Treatment Response Predictors of Neoadjuvant Therapy for Locally Advanced Gastric Cancer: Current Status and Future Perspectives
Abstract
1. Introduction
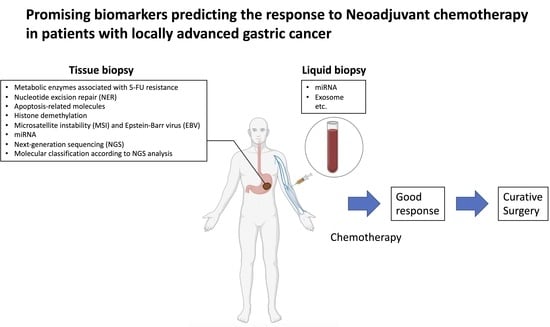
2. Biomarkers Involved in NAC
3. Metabolic Enzymes Associated with 5-FU Resistance
4. Nucleotide Excision Repair (NER)
5. Apoptosis-Related Molecules
6. Histone Demethylation
7. Microsatellite Instability (MSI) and Epstein-Barr Virus (EBV)
8. miRNA and Exosomes
9. Molecular Classification According to NGS Analysis
10. Future Perspectives
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Khan, U.; Shah, M.A. Optimizing Therapies in the Perioperative Management of Gastric Cancer. Curr. Treat. Options Oncol. 2019, 20, 57. [Google Scholar] [CrossRef] [PubMed]
- Petrillo, A.; Smyth, E.C. Multimodality treatment for localized gastric cancer: State of the art and new insights. Curr. Opin. Oncol. 2020, 32, 347–355. [Google Scholar] [CrossRef] [PubMed]
- D’Ugo, D.; Rausei, S.; Biondi, A.; Persiani, R. Preoperative treatment and surgery in gastric cancer: Friends or foes? Lancet. Oncol. 2009, 10, 191–195. [Google Scholar] [CrossRef]
- Moehler, M.; Schimanski, C.C.; Gockel, I.; Junginger, T.; Galle, P.R. (Neo)adjuvant strategies of advanced gastric carcinoma: Time for a change? Dig. Dis. 2004, 22, 345–350. [Google Scholar] [CrossRef]
- Fujitani, K. Overview of Adjuvant and Neoadjuvant Therapy for Resectable Gastric Cancer in the East. Dig. Surg. 2013, 30, 119–129. [Google Scholar] [CrossRef]
- Terashima, M.; Iwasaki, Y.; Mizusawa, J.; Katayama, H.; Nakamura, K.; Katai, H.; Yoshikawa, T.; Ito, Y.; Kaji, M.; Kimura, Y.; et al. Randomized phase III trial of gastrectomy with or without neoadjuvant S-1 plus cisplatin for type 4 or large type 3 gastric cancer, the short-term safety and surgical results: Japan Clinical Oncology Group Study (JCOG0501). Gastric Cancer 2019, 22, 1044–1052. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Terashima, M.; Mizusawa, J.; Katayama, H.; Nakamura, K.; Katai, H.; Yoshikawa, T.; Ito, S.; Kaji, M.; Kimura, Y.; et al. Gastrectomy with or without neoadjuvant S-1 plus cisplatin for type 4 or large type 3 gastric cancer (JCOG0501): An open-label, phase 3, randomized controlled trial. Gastric Cancer 2021, 24, 492–502. [Google Scholar] [CrossRef]
- Cunningham, D.; Allum, W.H.; Stenning, S.P.; Thompson, J.N.; Van de Velde, C.J.H.; Nicolson, M.; Scarffe, J.H.; Lofts, F.J.; Falk, S.J.; Iveson, T.J.; et al. Perioperative Chemotherapy versus Surgery Alone for Resectable Gastroesophageal Cancer. N. Engl. J. Med. 2006, 355, 11–20. [Google Scholar] [CrossRef]
- Al-Batran, S.-E.; Hofheinz, R.D.; Pauligk, C.; Kopp, H.-G.; Haag, G.M.; Luley, K.B.; Meiler, J.; Homann, N.; Lorenzen, S.; Schmalenberg, H.; et al. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): Results from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial. Lancet Oncol. 2016, 17, 1697–1708. [Google Scholar] [CrossRef]
- Al-Batran, S.-E.; Homann, N.; Pauligk, C.; Goetze, T.O.; Meiler, J.; Kasper, S.; Kopp, H.-G.; Mayer, F.; Haag, G.M.; Luley, K.; et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): A randomised, phase 2/3 trial. Lancet 2019, 393, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.S.; Badgwell, B.D. Current treatment and recent progress in gastric cancer. CA Cancer J. Clin. 2021, 71, 264–279. [Google Scholar] [CrossRef] [PubMed]
- Ajani, J.A.; Mansfield, P.F.; Crane, C.H.; Wu, T.T.; Lunagomez, S.; Lynch, P.M.; Janjan, N.; Feig, B.; Faust, J.; Yao, J.C.; et al. Paclitaxel-Based Chemoradiotherapy in Localized Gastric Carcinoma: Degree of Pathologic Response and Not Clinical Parameters Dictated Patient Outcome. J. Clin. Oncol. 2005, 23, 1237–1244. [Google Scholar] [CrossRef]
- Gervaso, L.; Pellicori, S.; Cella, C.A.; Bagnardi, V.; Lordick, F.; Fazio, N. Biomarker evaluation in radically resectable locally advanced gastric cancer treated with neoadjuvant chemotherapy: An evidence reappraisal. Ther. Adv. Med. Oncol. 2021, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-Z.; Zeng, Z.-Y.; Ye, X.; Sun, J.; Zhang, Z.-M.; Kang, W.-M. Interpretation of the development of neoadjuvant therapy for gastric cancer based on the vicissitudes of the NCCN guidelines. World J. Gastrointest. Oncol. 2020, 12, 37–53. [Google Scholar] [CrossRef]
- Wagner, A.D.; Syn, N.L.; Moehler, M.; Grothe, W.; Yong, W.P.; Tai, B.-C.; Ho, J.; Unverzagt, S. Chemotherapy for advanced gastric cancer. Cochrane Database Syst. Rev. 2017, 8, CD004064. [Google Scholar] [CrossRef]
- Napieralski, R.; Ott, K.; Kremer, M.; Specht, K.; Vogelsang, H.; Becker, K.; Müller, M.; Lordick, F.; Fink, U.; Rüdiger Siewert, J.; et al. Combined GADD45A and Thymidine Phosphorylase Expression Levels Predict Response and Survival of Neoadjuvant-Treated Gastric Cancer Patients. Clin. Cancer Res. 2005, 11, 3025–3031. [Google Scholar] [CrossRef]
- Ott, K.; Vogelsang, H.; Marton, N.; Becker, K.; Lordick, F.; Kobl, M.; Schuhmacher, C.; Novotny, A.; Mueller, J.; Fink, U.; et al. Thethymidylate synthase tandem repeat promoter polymorphism: A predictor for tumor-related survival in neoadjuvant treated locally advanced gastric cancer. Int. J. Cancer 2006, 119, 2885–2894. [Google Scholar] [CrossRef]
- Metzger, R.; Leichman, C.G.; Danenberg, K.D.; Danenberg, P.V.; Lenz, H.J.; Hayashi, K.; Groshen, S.; Salonga, D.; Cohen, H.; Laine, L.; et al. ERCC1 mRNA levels complement thymidylate synthase mRNA levels in predicting response and survival for gastric cancer patients receiving combination cisplatin and fluorouracil chemotherapy. J. Clin. Oncol. 1998, 16, 309–316. [Google Scholar] [CrossRef]
- Fareed, K.R.; Al-Attar, A.; Soomro, I.N.; Kaye, P.V.; Patel, J.; Lobo, D.N.; Parsons, S.L.; Madhusudan, S. Tumour regression and ERCC1 nuclear protein expression predict clinical outcome in patients with gastro-oesophageal cancer treated with neoadjuvant chemotherapy. Br. J. Cancer 2010, 102, 1600–1607. [Google Scholar] [CrossRef]
- Hirakawa, M.; Sato, Y.; Ohnuma, H.; Takayama, T.; Sagawa, T.; Nobuoka, T.; Harada, K.; Miyamoto, H.; Sato, Y.; Takahashi, Y.; et al. A phase II study of neoadjuvant combination chemotherapy with docetaxel, cisplatin, and S-1 for locally advanced resectable gastric cancer: Nucleotide excision repair (NER) as potential chemoresistance marker. Cancer Chemother. Pharmacol. 2013, 71, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Kubo, T.; Kawano, Y.; Himuro, N.; Sugita, S.; Sato, Y.; Ishikawa, K.; Takada, K.; Murase, K.; Miyanishi, K.; Sato, T.; et al. BAK is a predictive and prognostic biomarker for the therapeutic effect of docetaxel treatment in patients with advanced gastric cancer. Gastric Cancer 2016, 19, 827–838. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hashimoto, T.; Kurokawa, Y.; Takahashi, T.; Miyazaki, Y.; Tanaka, K.; Makino, T.; Yamasaki, M.; Nakajima, K.; Ikeda, J.; Mori, M.; et al. Predictive value of MLH1 and PD-L1 expression for prognosis and response to preoperative chemotherapy in gastric cancer. Gastric Cancer 2019, 22, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Haag, G.M.; Czink, E.; Ahadova, A.; Schmidt, T.; Sisic, L.; Blank, S.; Heger, U.; Apostolidis, L.; Berger, A.K.; Springfeld, C.; et al. Prognostic significance of microsatellite-instability in gastric and gastroesophageal junction cancer patients undergoing neoadjuvant chemotherapy. Int. J. Cancer 2019, 144, 1697–1703. [Google Scholar] [CrossRef]
- Liu, K.; Qian, T.; Tang, L.; Wang, J.; Yang, H.; Ren, J. Decreased expression of microRNA let-7i and its association with chemotherapeutic response in human gastric cancer. World J. Surg. Oncol. 2012, 10, 3–8. [Google Scholar] [CrossRef]
- Diasio, R.B. Oral DPD-inhibitory fluoropyrimidine drugs. Oncology 2000, 14, 19–23. [Google Scholar]
- Terashima, M.; Irinoda, T.; Fujiwara, H.; Nakaya, T.; Takagane, A.; Abe, K.; Yonezawa, H.; Oyama, K.; Inaba, T.; Saito, K.; et al. Roles of thymidylate synthase and dihydropyrimidine dehydrogenase in tumor progression and sensitivity to 5-fluorouracil in human gastric cancer. Anticancer Res. 2002, 22, 761–768. [Google Scholar]
- Wang, W.; Cassidy, J.; O’Brien, V.; Ryan, K.M.; Collie-Duguid, E. Mechanistic and Predictive Profiling of 5-Fluorouracil Resistance in Human Cancer Cells. Cancer Res. 2004, 64, 8167–8176. [Google Scholar] [CrossRef]
- Wang, D.; Yu, X.; Wang, X. High/Positive Expression of 5-Fluorouracil Metabolic Enzymes Predicts Better Response to S-1 in Patients with Gastric Cancer: A Meta-Analysis. Int. J. Biol. Mark. 2016, 31, 101–109. [Google Scholar] [CrossRef]
- Marteijn, J.A.; Lans, H.; Vermeulen, W.; Hoeijmakers, J.H.J. Understanding nucleotide excision repair and its roles in cancer and ageing. Nat. Rev. Mol. Cell Biol. 2014, 15, 465–481. [Google Scholar] [CrossRef]
- Jeggo, P.A.; Pearl, L.H.; Carr, A.M. DNA repair, genome stability and cancer: A historical perspective. Nat. Rev. Cancer 2016, 16, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Sugasawa, K. Regulation of damage recognition in mammalian global genomic nucleotide excision repair. Mutat. Res. 2010, 685, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zou, Z.; Qian, X.; Ding, Y.; Xie, L.; Sanchez, J.J.; Zhao, Y.; Feng, J.; Ling, Y.; Liu, Y.; et al. ERCC1 mRNA levels and survival of advanced gastric cancer patients treated with a modified FOLFOX regimen. Br. J. Cancer 2008, 98, 1398–1402. [Google Scholar] [CrossRef]
- Kwon, H.-C.; Roh, M.S.; Oh, S.Y.; Kim, S.-H.; Kim, M.C.; Kim, J.-S.; Kim, H.-J. Prognostic value of expression of ERCC1, thymidylate synthase, and glutathione S-transferase P1 for 5-fluorouracil/oxaliplatin chemotherapy in advanced gastric cancer. Ann. Oncol. 2007, 18, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Takayama, T.; Sagawa, T.; Takahashi, Y.; Ohnuma, H.; Okubo, S.; Shintani, N.; Tanaka, S.; Kida, M.; Sato, Y.; et al. Phase II study of S-1, docetaxel and cisplatin combination chemotherapy in patients with unresectable metastatic gastric cancer. Cancer Chemother. Pharmacol. 2010, 66, 721–728. [Google Scholar] [CrossRef]
- Takimoto, R.; MacLachlan, T.K.; Dicker, D.T.; Niitsu, Y.; Mori, T.; El-Deiry, W.S. BRCA1 Transcriptionally Regulates Damaged DNA Binding Protein (DDB2) In the DNA Repair Response Following UV-Irradiation. Cancer Biol. Ther. 2002, 1, 177–186. [Google Scholar] [CrossRef][Green Version]
- Wang, X.; Lin, Y.; Lan, F.; Yu, Y.; Ouyang, X.; Liu, W.; Xie, F.; Wang, X.; Huang, Q. BAX and CDKN1A polymorphisms correlated with clinical outcomes of gastric cancer patients treated with postoperative chemotherapy. Med. Oncol. 2014, 31, 249. [Google Scholar] [CrossRef]
- Jeong, S.H.; Han, J.H.; Kim, J.H.; Ahn, M.S.; Hwang, Y.H.; Lee, H.W.; Kang, S.Y.; Park, J.S.; Choi, J.-H.; Lee, K.J.; et al. Bax Predicts Outcome in Gastric Cancer Patients Treated with 5-fluorouracil, Leucovorin, and Oxaliplatin Palliative Chemotherapy. Dig. Dis. Sci. 2011, 56, 131–138. [Google Scholar] [CrossRef]
- Chonghaile, T.N.; Sarosiek, K.A.; Vo, T.-T.; Ryan, J.A.; Tammareddi, A.; Moore, V.D.G.; Deng, J.; Anderson, K.C.; Richardson, P.; Tai, Y.-T.; et al. Pretreatment Mitochondrial Priming Correlates with Clinical Response to Cytotoxic Chemotherapy. Science 2011, 334, 1129–1133. [Google Scholar] [CrossRef]
- Vo, T.-T.; Ryan, J.; Carrasco, R.; Neuberg, D.; Rossi, D.J.; Stone, R.M.; DeAngelo, D.J.; Frattini, M.G.; Letai, A. Relative Mitochondrial Priming of Myeloblasts and Normal HSCs Determines Chemotherapeutic Success in AML. Cell 2012, 151, 344–355. [Google Scholar] [CrossRef]
- Wu, N.; Huang, Y.; Zou, Z.; Gimenez-Capitan, A.; Yu, L.; Hu, W.; Zhu, L.; Sun, X.; Sanchez, J.J.; Guan, W.; et al. High BIM mRNA levels are associated with longer survival in advanced gastric cancer. Oncol. Lett. 2017, 13, 1826–1834. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chi, P.; Allis, C.D.; Wang, G.G. Covalent histone modifications—Miswritten, misinterpreted and mis-erased in human cancers. Nat. Rev. Cancer 2010, 10, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Kogure, M.; Takawa, M.; Cho, H.-S.; Toyokawa, G.; Hayashi, K.; Tsunoda, T.; Kobayashi, T.; Daigo, Y.; Sugiyama, M.; Atomi, Y.; et al. Deregulation of the histone demethylase JMJD2A is involved in human carcinogenesis through regulation of the G1/S transition. Cancer Lett. 2013, 336, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Berry, W.L.; Shin, S.; Lightfoot, S.A.; Janknecht, R. Oncogenic features of the JMJD2A histone demethylase in breast cancer. Int. J. Oncol. 2012, 41, 1701–1706. [Google Scholar] [CrossRef]
- Hu, C.-E.; Liu, Y.-C.; Zhang, H.-D.; Huang, G.-J. JMJD2A predicts prognosis and regulates cell growth in human gastric cancer. Biochem. Biophys. Res. Commun. 2014, 449, 1–7. [Google Scholar] [CrossRef]
- Kitamura, S.; Tanahashi, T.; Aoyagi, E.; Nakagawa, T.; Okamoto, K.; Kimura, T.; Miyamoto, H.; Mitsui, Y.; Rokutan, K.; Muguruma, N.; et al. Response Predictors of S-1, Cisplatin, and Docetaxel Combination Chemotherapy for Metastatic Gastric Cancer: Microarray Analysis of Whole Human Genes. Oncology 2017, 93, 127–135. [Google Scholar] [CrossRef]
- Nakagawa, T.; Sato, Y.; Tanahashi, T.; Mitsui, Y.; Kida, Y.; Fujino, Y.; Hirata, M.; Kitamura, S.; Miyamoto, H.; Okamoto, K.; et al. JMJD2A sensitizes gastric cancer to chemotherapy by cooperating with CCDC8. Gastric Cancer 2020, 23, 426–436. [Google Scholar] [CrossRef]
- Baretti, M.; Le, D.T. DNA mismatch repair in cancer. Pharmacol. Ther. 2018, 189, 45–62. [Google Scholar] [CrossRef]
- Biesma, H.D.; Soeratram, T.T.D.; Sikorska, K.; Caspers, I.A.; van Essen, H.F.; Egthuijsen, J.M.P.; Mookhoek, A.; van Laarhoven, H.W.M.; van Berge Henegouwen, M.I.; Nordsmark, M.; et al. Response to neoadjuvant chemotherapy and survival in molecular subtypes of resectable gastric cancer: A post hoc analysis of the D1/D2 and CRITICS trials. Gastric Cancer 2022, 25, 640–651. [Google Scholar] [CrossRef]
- Pietrantonio, F.; Miceli, R.; Raimondi, A.; Kim, Y.W.; Kang, W.K.; Langley, R.E.; Choi, Y.Y.; Kim, K.-M.; Nankivell, M.G.; Morano, F.; et al. Individual Patient Data Meta-Analysis of the Value of Microsatellite Instability As a Biomarker in Gastric Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2019, 37, 3392–3400. [Google Scholar] [CrossRef]
- Cats, A.; Jansen, E.P.M.; van Grieken, N.C.T.; Sikorska, K.; Lind, P.; Nordsmark, M.; Meershoek-Klein Kranenbarg, E.; Boot, H.; Trip, A.K.; Swellengrebel, H.A.M.; et al. Chemotherapy versus chemoradiotherapy after surgery and preoperative chemotherapy for resectable gastric cancer (CRITICS): An international, open-label, randomised phase 3 trial. Lancet Oncol. 2018, 19, 616–628. [Google Scholar] [CrossRef]
- Songun, I.; Putter, H.; Kranenbarg, E.M.K.; Sasako, M.; van de Velde, C.J.H. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010, 11, 439–449. [Google Scholar] [CrossRef]
- Bass, A.J.; Thorsson, V.; Shmulevich, I.; Reynolds, S.M.; Miller, M.; Bernard, B.; Hinoue, T.; Laird, P.W.; Curtis, C.; Shen, H.; et al. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014, 513, 202–209. [Google Scholar] [CrossRef]
- Kohlruss, M.; Grosser, B.; Krenauer, M.; Slotta-Huspenina, J.; Jesinghaus, M.; Blank, S.; Novotny, A.; Reiche, M.; Schmidt, T.; Ismani, L.; et al. Prognostic implication of molecular subtypes and response to neoadjuvant chemotherapy in 760 gastric carcinomas: Role of Epstein–Barr virus infection and high- and low-microsatellite instability. J. Pathol. Clin. Res. 2019, 5, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, D.; Polom, K.; Pascale, V.; Vindigni, C.; Piagnerelli, R.; De Franco, L.; Ferrara, F.; Roviello, G.; Garosi, L.; Petrioli, R.; et al. Strong Prognostic Value of Microsatellite Instability in Intestinal Type Non-cardia Gastric Cancer. Ann. Surg. Oncol. 2016, 23, 943–950. [Google Scholar] [CrossRef]
- Van Beek, J.; zur Hausen, A.; Kranenbarg, E.K.; van de Velde, C.J.H.; Middeldorp, J.M.; van den Brule, A.J.C.; Meijer, C.J.L.M.; Bloemena, E. EBV-positive gastric adenocarcinomas: A distinct clinicopathologic entity with a low frequency of lymph node involvement. J. Clin. Oncol. 2004, 22, 664–670. [Google Scholar] [CrossRef]
- Ignatova, E.; Seriak, D.; Fedyanin, M.; Tryakin, A.; Pokataev, I.; Menshikova, S.; Vakhabova, Y.; Smirnova, K.; Tjulandin, S.; Ajani, J.A. Epstein–Barr virus-associated gastric cancer: Disease that requires special approach. Gastric Cancer 2020, 23, 951–960. [Google Scholar] [CrossRef]
- Hussen, B.M.; Hidayat, H.J.; Salihi, A.; Sabir, D.K.; Taheri, M.; Ghafouri-Fard, S. MicroRNA: A signature for cancer progression. Biomed. Pharmacother. 2021, 138, 111528. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 00402. [Google Scholar] [CrossRef]
- Yao, Y.; Ding, Y.; Bai, Y.; Zhou, Q.; Lee, H.; Li, X.; Teng, L. Identification of Serum Circulating MicroRNAs as Novel Diagnostic Biomarkers of Gastric Cancer. Front. Genet. 2020, 11, 591515. [Google Scholar] [CrossRef]
- So, J.B.Y.; Kapoor, R.; Zhu, F.; Koh, C.; Zhou, L.; Zou, R.; Tang, Y.C.; Goo, P.C.K.; Rha, S.Y.; Chung, H.C.; et al. Development and validation of a serum microRNA biomarker panel for detecting gastric cancer in a high-risk population. Gut 2021, 70, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Ueda, T.; Volinia, S.; Okumura, H.; Shimizu, M.; Taccioli, C.; Rossi, S.; Alder, H.; Liu, C.; Oue, N.; Yasui, W.; et al. Relation between microRNA expression and progression and prognosis of gastric cancer: A microRNA expression analysis. Lancet Oncol. 2010, 11, 136–146. [Google Scholar] [CrossRef]
- Tan, B.; Li, Y.; Di, Y.; Fan, L.; Zhao, Q.; Liu, Q.; Wang, D.; Jia, N. Clinical value of peripheral blood microRNA detection in evaluation of SOX regimen as neoadjuvant chemotherapy for gastric cancer. J. Clin. Lab. Anal. 2018, 32, e22363. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Li, D.; Hou, S.; Zhu, X. The cancer exosomes: Clinical implications, applications and challenges. Int. J. Cancer 2020, 146, 2946–2959. [Google Scholar] [CrossRef]
- Liu, J.; Ren, L.; Li, S.; Li, W.; Zheng, X.; Yang, Y.; Fu, W.; Yi, J.; Wang, J.; Du, G. The biology, function, and applications of exosomes in cancer. Acta Pharm. Sin. B 2021, 11, 2783–2797. [Google Scholar] [CrossRef]
- Uchôa Guimarães, C.T.; Ferreira Martins, N.N.; Cristina da Silva Oliveira, K.; Almeida, C.M.; Pinheiro, T.M.; Gigek, C.O.; Roberto de Araújo Cavallero, S.; Assumpção, P.P.; Cardoso Smith, M.A.; Burbano, R.R.; et al. Liquid biopsy provides new insights into gastric cancer. Oncotarget 2018, 9, 15144–15156. [Google Scholar] [CrossRef]
- Lengyel, C.G.; Hussain, S.; Trapani, D.; El Bairi, K.; Altuna, S.C.; Seeber, A.; Odhiambo, A.; Habeeb, B.S.; Seid, F. The emerging role of liquid biopsy in gastric cancer. J. Clin. Med. 2021, 10, 2108. [Google Scholar] [CrossRef]
- Huang, T.; Song, C.; Zheng, L.; Xia, L.; Li, Y.; Zhou, Y. The roles of extracellular vesicles in gastric cancer development, microenvironment, anti-cancer drug resistance, and therapy. Mol. Cancer 2019, 18, 62. [Google Scholar] [CrossRef]
- Zhang, H.; Deng, T.; Liu, R.; Ning, T.; Yang, H.; Liu, D.; Zhang, Q.; Lin, D.; Ge, S.; Bai, M.; et al. CAF secreted miR-522 suppresses ferroptosis and promotes acquired chemo-resistance in gastric cancer. Mol. Cancer 2020, 19, 43. [Google Scholar] [CrossRef]
- Wang, M.; Qiu, R.; Yu, S.; Xu, X.; Li, G.; Gu, R.; Tan, C.; Zhu, W.; Shen, B. Paclitaxel-resistant gastric cancer MGC-803 cells promote epithelial-to-mesenchymal transition and chemoresistance in paclitaxel-sensitive cells via exosomal delivery of miR-155-5p. Int. J. Oncol. 2018, 54, 326–338. [Google Scholar] [CrossRef]
- Verma, R.; Sharma, P.C. Next generation sequencing-based emerging trends in molecular biology of gastric cancer. Am. J. Cancer Res. 2018, 8, 207–225. [Google Scholar] [PubMed]
- Nemtsova, M.V.; Kalinkin, A.I.; Kuznetsova, E.B.; Bure, I.V.; Alekseeva, E.A.; Bykov, I.I.; Khorobrykh, T.V.; Mikhaylenko, D.S.; Tanas, A.S.; Kutsev, S.I.; et al. Clinical relevance of somatic mutations in main driver genes detected in gastric cancer patients by next-generation DNA sequencing. Sci. Rep. 2020, 10, 504. [Google Scholar] [CrossRef] [PubMed]
- Fassan, M.; Simbolo, M.; Bria, E.; Mafficini, A.; Pilotto, S.; Capelli, P.; Bencivenga, M.; Pecori, S.; Luchini, C.; Neves, D.; et al. High-throughput mutation profiling identifies novel molecular dysregulation in high-grade intraepithelial neoplasia and early gastric cancers. Gastric Cancer 2014, 17, 442–449. [Google Scholar] [CrossRef]
- Bria, E.; Pilotto, S.; Simbolo, M.; Fassan, M.; de Manzoni, G.; Carbognin, L.; Sperduti, I.; Brunelli, M.; Cataldo, I.; Tomezzoli, A.; et al. Comprehensive molecular portrait using next generation sequencing of resected intestinal-type gastric cancer patients dichotomized according to prognosis. Sci. Rep. 2016, 6, 22982. [Google Scholar] [CrossRef] [PubMed]
- Al-Batran, S.-E.; Homann, N.; Pauligk, C.; Illerhaus, G.; Martens, U.M.; Stoehlmacher, J.; Schmalenberg, H.; Luley, K.B.; Prasnikar, N.; Egger, M.; et al. Effect of Neoadjuvant Chemotherapy Followed by Surgical Resection on Survival in Patients With Limited Metastatic Gastric or Gastroesophageal Junction Cancer: The AIO-FLOT3 Trial. JAMA Oncol. 2017, 3, 1237–1244. [Google Scholar] [CrossRef] [PubMed]
- Eveno, C.; Adenis, A.; Bouche, O.; Le Malicot, K.; Hautefeuille, V.; Faroux, R.; Thirot Bidault, A.; Egreteau, J.; Meunier, B.; Mabro, M.; et al. Adjuvant chemotherapy versus perioperative chemotherapy (CTx) for resectable gastric signet ring cell (SRC) gastric cancer: A multicenter, randomized phase II study (PRODIGE 19). J. Clin. Oncol. 2019, 37, 4019. [Google Scholar] [CrossRef]
- Cristescu, R.; Lee, J.; Nebozhyn, M.; Kim, K.M.; Ting, J.C.; Wong, S.S.; Liu, J.; Yue, Y.G.; Wang, J.; Yu, K.; et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat. Med. 2015, 21, 449–456. [Google Scholar] [CrossRef]
- Murugaesu, N.; Wilson, G.A.; Birkbak, N.J.; Watkins, T.B.K.; McGranahan, N.; Kumar, S.; Abbassi-Ghadi, N.; Salm, M.; Mitter, R.; Horswell, S.; et al. Tracking the genomic evolution of esophageal adenocarcinoma through neoadjuvant chemotherapy. Cancer Discov. 2015, 5, 821–832. [Google Scholar] [CrossRef]
- Paschold, L.; Binder, M. Circulating Tumor DNA in Gastric and Gastroesophageal Junction Cancer. Curr. Oncol. 2022, 29, 1430–1441. [Google Scholar] [CrossRef]
| Possible Advantages | Possible Disadvantages |
|---|---|
| Downsizing or downstaging of the primary tumor | Delayed definitive surgery |
| Improvement of the possibility of subsequent R0 resection | Worsening general performance status |
| Eliminating systemic micrometastases | Chemotherapy-related peritumoral fibrotic reaction |
| Evaluation of a chemosensitivity-guide for adjuvant chemotherapy | Perioperative complication |
| More efficient delivery of chemotherapy due to prior surgical disruption of the vasculature | Disease progression (leads to inoperable disease) |
| Better tolerability than postoperative chemotherapy |
| Biomarker | Chemotherapy | Samples | Cases | Method | Results | Author |
|---|---|---|---|---|---|---|
| DPD, TP, GADD45A | 5-FU/cisplatin | Biopsy | 61 | Real-time PCR | High DPD levels were found more frequently in non-responding patients and were associated with worse survival. The combination of GADD45A and TP revealed the strongest predictive effect. | Napieralski et al. [17] |
| TS, MTHFR | 5-FU | Blood | 238 | PCR | A significant survival benefit for the patients with NAC was found for the 2rpt/2rpt and 2rpt/3rpt genotypes | Ott et al. [18] |
| ERCC1 | 5-FU/cisplatin | Biopsy | 38 | PCR | ERCC1 mRNA levels had a statistically significant association with survival | Metzger et al. [19] |
| ERCC1 | Platinum-based chemotherapy | Tissue | 142 | Immunohistochemistry | ERCC1 expression correlated with lack of histopathological response to NAC and was associated with OS | Fareed et al. [20] |
| DDB2/ERCC1 | Docetaxel, cisplatin, S-1 | Biopsy | 43 | Immunohistochemistry | DDB2- and/or ERCC1-high phenotype was significantly correlated with non-responding patients | Hirakawa et al. [21] |
| BAK | Docetaxel, cisplatin, S-1 | Biopsy | 69 | Immunohistochemistry | BAK expression was predictive of chemotherapeutic responses and survival. | Kubo et al. [22] |
| MLH1 | Fluorouracil-based doublet or triplet chemotherapy | Tissue | 285 | Immunohistochemistry | Loss of MLH1 was associated with chemoresistance and did not prolong survival following neoadjuvant chemotherapy. | Hashimoto et al. [23] |
| MSI | Platinum-based chemotherapy | Tissue | 101 | Immunohistochemistry | MSI-H phenotype was a favorable prognostic marker in patients with gastric cancer receiving NAC | Haag et al. [24] |
| MicroRNA (let-7i) | Folinic acid, fluorouracil, and oxaliplatin | Tissue | 68 | Quantitative RT-PCR. | Low let-7i expression was an unfavorable prognostic factor of OS. | Liu et al. [25] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, Y.; Okamoto, K.; Kawaguchi, T.; Nakamura, F.; Miyamoto, H.; Takayama, T. Treatment Response Predictors of Neoadjuvant Therapy for Locally Advanced Gastric Cancer: Current Status and Future Perspectives. Biomedicines 2022, 10, 1614. https://doi.org/10.3390/biomedicines10071614
Sato Y, Okamoto K, Kawaguchi T, Nakamura F, Miyamoto H, Takayama T. Treatment Response Predictors of Neoadjuvant Therapy for Locally Advanced Gastric Cancer: Current Status and Future Perspectives. Biomedicines. 2022; 10(7):1614. https://doi.org/10.3390/biomedicines10071614
Chicago/Turabian StyleSato, Yasushi, Koichi Okamoto, Tomoyuki Kawaguchi, Fumika Nakamura, Hiroshi Miyamoto, and Tetsuji Takayama. 2022. "Treatment Response Predictors of Neoadjuvant Therapy for Locally Advanced Gastric Cancer: Current Status and Future Perspectives" Biomedicines 10, no. 7: 1614. https://doi.org/10.3390/biomedicines10071614
APA StyleSato, Y., Okamoto, K., Kawaguchi, T., Nakamura, F., Miyamoto, H., & Takayama, T. (2022). Treatment Response Predictors of Neoadjuvant Therapy for Locally Advanced Gastric Cancer: Current Status and Future Perspectives. Biomedicines, 10(7), 1614. https://doi.org/10.3390/biomedicines10071614