 | CD | Biopsy | RT-qPCR | MiR-146a and -155 showed increased duodenal expression in pediatric CD. |
| 22 | miR-223-3p, miR-31-5p | CD, HC | Biopsy | Nanostring | Mir-223-3p expression showed age- and sex-related effects and miR-31-5p expression was driven by location |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alghoul, Z.; Yang, C.; Merlin, D. The Current Status of Molecular Biomarkers for Inflammatory Bowel Disease. Biomedicines 2022, 10, 1492. https://doi.org/10.3390/biomedicines10071492
Alghoul Z, Yang C, Merlin D. The Current Status of Molecular Biomarkers for Inflammatory Bowel Disease. Biomedicines. 2022; 10(7):1492. https://doi.org/10.3390/biomedicines10071492
Chicago/Turabian StyleAlghoul, Zahra, Chunhua Yang, and Didier Merlin. 2022. "The Current Status of Molecular Biomarkers for Inflammatory Bowel Disease" Biomedicines 10, no. 7: 1492. https://doi.org/10.3390/biomedicines10071492
APA StyleAlghoul, Z., Yang, C., & Merlin, D. (2022). The Current Status of Molecular Biomarkers for Inflammatory Bowel Disease. Biomedicines, 10(7), 1492. https://doi.org/10.3390/biomedicines10071492








