Novel Phenotyping for Acute Heart Failure—Unsupervised Machine Learning-Based Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Machine Learning and Statistical Analysis
- EuclideanDistance;
- CamberraDistance;
- ChebychevDistance;
- CorrelationSimilarity;
- CosineSimilarity;
- DiceSimilarity;
- DynamicTimeWarpingDistance;
- InnerProductSimilarity;
- JaccardSimilarity;
- KernelEuclideanDistance;
- ManhattanDistance;
- MaxProductSimilarity;
- OverlapSimilarity.
3. Results
3.1. Patients Characteristics
3.2. Clustering
3.3. Prognostic Significance of Clusters
4. Discussion
4.1. Clusters 1 and 4
4.2. Cluster 2
4.3. Cluster 3
4.4. Cluster 1 and Cluster 5
4.5. Novelty and Clinical Implications
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Ezekowitz, J.A.; Kaul, P.; Bakal, J.A.; Armstrong, P.W.; Welsh, R.C.; McAlister, F.A. Declining In-Hospital Mortality and Increasing Heart Failure Incidence in Elderly Patients with First Myocardial Infarction. J. Am. Coll. Cardiol. 2009, 53, 13–20. [Google Scholar] [CrossRef] [Green Version]
- Nohria, A.; Tsang, S.W.; Fang, J.C.; Lewis, E.F.; Jarcho, J.A.; Mudge, G.H.; Stevenson, L.W. Clinical Assessment Identifies Hemodynamic Profiles That Predict Outcomes in Patients Admitted with Heart Failure. J. Am. Coll. Cardiol. 2003, 41, 1797–1804. [Google Scholar] [CrossRef] [Green Version]
- Horiuchi, Y.; Tanimoto, S.; Latif, A.H.M.M.; Urayama, K.Y.; Aoki, J.; Yahagi, K.; Okuno, T.; Sato, Y.; Tanaka, T.; Koseki, K.; et al. Identifying Novel Phenotypes of Acute Heart Failure Using Cluster Analysis of Clinical Variables. Int. J. Cardiol. 2018, 262, 57–63. [Google Scholar] [CrossRef]
- Shah, S.J.; Katz, D.H.; Selvaraj, S.; Burke, M.A.; Yancy, C.W.; Gheorghiade, M.; Bonow, R.O.; Huang, C.C.; Deo, R.C. Phenomapping for Novel Classification of Heart Failure with Preserved Ejection Fraction. Circulation 2015, 131, 269–279. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, J.P.; Duarte, K.; McMurray, J.J.V.; Pitt, B.; van Veldhuisen, D.J.; Vincent, J.; Ahmad, T.; Tromp, J.; Rossignol, P.; Zannad, F. Data-Driven Approach to Identify Subgroups of Heart Failure with Reduced Ejection Fraction Patients with Different Prognoses and Aldosterone Antagonist Response Patterns. Circ. Heart Fail. 2018, 11, e004926. [Google Scholar] [CrossRef]
- Segar, M.W.; Patel, K.V.; Ayers, C.; Basit, M.; Tang, W.H.W.; Willett, D.; Berry, J.; Grodin, J.L.; Pandey, A. Phenomapping of Patients with Heart Failure with Preserved Ejection Fraction Using Machine Learning-Based Unsupervised Cluster Analysis. Eur. J. Heart Fail. 2020, 22, 148–158. [Google Scholar] [CrossRef]
- Yagi, R.; Takei, M.; Kohsaka, S.; Shiraishi, Y.; Ikemura, N.; Shoji, S.; Niimi, N.; Higuchi, S.; Goda, A.; Kohno, T.; et al. Phenomapping in Patients Experiencing Worsening Renal Function during Hospitalization for Acute Heart Failure. ESC Heart Fail. 2021, 8, 5192. [Google Scholar] [CrossRef]
- Ahmad, T.; Pencina, M.J.; Schulte, P.J.; O’Brien, E.; Whellan, D.J.; Piña, I.L.; Kitzman, D.W.; Lee, K.L.; O’Connor, C.M.; Felker, G.M. Clinical Implications of Chronic Heart Failure Phenotypes Defined by Cluster Analysis. J. Am. Coll. Cardiol. 2014, 64, 1765–1774. [Google Scholar] [CrossRef] [Green Version]
- Zymliński, R.; Sokolski, M.; Siwolowski, P.; Biegus, J.; Nawrocka, S.; Jankowska, E.A.; Todd, J.; Yerramilli, R.; Estis, J.; Banasiak, W.; et al. Elevated Troponin I Level Assessed by a New High-Sensitive Assay and the Risk of Poor Outcomes in Patients with Acute Heart Failure. Int. J. Cardiol. 2017, 230, 646–652. [Google Scholar] [CrossRef]
- Henneges, C.; Morbach, C.; Sahiti, F.; Scholz, N.; Frantz, S.; Ertl, G.; Angermann, C.E.; Störk, S. Sex-Specific Bimodal Clustering of Left Ventricular Ejection Fraction in Patients with Acute Heart Failure. ESC Heart Fail. 2021, 9, 786–790. [Google Scholar] [CrossRef]
- Nowak, R.M.; Reed, B.P.; DiSomma, S.; Nanayakkara, P.; Moyer, M.; Millis, S.; Levy, P. Presenting Phenotypes of Acute Heart Failure Patients in the ED: Identification and Implications. Am. J. Emerg Med. 2017, 35, 536–542. [Google Scholar] [CrossRef]
- Ahmad, T.; Desai, N.; Wilson, F.; Schulte, P.; Dunning, A.; Jacoby, D.; Allen, L.; Fiuzat, M.; Rogers, J.; Felker, G.M.; et al. Clinical Implications of Cluster Analysis-Based Classification of Acute Decompensated Heart Failure and Correlation with Bedside Hemodynamic Profiles. PLoS ONE 2016, 11, e0145881. [Google Scholar] [CrossRef]
- Peters, S.A.E.; Muntner, P.; Woodward, M. Sex Differences in the Prevalence of, and Trends in, Cardiovascular Risk Factors, Treatment, and Control in the United States, 2001 to 2016. Circulation 2019, 139, 1025–1035. [Google Scholar] [CrossRef]
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-Density Lipoproteins Cause Atherosclerotic Cardiovascular Disease. 1. Evidence from Genetic, Epidemiologic, and Clinical Studies. A Consensus Statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017, 38, 2459. [Google Scholar] [CrossRef] [Green Version]
- Nordestgaard, B.G.; Chapman, M.J.; Ray, K.; Borén, J.; Andreotti, F.; Watts, G.F.; Ginsberg, H.; Amarenco, P.; Catapano, A.; Descamps, O.S.; et al. Lipoprotein(a) as a Cardiovascular Risk Factor: Current Status. Eur. Heart J. 2010, 31, 2844. [Google Scholar] [CrossRef]
- Iorga, A.; Cunningham, C.M.; Moazeni, S.; Ruffenach, G.; Umar, S.; Eghbali, M. The Protective Role of Estrogen and Estrogen Receptors in Cardiovascular Disease and the Controversial Use of Estrogen Therapy. Biol. Sex Differ. 2017, 8, 33. [Google Scholar] [CrossRef] [Green Version]
- Xiang, D.; Liu, Y.; Zhou, S.; Zhou, E.; Wang, Y. Protective Effects of Estrogen on Cardiovascular Disease Mediated by Oxidative Stress. Oxidative Med. Cell. Longev. 2021, 2021, 5523516. [Google Scholar] [CrossRef]
- Shekhar, S.; Travis, O.K.; He, X.; Roman, R.J.; Fan, F. Menopause and Ischemic Stroke: A Brief Review. MOJ Toxicol. 2017, 3, 59. [Google Scholar] [CrossRef] [Green Version]
- Lisabeth, L.; Bushnell, C. Menopause and Stroke: An Epidemiologic Review. Lancet Neurol. 2012, 11, 82. [Google Scholar] [CrossRef] [Green Version]
- Ronco, C.; Haapio, M.; House, A.A.; Anavekar, N.; Bellomo, R. Cardiorenal Syndrome. J. Am. Coll. Cardiol. 2008, 52, 1527–1539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dignass, A.; Farrag, K.; Stein, J. Limitations of Serum Ferritin in Diagnosing Iron Deficiency in Inflammatory Conditions. Int. J. Chronic Dis. 2018, 2018, 9394060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loncar, G.; Obradovic, D.; Thiele, H.; von Haehling, S.; Lainscak, M. Iron Deficiency in Heart Failure. ESC Heart Fail. 2021, 8, 2368. [Google Scholar] [CrossRef]
- Alnuwaysir, R.I.S.; Hoes, M.F.; van Veldhuisen, D.J.; van der Meer, P.; Beverborg, N.G. Iron Deficiency in Heart Failure: Mechanisms and Pathophysiology. J. Clin. Med. 2022, 11, 125. [Google Scholar] [CrossRef] [PubMed]
- von Haehling, S.; Ebner, N.; Evertz, R.; Ponikowski, P.; Anker, S.D. Iron Deficiency in Heart Failure: An Overview. JACC Heart Fail. 2019, 7, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Hoes, M.F.; Grote Beverborg, N.; Kijlstra, J.D.; Kuipers, J.; Swinkels, D.W.; Giepmans, B.N.G.; Rodenburg, R.J.; van Veldhuisen, D.J.; de Boer, R.A.; van der Meer, P. Iron Deficiency Impairs Contractility of Human Cardiomyocytes through Decreased Mitochondrial Function. Eur. J. Heart Fail. 2018, 20, 910. [Google Scholar] [CrossRef] [Green Version]
- Anand, I.S.; Gupta, P. Anemia and Iron Deficiency in Heart Failure. Circulation 2018, 138, 80–98. [Google Scholar] [CrossRef]
- Bornstein, A.B.; Rao, S.S.; Marwaha, K. Left Ventricular Hypertrophy; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Dunlay, S.M.; Roger, V.L.; Redfield, M.M. Epidemiology of Heart Failure with Preserved Ejection Fraction. Nat. Rev. Cardiol 2017, 14, 591–602. [Google Scholar] [CrossRef]
- Ather, S.; Chan, W.; Bozkurt, B.; Aguilar, D.; Ramasubbu, K.; Zachariah, A.A.; Wehrens, X.H.T.; Deswal, A. Impact of Noncardiac Comorbidities on Morbidity and Mortality in a Predominantly Male Population with Heart Failure and Preserved versus Reduced Ejection Fraction. J. Am. Coll. Cardiol. 2012, 59, 998–1005. [Google Scholar] [CrossRef] [Green Version]
- Biegus, J.; Zymliński, R.; Sokolski, M.; Todd, J.; Cotter, G.; Metra, M.; Jankowska, E.A.; Banasiak, W.; Ponikowski, P. Serial Assessment of Spot Urine Sodium Predicts Effectiveness of Decongestion and Outcome in Patients with Acute Heart Failure. Eur. J. Heart Fail. 2019, 21, 624–633. [Google Scholar] [CrossRef]
- Kamper, R.S.; Alcazar, J.; Andersen, L.L.; Haddock, B.; Jørgensen, N.R.; Hovind, P.; Suetta, C. Associations between Inflammatory Markers, Body Composition, and Physical Function: The Copenhagen Sarcopenia Study. J. Cachexia Sarcopenia Muscle 2021, 12, 1641–1652. [Google Scholar] [CrossRef] [PubMed]
- Milo-Cotter, O.; Cotter-Davison, B.; Lombardi, C.; Sun, H.; Bettari, L.; Bugatti, S.; Rund, M.; Metra, M.; Kaluski, E.; Kobrin, I.; et al. Neurohormonal Activation in Acute Heart Failure: Results from VERITAS. Cardiology 2011, 119, 96–105. [Google Scholar] [CrossRef]
- Amor-Salamanca, A.; Guzzo-Merello, G.; González-López, E.; Domínguez, F.; Restrepo-Córdoba, A.; Cobo-Marcos, M.; Gómez-Bueno, M.; Segovia-Cubero, J.; Alonso-Pulpón, L.; García-Pavía, P. Prognostic Impact and Predictors of Ejection Fraction Recovery in Patients with Alcoholic Cardiomyopathy. Rev. Esp. Cardiol. 2018, 71, 612–619, (In English and Spanish). [Google Scholar] [CrossRef]


| Demographics | Age, Sex |
| HF characteristics | De novo or chronic HF, Etiology |
| Comorbidities | Coronary artery disease (0 or 1), myocardial infarction (0 or 1), PCI/CABG (0 or 1), hypertension (0 or 1), valvular heart disease (0 or 1), diabetes (0 or 1), diabetes treated with: insulin = 1 Oral drugs = 2, diet = 3, stroke (0 or 1),COPD (0 or 1) |
| Clinical status | Dyspnoea at rest (0 or 1), Dyspnoea at rest lasts since (number) days, NYHA at admission, swelling of the lower limbs (lack = 0, 1 + (10–15 s) = 1, 2 + (15–30 s) = 2, 3 + (>30 s) = 3), Decrease in exercise tolerance (0 or 1), decrease in exercise tolerance (for how many days), body weight, systolic pressure, diastolic pressure, heart rate, jugular veins pressure (<6 cm = 1, 6–10 cm = 2, >10 cm = 3, not to be assessed = 4), pulmonary congestion (no—0; up to 1/3 of lungs—1; up to 2/3—2; >2/3—3), pulmonary congestion (0 or 1), ascites (0 or 1), hepatomegaly (0 or 1), implantable device, none = 0, 1-PM, 2-ICD, 3-CRT2 |
| Lifestyle factors | Smoking status (0 = never, 1 = now, 2 = in the past). If smoking in the past, how many cigarettes did the patient smoke? Alcohol (0 or 1), How many cigarettes do the patients smoke daily, How many years did the patient smoke/does the patient smoke cigarettes? |
| Laboratory parameters | HGB, HCT, RBC, MCV, MCH, MCHC, RDW, WBC, LYMPH, MONO, NEUTR, PLT, serum PH, pCO2, pO2, ctO2, BO2, HCO3, HCO3std, ctCO2, BE, sO2, FO2Hb, FHHb, ctHb, Lac, mOsm, Na in serum, K in serum, Creatinine in serum, Urea in serum, Glucose in serum, Ast, Alt, CRP, GGTP, NTproBNP, Total_bilirubin, INR, Albumin in serum, Troponin in serum, Urine Na, Urine K, Urine Urea, Urine Creatinine, Fe, TIBC, Tsat, sTfR, Ferritin, IL-6, eGFR |
| Echocardiography | Reduced ejection fraction (0 or 1); ejection fraction |
| Parameter | Cluster_0 | Cluster_1 | Cluster_2 | Cluster_3 | Cluster_4 | Cluster_5 | Global | p |
|---|---|---|---|---|---|---|---|---|
| Demographics | ||||||||
| n | 86 | 50 | 70 | 71 | 50 | 54 | 381 | - |
| Sex, male (n) | 78 (90.698%) | 23 (46%) | 58 (82.857%) | 53 (74.648%) | 49 (98%) | 24 (44.444%) | 285 (74.803%) | <0.001 |
| Age (years) | 67.293 [59–79] | 76.1 [68–81] | 58.821 [51.279–67.003] | 72 [63–80] | 66 [60.29–74.521] | 76.111 [64–82.992] | 68 [60–79] | <0.001 |
| aHF charcteristics | ||||||||
| Ejection fraction | 34 [28–43] | 47.5 [39–55] | 28 [20–40] | 30 [25–35] | 28 [20–35] | 50 [30–60] | 33 [25–45] | <0.001 |
| Chronic HF (n) | 32 (37.209%) | 22 (44%) | 34 (48.571%) | 69 (97.183%) | 47 (94%) | 38 (70.37%) | 242 (63.517%) | <0.001 |
| Reduced EF (n) | 67 (77.907%) | 16 (32%) | 58 (82.857%) | 66 (92.958%) | 45 (90%) | 17 (31.481%) | 269 (70.604%) | <0.001 |
| Etiology | <0.001 | |||||||
| Coronary artery disease (n) | 41 (47.674%) | 28 (56%) | 3 (4.286%) | 61 (85.915%) | 43 (86%) | 20 (37.037%) | 178 (46.719%) | |
| Valvular (n) | 5 (5.814%) | 2 (4%) | 15 (21.429%) | 3 (4.225%) | 1 (2%) | 2 (3.704%) | 46 (12.073%) | |
| Hypertension (n) | 1 (1.163%) | 5 (10%) | 1 (1.429%) | 1 (1.408%) | 1 (2%) | 4 (7.407%) | 13 (3.412%) | |
| Other (n) | 39 (45.349%) | 15 (30%) | 51 (72.857%) | 6 (8.451%) | 5 (10%) | 28 (51.852%) | 144 (37.795%) | |
| Comorbidites | ||||||||
| Coronary artery disease (n) | 56 (65.116%) | 38 (76%) | 1 (1.429%) | 69 (97.183%) | 49 (98%) | 5 (9.259%) | 218 (57.218%) | <0.001 |
| Myocardial infarction in the past (n) | 17 (19.767%) | 20 (40%) | 1 (1.429%) | 33 (46.479%) | 44 (88%) | 3 (5.556%) | 118 (30.971%) | <0.001 |
| PCI/CABG in the past (n) | 9 (10.465%) | 27 (54%) | 0 (0%) | 50 (70.423%) | 37 (74%) | 0 (0%) | 123 (32.283%) | <0.001 |
| Hypertension (n) | 72 (83.721%) | 47 (94%) | 27 (38.571%) | 56 (78.873%) | 38 (76%) | 47 (87.037%) | 286 (75.066%) | <0.001 |
| Valvular disease (n) | 52 (60.465%) | 16 (32%) | 43 (61.429%) | 57 (80.282%) | 38 (76%) | 38 (70.37%) | 244 (64.042%) | <0.001 |
| Diabetes mellitus (n) | 30 (34.884%) | 46 (92%) | 13 (18.571%) | 22 (30.986%) | 27 (54%) | 14 (25.926%) | 152 (39.895%) | <0.001 |
| Diabetes treatment (n) | ||||||||
| Insulin | 5 (5.814%) | 20 (40%) | 1 (1.429%) | 7 (9.859%) | 9 (18%) | 1 (1.852%) | 43 (11.286%) | |
| Oral drugs | 11 (12.791%) | 17 (34%) | 7 (10%) | 10 (14.085%) | 13 (26%) | 11 (20.37%) | 69 (18.11%) | |
| Diet | 5 (5.814%) | 4 (8%) | 0 (0%) | 1 (1.408%) | 4 (8%) | 0 (0%) | 14 (3.675%) | |
| Stroke (n) | 7 (8.14%) | 11 (22%) | 8 (11.429%) | 12 (16.901%) | 9 (18%) | 6 (11.111%) | 53 (13.911%) | <0.001 |
| COPD (n) | 8 (9.302%) | 11 (22%) | 4 (5.714%) | 12 (16.901%) | 8 (16%) | 7 (12.963%) | 50 (13.123%) | <0.001 |
| Clinical status | ||||||||
| Dyspnoea at rest (n) | 76 (88.372%) | 42 (84%) | 40 (57.143%) | 56 (78.873%) | 43 (86%) | 50 (92.593%) | 307 (80.577%) | <0.001 |
| Dyspnoea at rest lasts since (n) days | 3 [1–8] | 3 [1–7] | 3.5 [1–8.5] | 3 [2–8.5] | 3 [2–7] | 3 [2–6] | 3 [1–7] | 0.8 |
| Decrease in exercise tolerance (n) days | 14 [7–21] | 7 [6.5–29] | 14 [7–29] | 14 [7–28] | 10 [7–21] | 14 [6.5–30] | 14 [7–28] | 0.6 |
| NYHA (n) | 0.243 | |||||||
| I | 4 (4.651%) | 1 (2%) | 3 (4.286%) | 2 (2.817%) | 2 (4%) | 1 (1.852%) | 13 (3.412%) | |
| II | 11 (12.791%) | 8 (16%) | 13 (18.571%) | 7 (9.859%) | 13 (26%) | 10 (18.519%) | 62 (16.273%) | |
| III | 12 (13.953%) | 8 (16%) | 23 (32.857%) | 26 (36.62%) | 9 (18%) | 9 (16.667%) | 87 (22.835%) | |
| IV | 46 (53.488%) | 27 (54%) | 23 (32.857%) | 36 (50.704%) | 26 (52%) | 31 (57.407%) | 189 (49.606%) | |
| Swelling of lower limbs (n) | 0.006 | |||||||
| Swelling of lower limbs 0 | 18 (20.93%) | 16 (32%) | 26 (37.143%) | 19 (26.761%) | 16 (32%) | 7 (12.963%) | 102 (26.772%) | |
| Swelling of lower limbs 1 | 15 (17.442%) | 15 (30%) | 16 (22.857%) | 18 (25.352%) | 10 (20%) | 16 (29.63%) | 90 (23.622%) | |
| Swelling of lower limbs 2 | 27 (31.395%) | 13 (26%) | 17 (24.286%) | 23 (32.394%) | 11 (22%) | 16 (29.63%) | 107 (28.084%) | |
| Swelling of lower limbs 3 | 26 (30.233%) | 6 (12%) | 10 (14.286%) | 11 (15.493%) | 13 (26%) | 15 (27.778%) | 81 (21.26%) | |
| Deterioration of Effort Tolerance (n) | 79 (91.86%) | 47 (94%) | 63 (90%) | 67 (94.366%) | 49 (98%) | 53 (98.148%) | 358 (93.963%) | 0.407 |
| JVP (n) | <0.001 | |||||||
| JVP 1 | 57 (66.279%) | 32 (64%) | 42 (60%) | 53 (74.648%) | 17 (34%) | 31 (57.407%) | 232 (60.892%) | |
| JVP 2 | 24 (27.907%) | 17 (34%) | 23 (32.857%) | 18 (25.352%) | 25 (50%) | 21 (38.889%) | 128 (33.596%) | |
| JVP 3 | 5 (5.814%) | 0 (0%) | 5 (7.143%) | 0 (0%) | 8 (16%) | 2 (3.704%) | 20 (5.249%) | |
| Pulmonary edema (n) | <0.001 | |||||||
| no | 11 (12.791%) | 1 (2%) | 12 (17.143%) | 2 (2.817%) | 7 (14%) | 6 (11.111%) | 39 (10.236%) | |
| up to 1/3 of lungs | 49 (56.977%) | 23 (46%) | 45 (64.286%) | 50 (70.423%) | 31 (62%) | 25 (46.296%) | 223 (58.53%) | |
| up to 2/3 | 20 (23.256%) | 14 (28%) | 9 (12.857%) | 13 (18.31%) | 11 (22%) | 16 (29.63%) | 83 (21.785%) | |
| >2/3 | 6 (6.977%) | 11 (22%) | 4 (5.714%) | 6 (8.451%) | 1 (2%) | 7 (12.963%) | 35 (9.186%) | |
| Pulmonary congestion (n) | 75 (87.209%) | 48 (96%) | 58 (82.857%) | 69 (97.183%) | 43 (86%) | 48 (88.889%) | 341 (89.501%) | 0.048 |
| Ascites (n) | 15 (17.442%) | 3 (6%) | 9 (12.857%) | 2 (2.817%) | 13 (26%) | 8 (14.815%) | 50 (13.123%) | 0.003 |
| Hepatomegaly (n) | 29 (33.721%) | 8 (16%) | 11 (15.714%) | 1 (1.408%) | 27 (54%) | 6 (11.111%) | 82 (21.522%) | <0.001 |
| Implantable device (n) | <0.001 | |||||||
| PM | 2 (2.326%) | 8 (16%) | 2 (2.857%) | 8 (11.268%) | 2 (4%) | 6 (11.111%) | 28 (7.349%) | |
| ICD | 3 (3.488%) | 1 (2%) | 8 (11.429%) | 31 (43.662%) | 9 (18%) | 3 (5.556%) | 55 (14.436%) | |
| CRT | 2 (2.326%) | 1 (2%) | 3 (4.286%) | 3 (4.225%) | 15 (30%) | 2 (3.704%) | 26 (6.824%) | |
| Systolic pressure (mmHg) | 140 [120–158] | 160 [135–180] | 120 [105–131] | 126.5 [110–137] | 120 [102–145] | 120 [107–142] | 130 [110–150] | <0.001 |
| Diastolic pressure (mmHg) | 80 [70–95.5] | 80 [70–95] | 77.5 [70–87] | 80 [70–85] | 70 [62–80] | 70 [65–80] | 79 [70–90] | <0.001 |
| Heart rate (bpm) | 90 [75–110] | 80 [70–100] | 90.5 [80–105] | 80 [70–100] | 78 [70–90] | 88 [72–110] | 82.5 [70–100] | <0.001 |
| Body weight (kg) | 85.3 [77–98] | 79 [69–90.95] | 77.6 [68.5–88.3] | 77.4 [70.4–91] | 80.5 [71–94] | 74.9 [65–82] | 79.6 [70–91.5] | <0.001 |
| Lifestyle factors | ||||||||
| Smoking status (n) | <0.001 | |||||||
| Never | 41 (47.674%) | 32 (64%) | 35 (50%) | 49 (69.014%) | 8 (16%) | 36 (66.667%) | 201 (52.756%) | |
| Active | 23 (26.744%) | 3 (6%) | 21 (30%) | 7 (9.859%) | 4 (8%) | 3 (5.556%) | 61 (16.01%) | |
| In the past | 22 (25.581%) | 15 (30%) | 14 (20%) | 15 (21.127%) | 38 (76%) | 15 (27.778%) | 119 (31.234%) | |
| How many cigarettes do patients smoke daily (n) | 0.08 [0–15] | 1 [0–8] | 0 [0–15] | 0 [0–9] | 15 [4–20] | 3 [0–12] | 2 [0–15] | 0.047 |
| How many years did the patient smoke/does the patient smoke cigarettes (n) | 22.5 [0–30] | 20 [0–30] | 11.5 [0–30] | 0 [0–30] | 20 [5–30] | 0 [0–30] | 20 [0–30] | 0.36 |
| Active alcohol use (n) | 20 (23.256%) | 8 (16%) | 31 (44.286%) | 16 (22.535%) | 19 (38%) | 12 (22.222%) | 106 (27.822%) | 0.002 |
| Laboratory parameters | ||||||||
| HGB (g/dL) | 13.727 ± 1.881 | 11.972 ± 1.81 | 13.975 ± 1.651 | 13.213 ± 1.817 | 13.194 ± 2.114 | 12.391 ± 1.801 | 13.184 ± 1.953 | <0.001 |
| HCT (%) | 41.232 ± 5.21 | 36.686 ± 5.191 | 41.684 ± 4.665 | 39.907 ± 5.163 | 40.066 ± 6.319 | 37.343 ± 4.854 | 39.759 ± 5.49 | <0.001 |
| RBC (× 1012/L) | 4.544 ± 0.662 | 4.18 ± 0.55 | 4.595 ± 0.495 | 4.499 ± 0.65 | 4.516 ± 0.716 | 4.226 ± 0.628 | 4.448 ± 0.636 | <0.001 |
| MCH (pg) | 30.333 ± 2.325 | 28.692 ± 2.728 | 30.457 ± 2.269 | 29.49 ± 2.261 | 29.255 ± 2.565 | 29.479 ± 2.986 | 29.718 ± 2.552 | <0.001 |
| MCV fL | 91.188 ± 6.241 | 87.846 ± 6.236 | 90.854 ± 5.707 | 89.057 ± 6.144 | 89.034 ± 6.797 | 88.834 ± 6.451 | 89.668 ± 6.31 | 0.02 |
| WBC (× 109/L) | 8.6 [6.8–10.68] | 9.35 [6.7–12.3] | 8.25 [6.3–9.85] | 7.8 [6.4–9.52] | 8.44 [7.1–10.4] | 8.3 [6.1–9.9] | 8.3 [6.6–10.35] | 0.01 |
| PLT (× 109/L) | 214 [152–252.5] | 211 [163–298] | 197.5 [164.5–233] | 192 [149–234] | 195 [159–250] | 203 [144–242] | 198 [155–245] | 0.04 |
| pH | 7.44 [7.415–7.47] | 7.4 [7.35–7.46] | 7.45 [7.42–7.48] | 7.45 [7.43–7.47] | 7.45 [7.415–7.485] | 7.45 [7.385–7.48] | 7.44 [7.41–7.47] | <0.001 |
| pCO2 (mmHg) | 34.4 [31.55–38.7] | 37.3 [32.7–42.9] | 34.55 [30.9–36.55] | 34.55 [32.2–37.5] | 33.6 [31.6–38.25] | 36.2 [33.05–39.45] | 35.1 [31.8–38.9] | <0.001 |
| HCO3std (mmol/L) | 24.016 ± 3.193 | 22.989 ± 3.657 | 24.592 ± 2.474 | 24.676 ± 2.684 | 24.602 ± 3.376 | 25.321 ± 3.688 | 24.367 ± 3.203 | 0.01 |
| pO2 (mmHg) | 64.4 [57.15–73.15] | 66.3 [61.2–78.7] | 70.2 [62.3–75.5] | 65.6 [58.2–74.3] | 67.3 [60.05–74.7] | 65.15 [57.65–71.8] | 66.1 [59–74.6] | 0.8 |
| sO2 (%) | 92.1 [89.15–95.05] | 93.45 [90.6–94.9] | 94.45 [91.45–95.95] | 92.8 [89.9–94.9] | 93.1 [90.4–96] | 93.05 [90.2–95.4] | 93.1 [90.1–95.4] | 0.9 |
| mOsm (Osm/L) | 282.5 [274–286] | 286.5 [279–291] | 283 [274–287] | 281 [274–286] | 277.5 [272–286] | 279.5 [270–287] | 282 [274–287] | 0.01 |
| K (mmol/L) | 4.187 ± 0.577 | 4.481 ± 0.788 | 4.197 ± 0.484 | 4.185 ± 0.521 | 4.197 ± 0.622 | 4.063 ± 0.694 | 4.21 ± 0.614 | 0.02 |
| Na (mmol/L) | 140 [137–142] | 140 [137–142] | 139 [135.5–141.5] | 139 [137–142] | 138 [135–140] | 138.5 [135–141] | 139 [136–142] | 0.145 |
| Glucose (mg/dL) | 124 [100–162] | 144 [121–212] | 110 [99.5–131] | 113 [101–139] | 126.5 [107–150] | 117 [105–143] | 121 [103–151.5] | <0.001 |
| INR | 1.26 [1.08–1.48] | 1.31 [1.09–1.99] | 1.31 [1.14–1.77] | 1.54 [1.18–2.24] | 1.42 [1.17–2.08] | 1.46 [1.2–2.21] | 1.35 [1.12–1.97] | 0.06 |
| Total bilirubin (mg/dL) | 0.96 [0.72–1.46] | 0.785 [0.505–1.275] | 1.25 [0.765–1.755] | 1.145 [0.775–1.945] | 1.225 [0.855–1.705] | 1.03 [0.79–1.9] | 1.07 [0.73–1.7] | 0.09 |
| Albumin (g/dL) | 3.675 ± 0.402 | 3.775 ± 0.342 | 3.755 ± 0.406 | 3.831 ± 0.328 | 3.766 ± 0.386 | 3.648 ± 0.466 | 3.739 ± 0.394 | 0.1 |
| Ast (IU/L) | 29 [21.5–44.5] | 26 [17–37] | 30 [22–40] | 26 [20–37] | 26.5 [18–34.5] | 27 [20.5–38.5] | 27 [20–40] | 0.5 |
| Alt (IU/L) | 28 [21.5–58] | 28 [17–41] | 34.5 [21.5–55] | 30.5 [21–53] | 27.5 [16.5–40.5] | 24.5 [15.5–32] | 29 [19–48] | 0.7 |
| GGTP (IU/L) | 70 [40–127] | 54.5 [39.5–102.5] | 82 [48–166] | 72 [48–133] | 104 [45–183] | 60.5 [28–113.5] | 71 [41–128] | 0.8 |
| TIBC (μg/dL) | 331.45 ± 63.813 | 336.5 ± 84.925 | 362.968 ± 66.412 | 364.09 ± 68.448 | 366.302 ± 60.677 | 338.765 ± 72.717 | 349.457 ± 70.214 | 0.007 |
| Fe (μg/dL) | 48 [36–66.5] | 47.5 [31.5–65.5] | 60 [47–84] | 55 [43–79] | 62 [43–83] | 50 [37–61] | 54 [40–71] | 0.009 |
| Ferritin (ng/mL) | 162.5 [85.325–252] | 147.5 [57–249] | 124 [52–224] | 92 [54–156] | 94.985 [53.68–146] | 119.6 [67.36–200] | 109.3 [61–224] | 0.02 |
| Tsat (%) | 15.25 [10.113–20.1] | 15.05 [9.263–19.057] | 16.958 [13.2–25.455] | 14.8 [11.4–21.4] | 17 [12.429–23.4] | 15.9 [12.4–18.3] | 15.654 [11.609–21.05] | 0.46 |
| sTfR (mg/L) | 1.72 [1.42–2.72] | 2.02 [1.445–2.635] | 1.73 [1.41–2.08] | 1.97 [1.69–2.51] | 1.905 [1.59–2.46] | 1.79 [1.3–2.73] | 1.87 [1.46–2.51] | 0.66 |
| NTproBNP (pg/mL) | 5218 [2674–12496] | 4191 [2025–6048] | 7189 [5023–12849] | 5437 [3612–10572] | 5712.5 [3452.5–11170.5] | 5337 [2398–8775] | 5580 [3169–10421] | 0.03 |
| Troponin (ng/mL) | 0.042 [0.022–0.12] | 0.049 [0.025–0.156] | 0.032 [0.017–0.094] | 0.058 [0.03–0.156] | 0.05 [0.029–0.13] | 0.05 [0.02–0.14] | 0.05 [0.022–0.127] | 0.03 |
| CRP (mg/L) | 8.6 [4.4–19.3] | 6.8 [3.05–27.25] | 6.15 [3.2–14.05] | 7.425 [3.8–14.5] | 6.95 [3.25–16.05] | 8.18 [3.86–19.4] | 7.395 [3.5–18] | 0.18 |
| IL6 (pg/mL) | 12.108 [4.428–26.822] | 10.999 [0.633–27.125] | 7.979 [0.5–19.923] | 8.315 [0.5–14.6] | 8 [4.851–16.927] | 13.82 [3.785–38.5] | 9.989 [2.528–22.89] | 0.29 |
| Lactates (mmol/L) | 2 [1.4–2.4] | 1.95 [1.5–2.7] | 2 [1.6–2.7] | 1.8 [1.5–2.4] | 2.1 [1.45–2.7] | 2 [1.5–2.75] | 2 [1.5–2.6] | 0.64 |
| Urea (mmol/L) | 47 [37–73] | 55 [39–78] | 49.5 [38–68] | 53.5 [43–74] | 64 [44–86] | 44 [35–65] | 51 [38–73] | 0.3 |
| Creatinine (mg/dL) | 1.16 [1.03–1.5] | 1.32 [0.93–1.7] | 1.1 [0.935–1.295] | 1.23 [1.03–1.49] | 1.355 [1.09–1.8] | 1.2 [0.95–1.44] | 1.225 [1–1.505] | 0.003 |
| eGFR (mL/min/1.73m2) | 84.463 ± 26.383 | 68.036 ± 29.564 | 94.693 ± 31.385 | 76.697 ± 22.711 | 77.859 ± 34.792 | 79.116 ± 43.668 | 81.074 ± 32.041 | <0.001 |
| Urine Urea (mmol/L) | 1131 [555.5–1585] | 512 [369–905] | 886 [484–1674] | 730 [442–1330] | 887 [487–1509] | 514 [339.5–981] | 780 [442–1403] | <0.001 |
| Urine Creatinine (mg/dL) | 80.55 [41.75–147.6] | 33.5 [21.7–79.2] | 73.2 [34.7–129.1] | 61.5 [28.9–105] | 52.9 [38.9–136.8] | 42 [23.55–80.65] | 59.1 [30.1–110] | <0.001 |
| Urine K (mmol/L) | 35.765 [20–49.04] | 22.75 [15–32] | 28.73 [20–41] | 27 [17.14–37] | 31.5 [27–50.44] | 29.5 [17–41.5] | 29.77 [19–42.59] | <0.001 |
| Urine Na(mmol/L) | 87.286 ± 39.226 | 95.432 ± 32.757 | 90.87 ± 42.771 | 87.594 ± 37.329 | 84.533 ± 34.78 | 96.269 ± 36.412 | 89.959 ± 37.886 | 0.55 |
| Cluster | Key Clinical Feature |
|---|---|
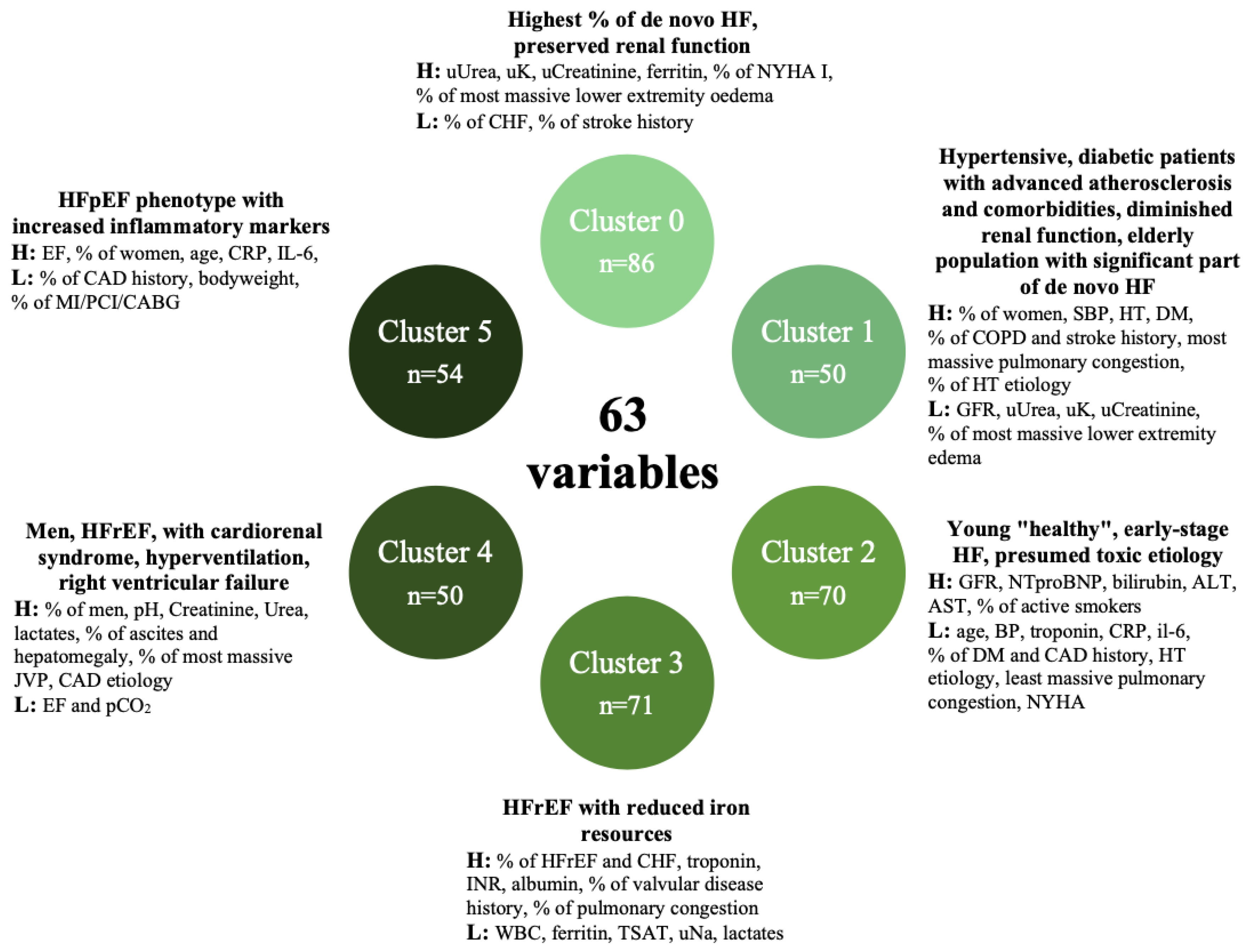
| Cluster 0 | Lowest % of chronic HF, most massive lower limbs oedema, highest urine urea, k, creatinine, highest ferritin, highest % of NYHA I, lowest % stroke history, better prognosis—highest % of de novo HF, with preserved renal function. |
| Cluster 1 | Higher % of women than in the rest of the population, highest systolic pressure, highest hypertension, diabetes, chronic obstructive pulmonary disease and stroke history (lowest GFR, lowest urine creatinine, urea and K, lowest NTproBNP), most massive pulmonary congestion and least massive peripheral oedema, highest hypertension etiology, better prognosis—hypertensive, diabetic patients with advanced atherosclerosis and comorbidities, diminished renal function, elderly population with a significant part of de novo HF. |
| Cluster 2 | Youngest patients, low NYHA and ejection fraction, lowest blood pressure, troponin, CRP and IL-6, lowest % diabetes history, lowest % of CAD history and etiology, lowest hypertension etiology, highest “other” etiology, highest GFR, NTproBNP, bilirubin, Alt, Ast, highest % of active smokers, least massive pulmonary congestion, better prognosis—young “healthy”, early-stage HF, presumed toxic etiology. |
| Cluster 3 | Lowest WBC, ferritin, urine Na, Tsat, lactates, highest troponin, INR, albumin, highest % of HFrEF and chronic HF, highest % of valvular disease history, highest % of pulmonary congestion (97%), mean prognosis—HFrEF with reduced iron resources. |
| Cluster 4 | Predominantly man, highest pH, creatinine, urea, lactates, lowest ejection fraction and pCO2, highest % of ascites and hepatomegaly, most massive JVP, highest CAD etiology, worse prognosis—men, HFrEF, with cardiorenal syndrome, hyperventilation, right ventricular failure. |
| Cluster 5 | Highest EF, no CAD history (0%), oldest population, highest % of women, highest CRP, IL6, lowest body weight, low % of MI/PCI/CABG, worst prognosis—HFpEF phenotype with increased inflammatory markers. |
| Cluster 5 | Cluster 4 | Cluster 3 | Cluster 2 | Cluster 1 | Cluster 0 | p | |
|---|---|---|---|---|---|---|---|
| One-year mortality | 45.3% | 40% | 21.1% | 17.1% | 22% | 25.6% | 0.002 |
| One-year mortality or HF rehospitalization | 68.1% | 77.3% | 55.7% | 63.2% | 55.3% | 53.5% | 0.112 |
| In-hospital deterioration | 8.5% | 16.3% | 8.2% | 3.1% | 15.2% | 7.8% | 0.1 |
| Duration of hosp. [days] | 9.3 ± 5.7 | 9.4 ± 6.8 | 6.7 ± 3.4 | 8.2 ± 7.5 | 9.7 ± 8.5 | 9.0 ± 7.3 | 0.1 |
| One-Year Mortality Risk | |||
|---|---|---|---|
| X2 | Hazard Ratio (95% Confidence Interval) | p | |
| Cluster 0 | 0.194 | 0.900 [0.562–1.441] | 0.662 |
| Cluster 1 | 0.679 | 0.776 [0.415–1.449] | 0.425 |
| Cluster 2 | 4.807 | 0.537 [0.294–0.979] | 0.043 |
| Cluster 3 | 1.964 | 0.688 [0.397–1.188] | 0.179 |
| Cluster 4 | 4.393 | 1.738 [1.067–2.831] | 0.026 |
| Cluster 5 | 8.753 | 2.095 [1.327–3.306] | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urban, S.; Błaziak, M.; Jura, M.; Iwanek, G.; Zdanowicz, A.; Guzik, M.; Borkowski, A.; Gajewski, P.; Biegus, J.; Siennicka, A.; et al. Novel Phenotyping for Acute Heart Failure—Unsupervised Machine Learning-Based Approach. Biomedicines 2022, 10, 1514. https://doi.org/10.3390/biomedicines10071514
Urban S, Błaziak M, Jura M, Iwanek G, Zdanowicz A, Guzik M, Borkowski A, Gajewski P, Biegus J, Siennicka A, et al. Novel Phenotyping for Acute Heart Failure—Unsupervised Machine Learning-Based Approach. Biomedicines. 2022; 10(7):1514. https://doi.org/10.3390/biomedicines10071514
Chicago/Turabian StyleUrban, Szymon, Mikołaj Błaziak, Maksym Jura, Gracjan Iwanek, Agata Zdanowicz, Mateusz Guzik, Artur Borkowski, Piotr Gajewski, Jan Biegus, Agnieszka Siennicka, and et al. 2022. "Novel Phenotyping for Acute Heart Failure—Unsupervised Machine Learning-Based Approach" Biomedicines 10, no. 7: 1514. https://doi.org/10.3390/biomedicines10071514
APA StyleUrban, S., Błaziak, M., Jura, M., Iwanek, G., Zdanowicz, A., Guzik, M., Borkowski, A., Gajewski, P., Biegus, J., Siennicka, A., Pondel, M., Berka, P., Ponikowski, P., & Zymliński, R. (2022). Novel Phenotyping for Acute Heart Failure—Unsupervised Machine Learning-Based Approach. Biomedicines, 10(7), 1514. https://doi.org/10.3390/biomedicines10071514






