Optogenetically Engineered Neurons Differentiated from Human SH-SY5Y Cells Survived and Expressed ChR2 in 3D Hydrogel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Modification of Alginate Hydrogels
2.3. Characterization of RGD-Alginate Hydrogels
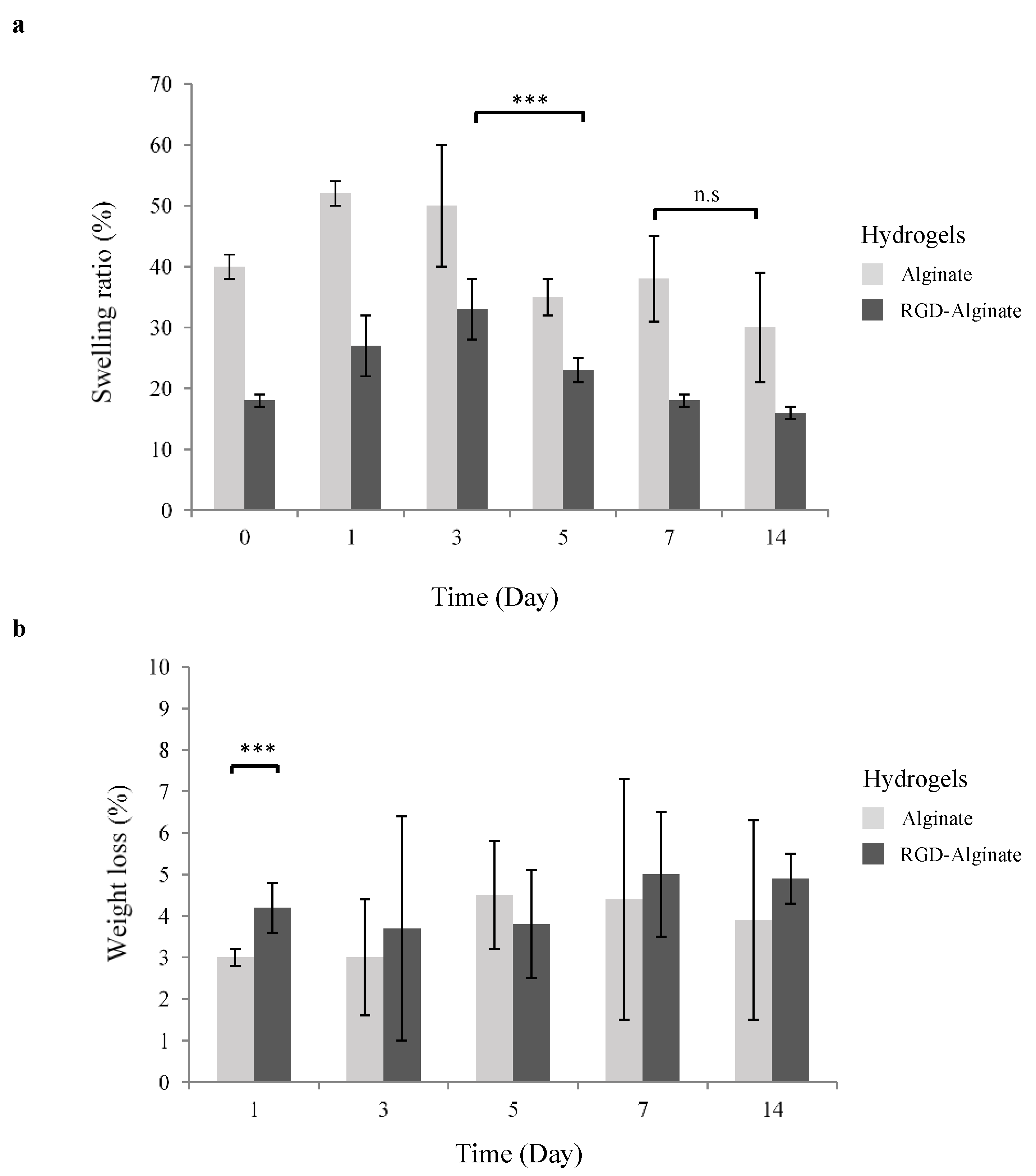
2.3.1. Swelling Test
2.3.2. Degradability Measurement
2.4. Optogenetic Modification and Neuronal Differentiation of Human SH-SY5Y Cells
2.4.1. Lentiviral Production
2.4.2. Neuronal Differentiation
2.5. Evaluation of Opsin Gene Expression and Differentiated Neuronal Population
2.5.1. Optogenetic Transduction and Efficiency
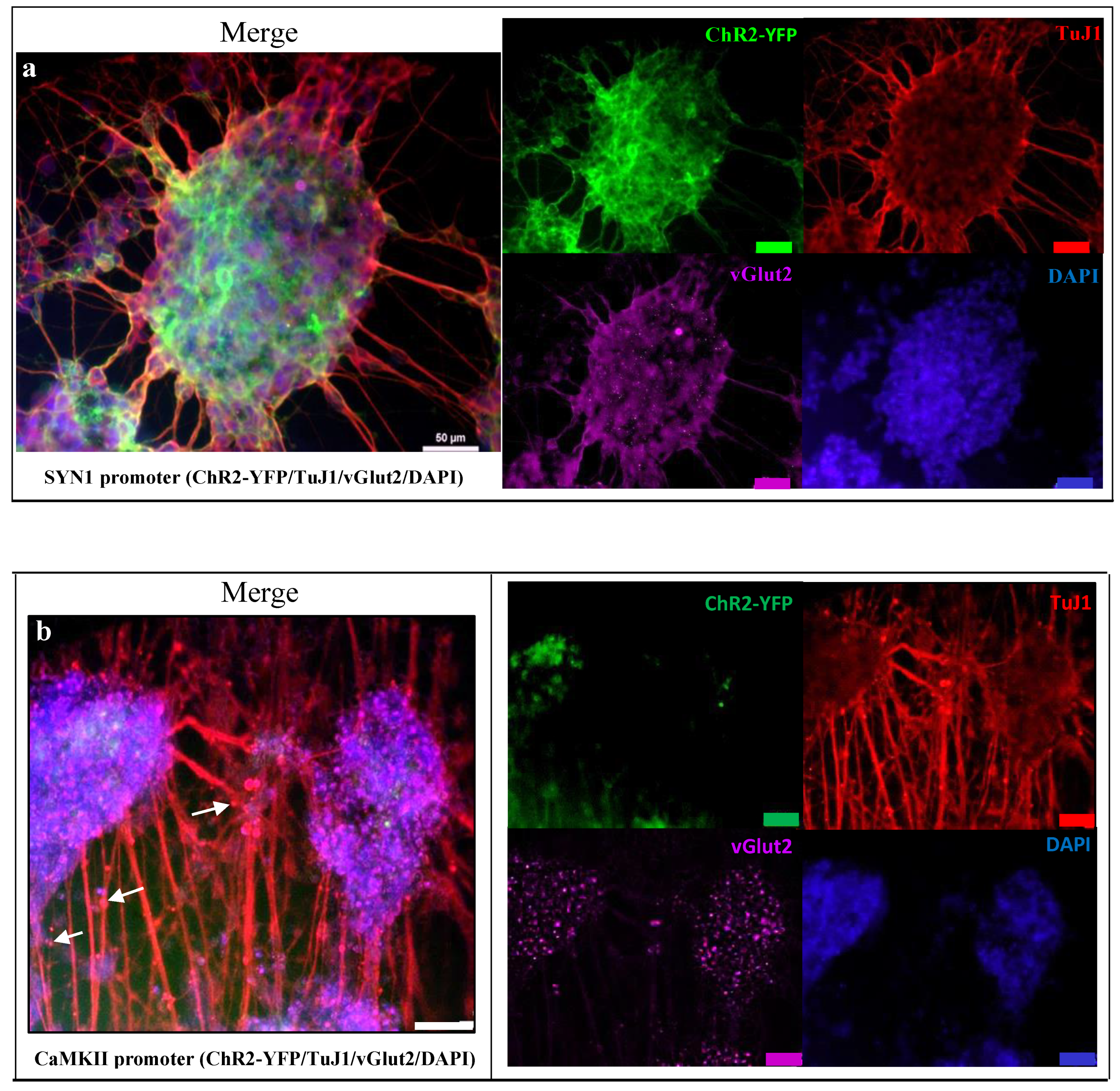
2.5.2. Immunofluorescent Staining for Neuronal Expression
2.5.3. Optogenetic Stimulation and Calcium Imaging
2.6. In Vitro Culture in RGD-Alginate Hydrogel and Evaluation
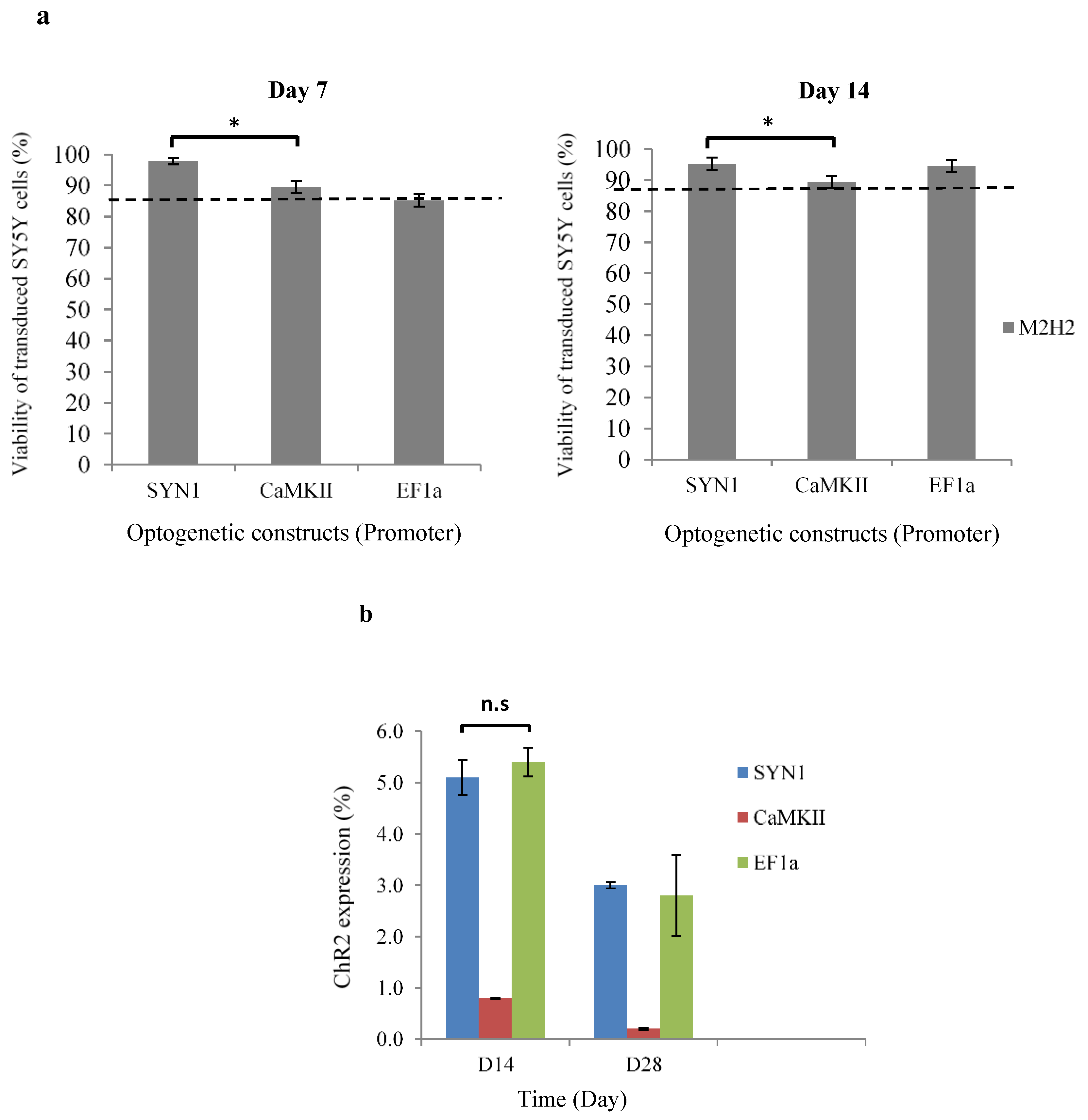
2.6.1. Cell Viability
2.6.2. ChR2 Expression
2.7. Statistical Analysis
3. Results and Discussion
3.1. Physical Properties of 3D Hydrogels
3.2. Expression of ChR2-YFP in Differentiated Human SH-SY5Y Cells
3.3. Neuronal Expression Post Optogenetically Modification and Differentiation
3.4. Effects of Optogenetic Stimulation
3.5. Cell Viability and ChR2 Expression in RGD-Alginate Hydrogels
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, W.; Li, Q.; Li, B.; Ma, K.; Zhang, C.; Fu, X. Optogenetics Sheds New Light on Tissue Engineering and Regenerative Medicine. Biomaterials 2020, 227, 119546. [Google Scholar] [CrossRef] [PubMed]
- Osaki, T.; Uzel, S.G.M.; Kamm, R.D. Microphysiological 3D Model of Amyotrophic Lateral Sclerosis (ALS) from Human IPS-Derived Muscle Cells and Optogenetic Motor Neurons. Sci. Adv. 2018, 4, eaat5847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.-Y.; Ma, J.; Khoo, T.S.; Abdullah, N.; Nik Md Noordin Kahar, N.N.F.; Abdul Hamid, Z.A.; Mustapha, M. Polysaccharide-Based Hydrogels for Microencapsulation of Stem Cells in Regenerative Medicine. Front. Bioeng. Biotechnol. 2021, 9, 1–19. [Google Scholar] [CrossRef]
- Andersen, T.; Auk-Emblem, P.; Dornish, M. 3D Cell Culture in Alginate Hydrogels. Microarrays 2015, 4, 133–161. [Google Scholar] [CrossRef] [PubMed]
- Pawar, S.N.; Edgar, K.J. Alginate Derivatization: A Review of Chemistry, Properties and Applications. Biomaterials 2012, 33, 3279–3305. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [Green Version]
- Hasany, M.; Talebian, S.; Sadat, S.; Ranjbar, N.; Mehrali, M.; Wallace, G.G.; Mehrali, M. Synthesis, Properties, and Biomedical Applications of Alginate Methacrylate (ALMA)-Based Hydrogels: Current Advances and Challenges. Appl. Mater. Today 2021, 24, 101150. [Google Scholar] [CrossRef]
- Bordoni, M.; Karabulut, E.; Kuzmenko, V.; Fantini, V.; Pansarasa, O.; Cereda, C.; Gatenholm, P. 3D Printed Conductive Nanocellulose Scaffolds for the Differentiation of Human Neuroblastoma Cells. Cells 2020, 9, 682. [Google Scholar] [CrossRef] [Green Version]
- Rowley, J.A.; Mooney, D.J. Alginate Type and RGD Density Control Myoblast Phenotype. J. Biomed. Mater. Res. 2002, 60, 217–223. [Google Scholar] [CrossRef]
- Rowley, J.A.; Madlambayan, G.; Mooney, D.J. Alginate Hydrogels as Synthetic Extracellular Matrix Materials. Biomaterials 1999, 20, 45–53. [Google Scholar] [CrossRef]
- Maia, F.R.; Lourenço, A.H.; Granja, P.L.; Gonçalves, R.M.; Barrias, C.C. Effect of Cell Density on Mesenchymal Stem Cells Aggregation in RGD-Alginate 3D Matrices under Osteoinductive Conditions. Macromol. Biosci. 2014, 14, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Bidarra, S.J.; Barrias, C.C.; Fonseca, K.B.; Barbosa, M.A.; Soares, R.A.; Granja, P.L. Injectable in Situ Crosslinkable RGD-Modified Alginate Matrix for Endothelial Cells Delivery. Biomaterials 2011, 32, 7897–7904. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; George, J.H.; Nagel, D.A.; Ye, H.; Kueberuwa, G.; Seymour, L.W. Optogenetic Control of IPS Cell-derived Neurons in 2D and 3D Culture Systems Using Channelrhodopsin-2 Expression Driven by the Synapsin-1 and Calcium-calmodulin Kinase II Promoters. J. Tissue Eng. Regen. Med. 2019, 13, 369–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vila, O.F.; Chavez, M.; Ma, S.P.; Yeager, K.; Zholudeva, L.V.; Colón-Mercado, J.M.; Qu, Y.; Nash, T.R.; Lai, C.; Feliciano, C.M.; et al. Bioengineered Optogenetic Model of Human Neuromuscular Junction. Biomaterials 2021, 276, 121033. [Google Scholar] [CrossRef]
- Khwanraj, K.; Phruksaniyom, C.; Madlah, S.; Dharmasaroja, P. Differential Expression of Tyrosine Hydroxylase Protein and Apoptosis-Related Genes in Differentiated and Undifferentiated SH-SY5Y Neuroblastoma Cells Treated with MPP+. Neurol. Res. Int. 2015, 2015, 734703. [Google Scholar] [CrossRef] [Green Version]
- Jämsä, A.; Hasslund, K.; Cowburn, R.F.; Bäckström, A.; Vasänge, M. The Retinoic Acid and Brain-Derived Neurotrophic Factor Differentiated SH-SY5Y Cell Line as a Model for Alzheimer’s Disease-like Tau Phosphorylation. Biochem. Biophys. Res. Commun. 2004, 319, 993–1000. [Google Scholar] [CrossRef]
- Roy, D.S.; Arons, A.; Mitchell, T.I.; Pignatelli, M.; Ryan, T.J.; Tonegawa, S. Memory Retrieval by Activating Engram Cells in Mouse Models of Early Alzheimer’s Disease. Nature 2016, 531, 508–512. [Google Scholar] [CrossRef] [Green Version]
- Sanders, T.H.; Jaeger, D. Optogenetic Stimulation of Cortico-Subthalamic Projections Is Sufficient to Ameliorate Bradykinesia in 6-Ohda Lesioned Mice. Neurobiol. Dis. 2016, 95, 225–237. [Google Scholar] [CrossRef] [Green Version]
- Forster, J.I.; Köglsberger, S.; Trefois, C.; Boyd, O.; Baumuratov, A.S.; Buck, L.; Balling, R.; Antony, P.M.A. Characterization of Differentiated SH-SY5Y as Neuronal Screening Model Reveals Increased Oxidative Vulnerability. J. Biomol. Screen. 2016, 21, 496–509. [Google Scholar] [CrossRef] [Green Version]
- Shipley, M.M.; Mangold, C.A.; Szpara, M.L. Differentiation of the SH-SY5Y Human Neuroblastoma Cell Line. J. Vis. Exp. 2016, 2016, 53193. [Google Scholar] [CrossRef]
- Simões, R.F.; Ferrão, R.; Silva, M.R.; Pinho, S.L.C.; Ferreira, L.; Oliveira, P.J.; Cunha-Oliveira, T. Refinement of a Differentiation Protocol Using Neuroblastoma SH-SY5Y Cells for Use in Neurotoxicology Research. Food Chem. Toxicol. 2021, 149, 111967. [Google Scholar] [CrossRef] [PubMed]
- Encinas, M.; Iglesias, M.; Liu, Y.; Wang, H.; Muhaisen, A.; Ceña, V.; Gallego, C.; Comella, J.X. Sequential Treatment of SH-SY5Y Cells with Retinoic Acid and Brain-Derived Neurotrophic Factor Gives Rise to Fully Differentiated, Neurotrophic Factor-Dependent, Human Neuron-Like Cells. J. Neurochem. 2002, 75, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Gaharwar, A.K.; Dammu, S.A.; Canter, J.M.; Wu, C.-J.; Schmidt, G. Highly Extensible, Tough, and Elastomeric Nanocomposite Hydrogels from Poly(Ethylene Glycol) and Hydroxyapatite Nanoparticles. Biomacromolecules 2011, 12, 1641–1650. [Google Scholar] [CrossRef] [PubMed]
- Fedorovich, N.E.; Alblas, J.; de Wijn, J.R.; Hennink, W.E.; Verbout, A.J.; Dhert, W.J.A. Hydrogels as Extracellular Matrices for Skeletal Tissue Engineering: State-of-the-Art and Novel Application in Organ Printing. Tissue Eng. 2007, 13, 1905–1925. [Google Scholar] [CrossRef] [PubMed]
- Davidovich-Pinhas, M.; Bianco-Peled, H. A Quantitative Analysis of Alginate Swelling. Carbohydr. Polym. 2010, 79, 1020–1027. [Google Scholar] [CrossRef]
- Bajpai, S.K.; Sharma, S. Investigation of Swelling/Degradation Behaviour of Alginate Beads Crosslinked with Ca2+ and Ba2+ Ions. React. Funct. Polym. 2004, 59, 129–140. [Google Scholar] [CrossRef]
- Chawla, K.; Yu, T.; Liao, S.W.; Guan, Z. Biodegradable and Biocompatible Synthetic Saccharide−Peptide Hydrogels for Three-Dimensional Stem Cell Culture. Biomacromolecules 2011, 12, 560–567. [Google Scholar] [CrossRef] [Green Version]
- Ni, Y.; Tang, Z.; Yang, J.; Gao, Y.; Lin, H.; Guo, L.; Zhang, K.; Zhang, X. Collagen Structure Regulates MSCs Behavior by MMPs Involved Cell–Matrix Interactions. J. Mater. Chem. B 2018, 6, 312–326. [Google Scholar] [CrossRef]
- Xia, Z.; Dickens, M.; Raingeaud, J.; Davis, R.J.; Greenberg, M.E. Opposing Effects of ERK and JNK-P38 MAP Kinases on Apoptosis. Science 1995, 270, 1326–1331. [Google Scholar] [CrossRef]
- Dittgen, T.; Nimmerjahn, A.; Komai, S.; Licznerski, P.; Waters, J.; Margrie, T.W.; Helmchen, F.; Denk, W.; Brecht, M.; Osten, P. Lentivirus-Based Genetic Manipulations of Cortical Neurons and Their Optical and Electrophysiological Monitoring in Vivo. Proc. Natl. Acad. Sci. USA 2004, 101, 18206–18211. [Google Scholar] [CrossRef] [Green Version]
- Rein, M.L.; Deussing, J.M. The Optogenetic (r)Evolution. Mol. Genet. Genomics 2012, 287, 95–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaguchi, M.; Ohashi, Y.; Tsubota, T.; Sato, A.; Koyano, K.W.; Wang, N.; Miyashita, Y. Characterization of the Properties of Seven Promoters in the Motor Cortex of Rats and Monkeys After Lentiviral Vector-Mediated Gene Transfer. Hum. Gene Ther. Methods 2013, 24, 333–344. [Google Scholar] [CrossRef] [Green Version]
- Balachandar, L.; Borrego, D.; Diaz, J.R. Serotype-Based Evaluation of an Optogenetic Construct in Rat Cortical Astrocytes. Biochem. Biophys. Res. Commun. 2022, 593, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Kügler, S.; Kilic, E.; Bähr, M. Human Synapsin 1 Gene Promoter Confers Highly Neuron-Specific Long-Term Transgene Expression from an Adenoviral Vector in the Adult Rat Brain Depending on the Transduced Area. Gene Ther. 2003, 10, 337–347. [Google Scholar] [CrossRef] [Green Version]
- Neves, M.I.; Moroni, L.; Barrias, C.C. Modulating Alginate Hydrogels for Improved Biological Performance as Cellular 3D Microenvironments. Front. Bioeng. Biotechnol. 2020, 8, 665. [Google Scholar] [CrossRef] [PubMed]
- Dumbleton, J.; Agarwal, P.; Huang, H.; Hogrebe, N.; Han, R.; Gooch, K.J.; He, X. The Effect of RGD Peptide on 2D and Miniaturized 3D Culture of HEPM Cells, MSCs, and ADSCs with Alginate Hydrogel. Cell. Mol. Bioeng. 2016, 9, 277–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuda, S.; Kee, M.Z.L.; Cunha, C.; Kim, J.; Yan, P.; Loew, L.M.; Augustine, G.J. Probing the Function of Neuronal Populations: Combining Micromirror-Based Optogenetic Photostimulation with Voltage-Sensitive Dye Imaging. Neurosci. Res. 2013, 75, 76–81. [Google Scholar] [CrossRef] [Green Version]
- Arrenberg, A.B.; Stainier, D.Y.R.; Baier, H.; Huisken, J. Optogenetic Control of Cardiac Function. Science 2010, 330, 971–974. [Google Scholar] [CrossRef]
- Jeon, O.; Wolfson, D.W.; Alsberg, E. In-Situ Formation of Growth-Factor-Loaded Coacervate Microparticle-Embedded Hydrogels for Directing Encapsulated Stem Cell Fate. Adv. Mater. 2015, 27, 2216–2223. [Google Scholar] [CrossRef]
- Moxon, S.R.; Corbett, N.J.; Fisher, K.; Potjewyd, G.; Domingos, M.; Hooper, N.M. Blended Alginate/Collagen Hydrogels Promote Neurogenesis and Neuronal Maturation. Mater. Sci. Eng. C 2019, 104, 109904. [Google Scholar] [CrossRef]
- Distler, T.; Lauria, I.; Detsch, R.; Sauter, C.M.; Bendt, F.; Kapr, J.; Rütten, S.; Boccaccini, A.R.; Fritsche, E. Neuronal Differentiation from Induced Pluripotent Stem Cell-Derived Neurospheres by the Application of Oxidized Alginate-Gelatin-Laminin Hydrogels. Biomedicines 2021, 9, 261. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-Y.; George, J.; Nagel, D.; Ye, H.; Seymour, L. Optogenetically Engineered Neurons Differentiated from Human SH-SY5Y Cells Survived and Expressed ChR2 in 3D Hydrogel. Biomedicines 2022, 10, 1534. https://doi.org/10.3390/biomedicines10071534
Lee S-Y, George J, Nagel D, Ye H, Seymour L. Optogenetically Engineered Neurons Differentiated from Human SH-SY5Y Cells Survived and Expressed ChR2 in 3D Hydrogel. Biomedicines. 2022; 10(7):1534. https://doi.org/10.3390/biomedicines10071534
Chicago/Turabian StyleLee, Si-Yuen, Julian George, David Nagel, Hua Ye, and Leonard Seymour. 2022. "Optogenetically Engineered Neurons Differentiated from Human SH-SY5Y Cells Survived and Expressed ChR2 in 3D Hydrogel" Biomedicines 10, no. 7: 1534. https://doi.org/10.3390/biomedicines10071534





