Divergence of Chemerin Reduction by an ATS9R Nanoparticle Targeting Adipose Tissue In Vitro vs. In Vivo in the Rat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Use
2.2. Adipocyte and SVF Isolation
2.3. NP Formation
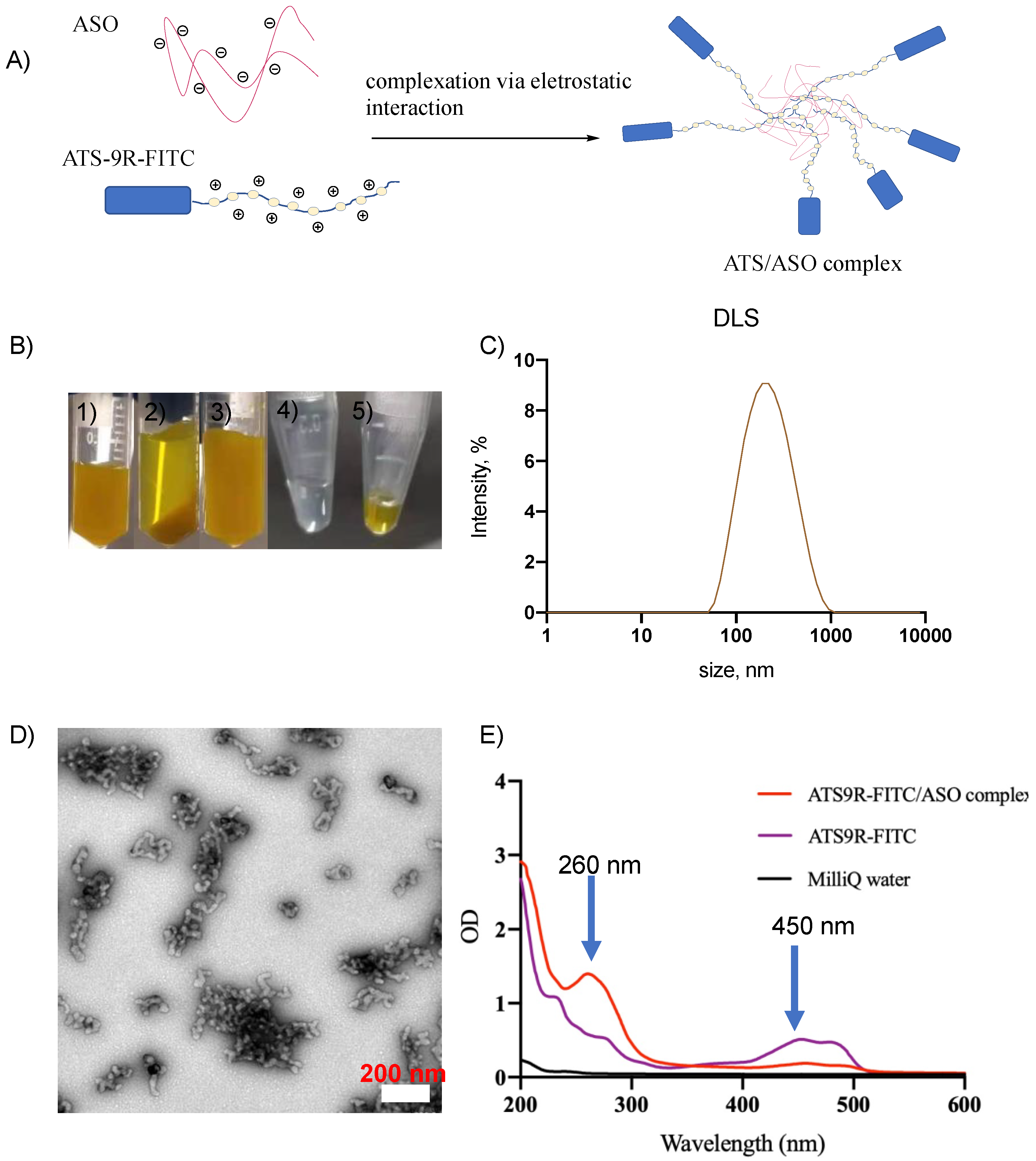
2.4. Characterization of NP Complex
2.5. RT-PCR
2.6. In Vitro NP Incubation
2.7. Ex Vivo NP Incubation
2.8. In Vivo NP Incubation
2.8.1. Subcutaneous Injections
2.8.2. Tail Vein Injections
2.9. IVIS Imaging
2.10. Data Analysis
3. Results
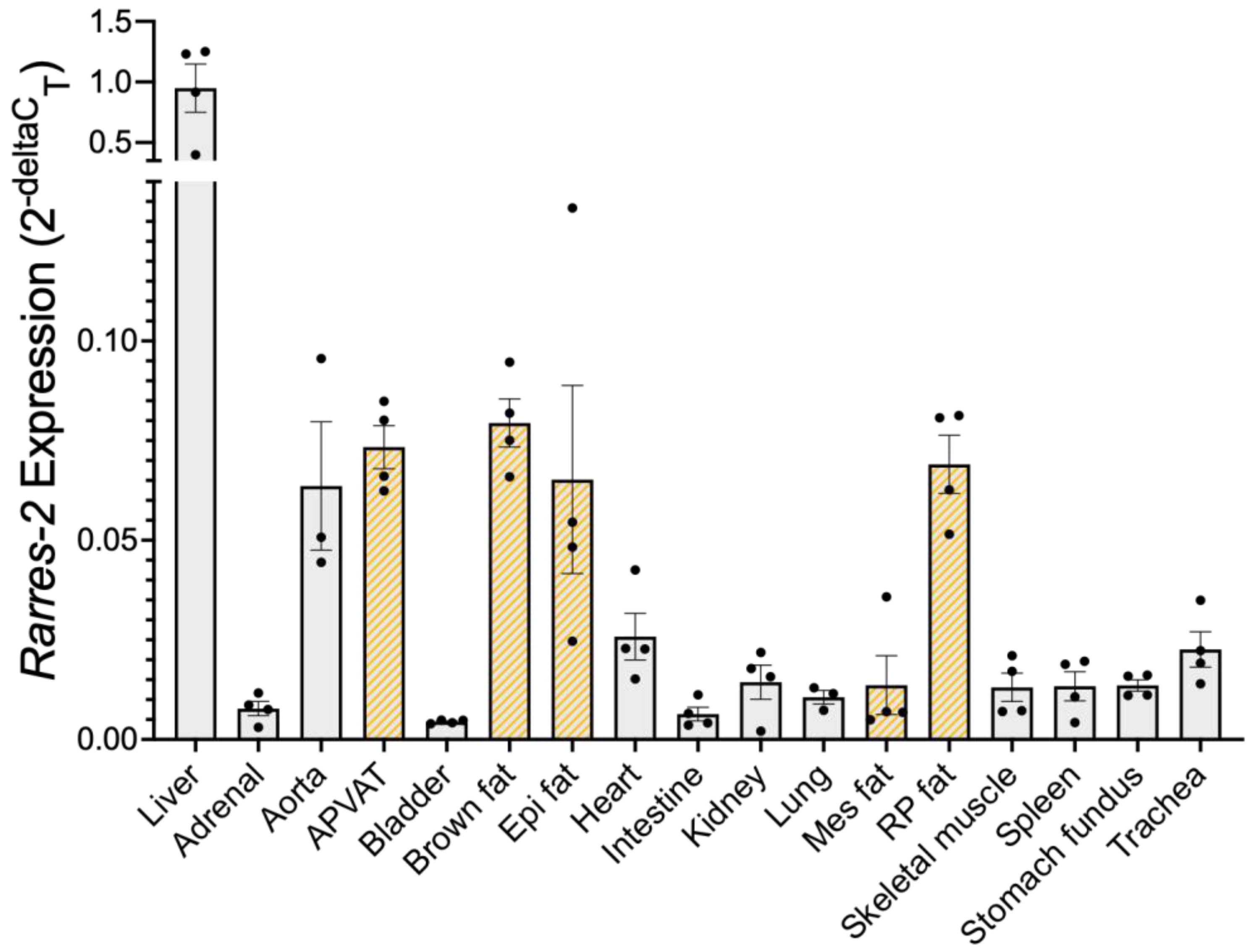
3.1. Liver Expressed the Most Chemerin mRNA, Followed by Adipose Tissue
3.2. NP Was Synthesized Reproducibly and Was Stable
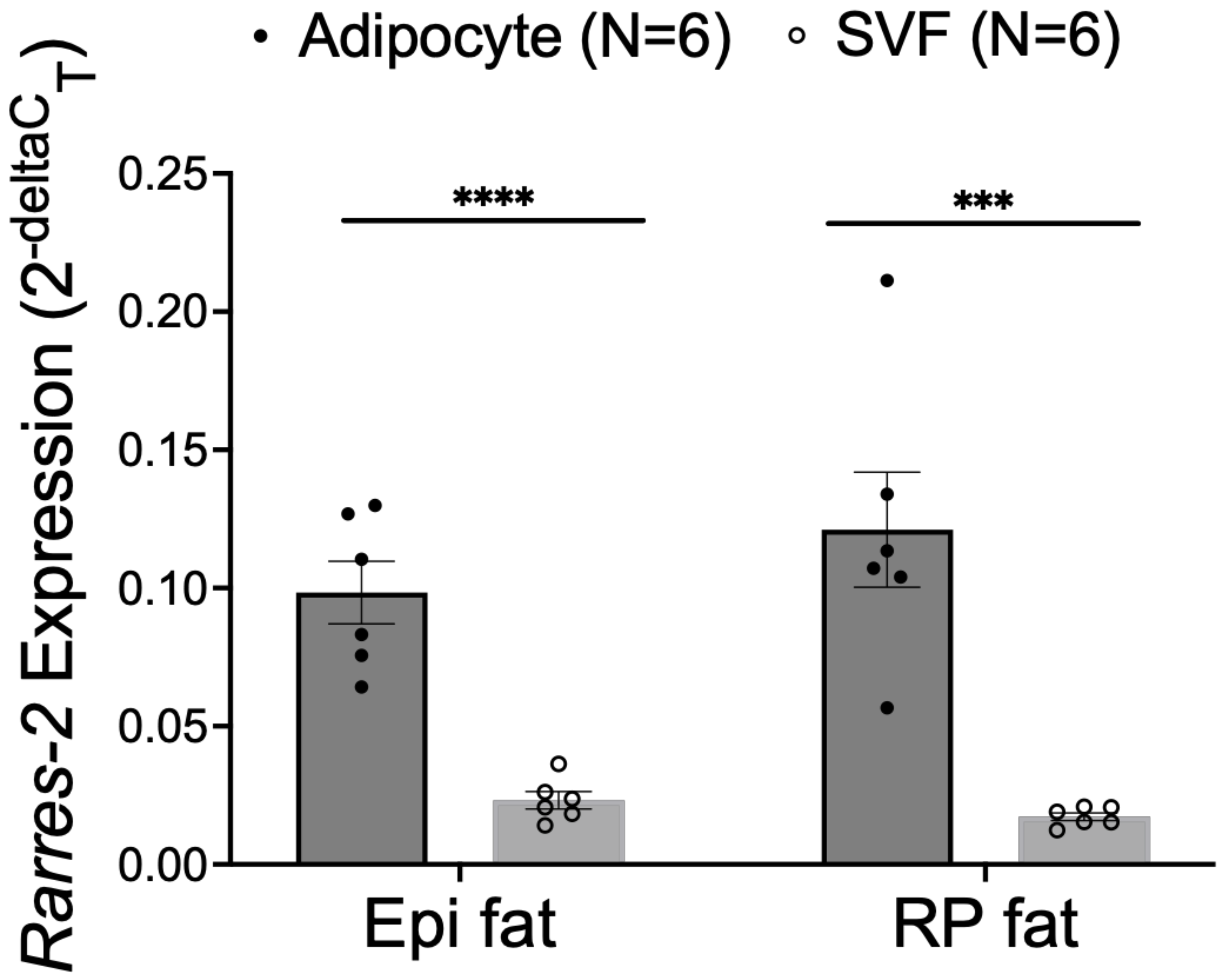
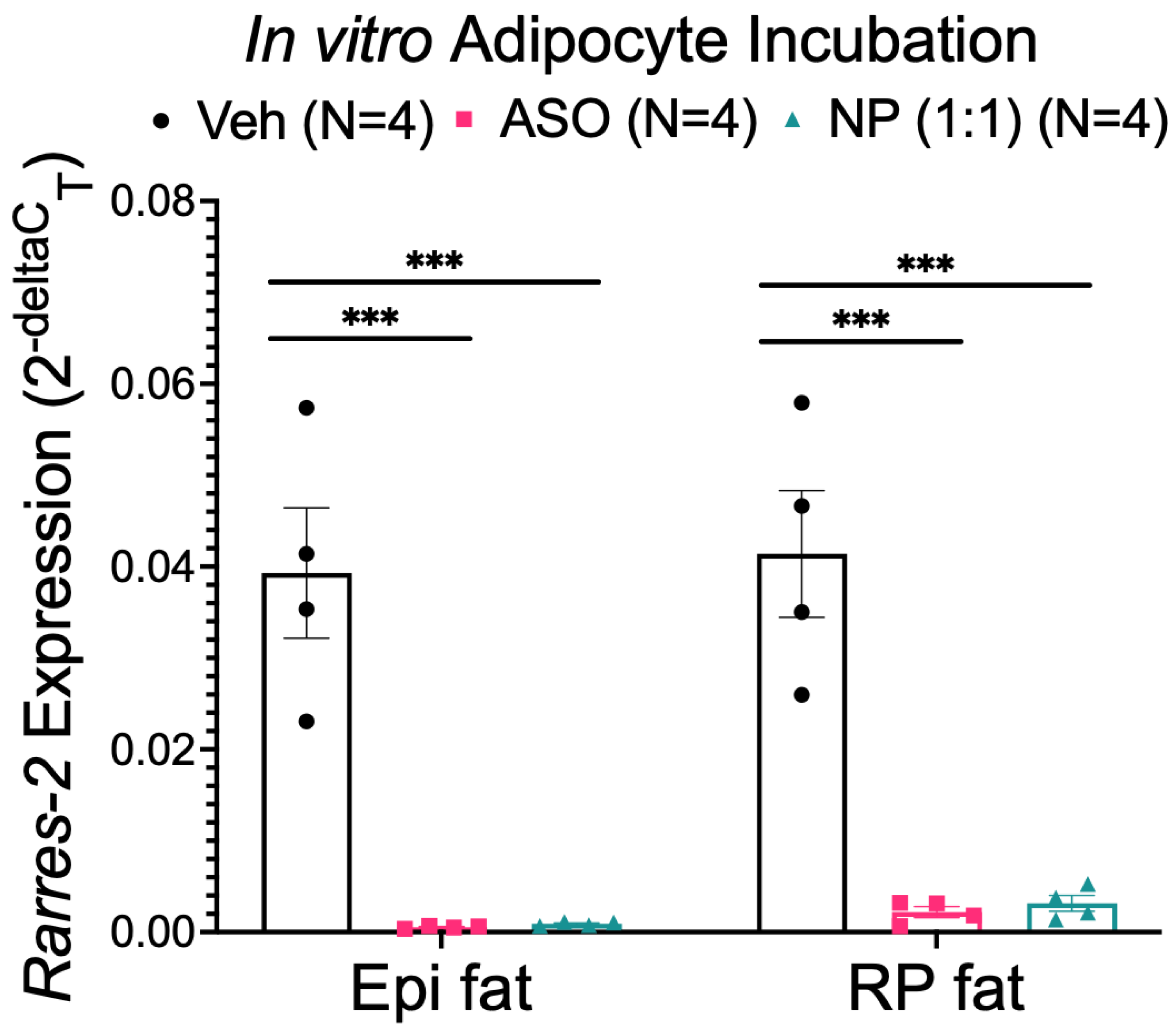
3.3. NP Functionally Reduced Chemerin Expression in Isolated Adipocytes
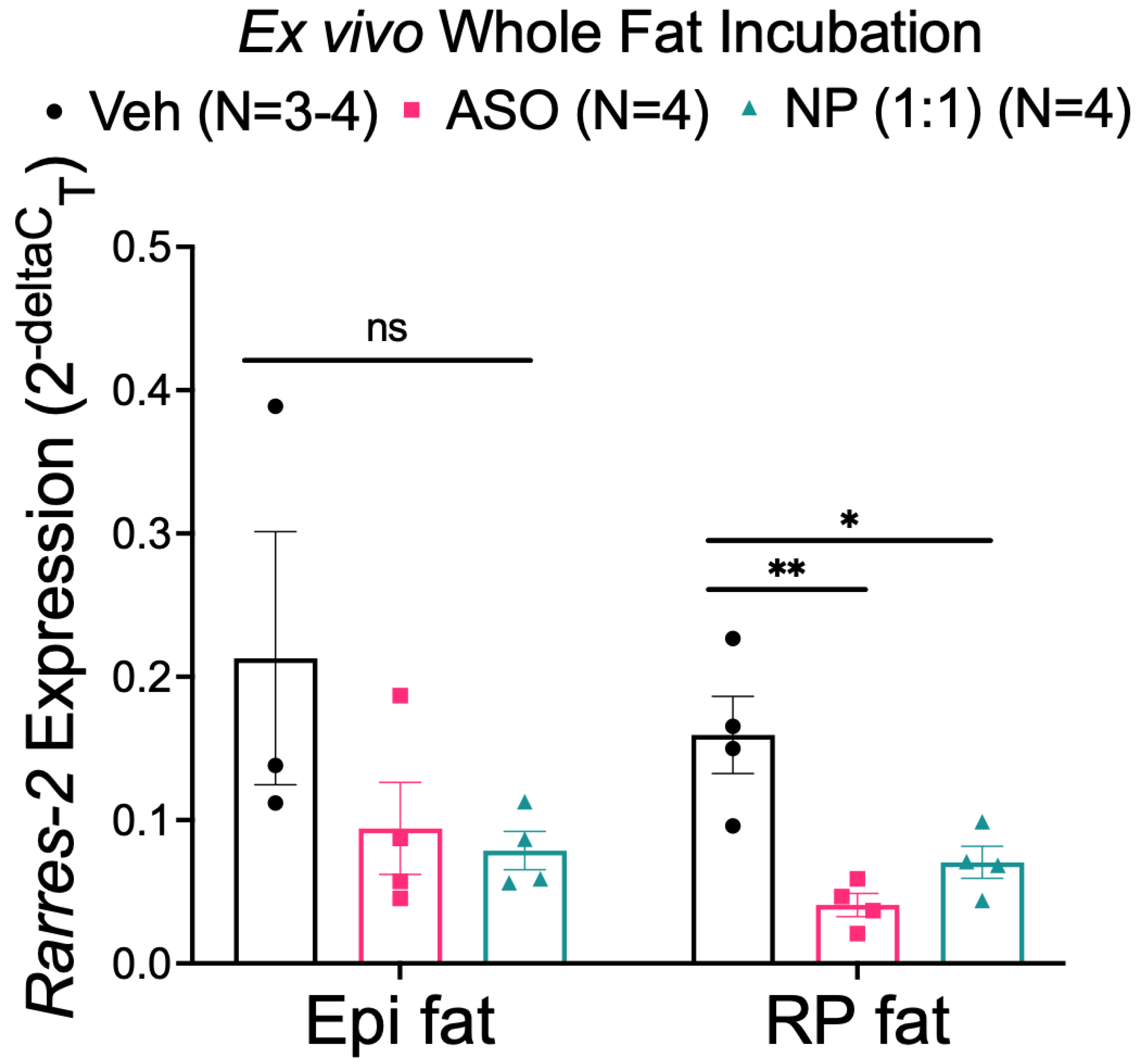
3.4. NP Reduced Chemerin Expression in Ex Vivo Incubated RP, but Not Epi Fat
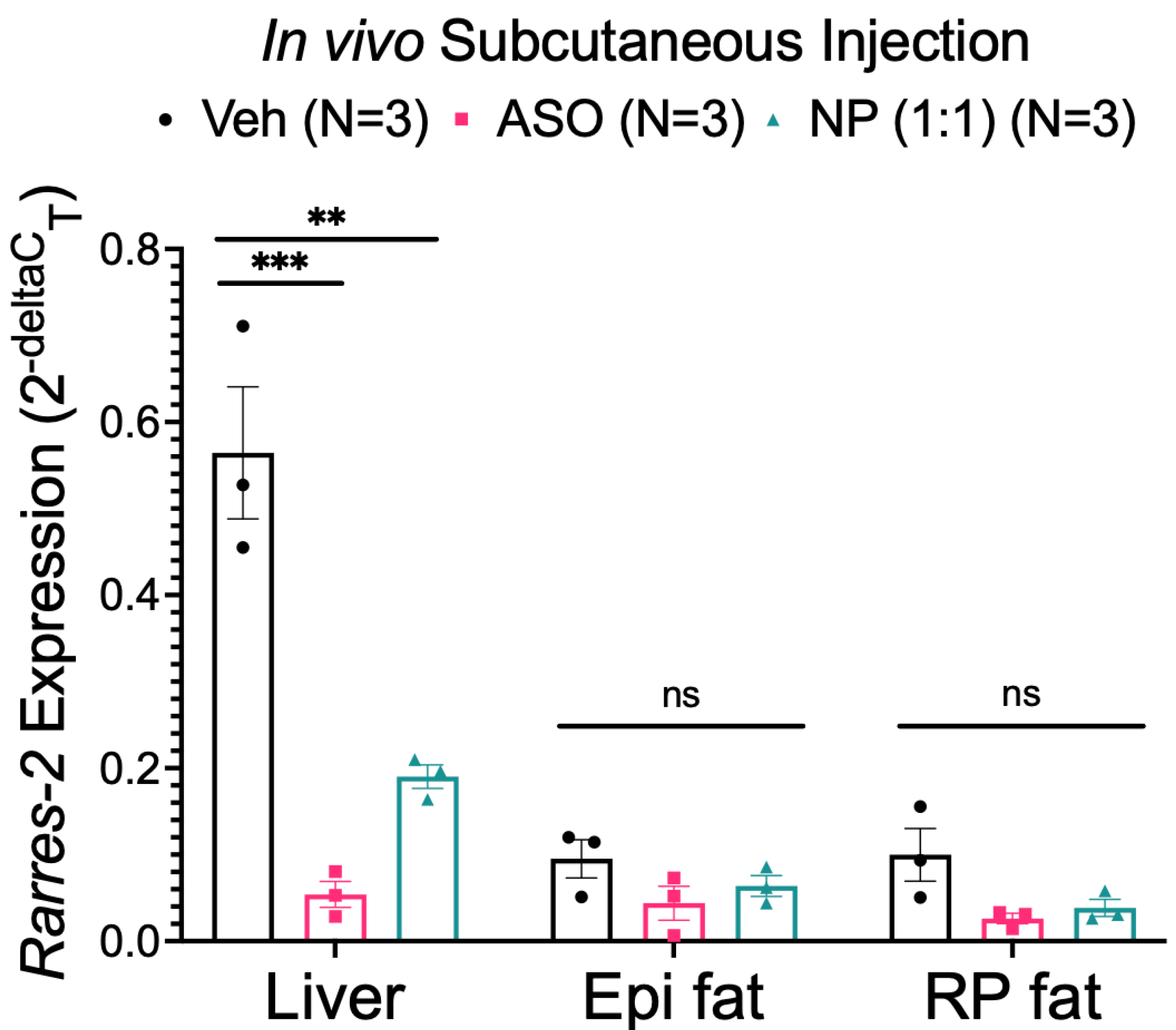
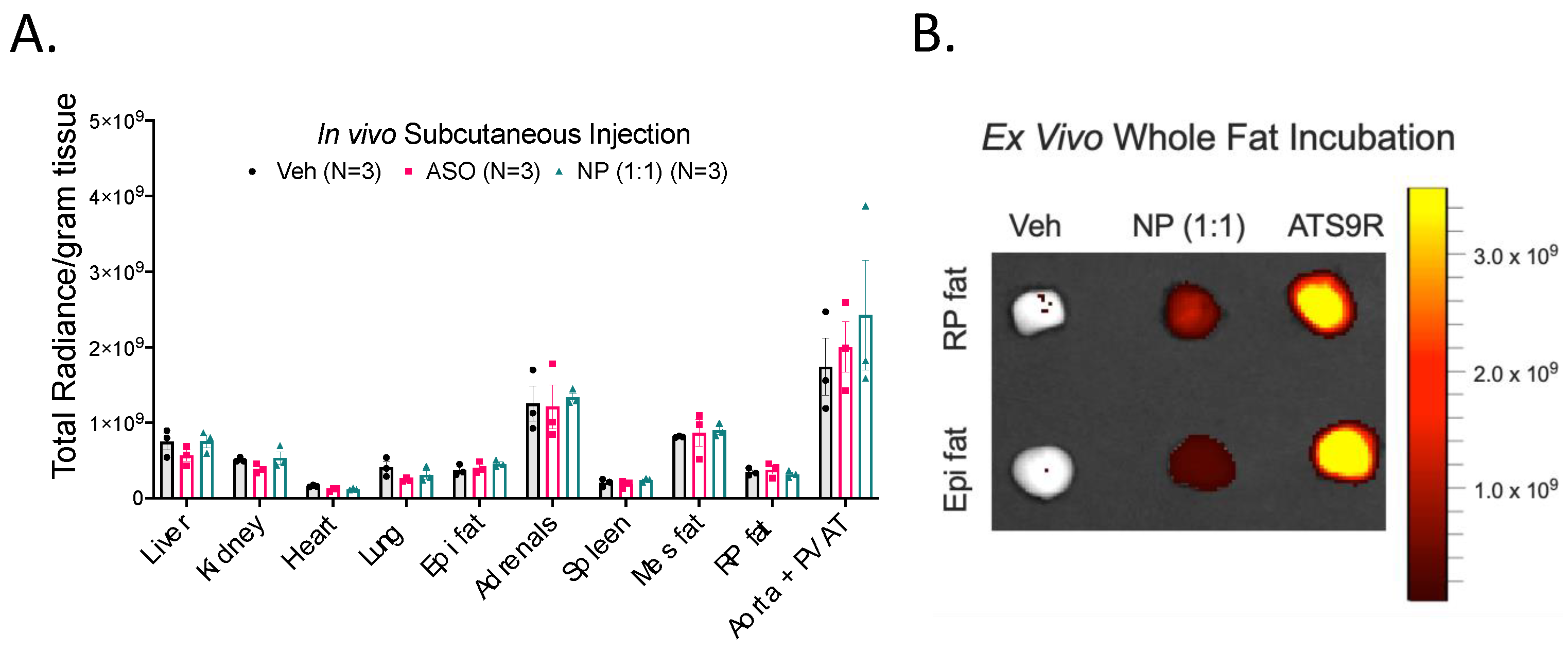
3.5. NP Was Unable to Selectively Deliver ASO to Fat In Vivo
3.6. FITC Labeling Was Not a Viable Method of Tracking the NP
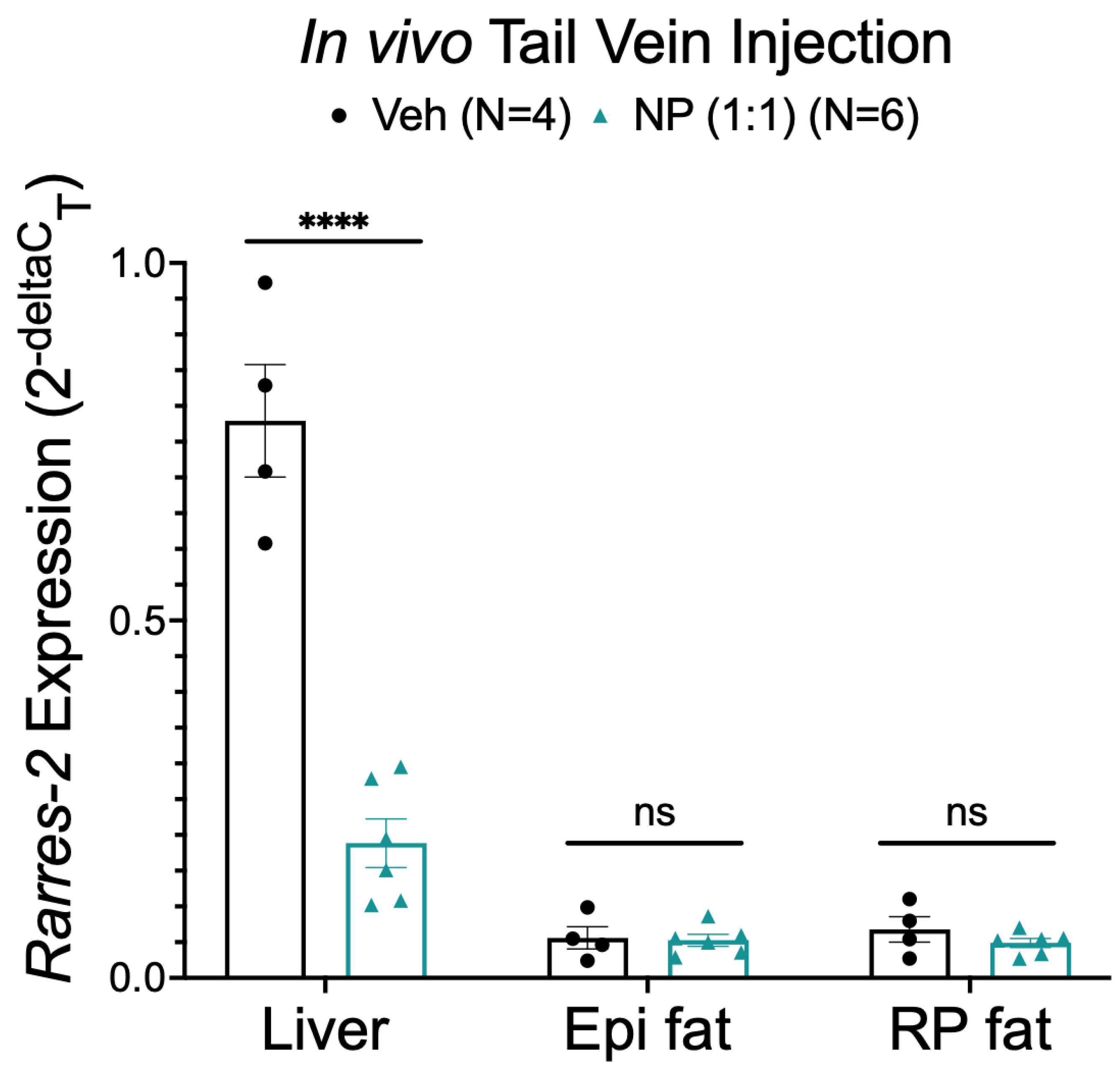
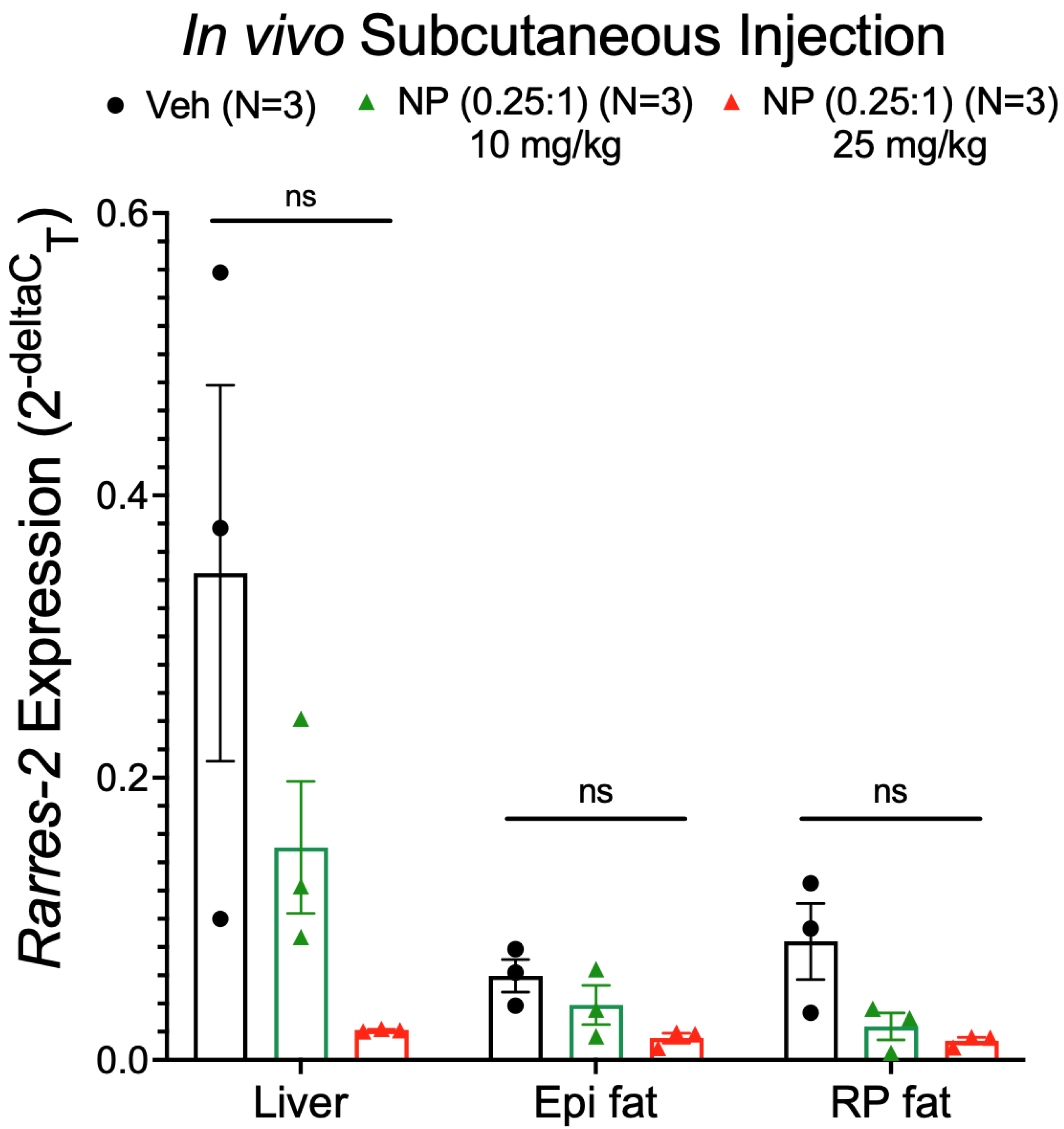
3.7. Alternative NP Administration Route and Concentrations Did Not Improve Selectivity
4. Discussion
4.1. Lack of Success of Adipose-Specific NP Delivery
4.2. Modifications of NP Administration and Formulation Did Not Improve Adipose-Specific Delivery
4.3. Challenges, Limitations, and Alternatives
4.4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Whitlock, G.; Lewington, S.; Sherliker, P.; Clarke, R.; Emberson, J.; Halsey, J.; Qizilbash, N.; Collins, R.; Peto, R.; Collaboration, P.S. Body-mass index and cause-specific mortality in 900,000 adults: Collaborative analyses of 57 prospective studies. Lancet 2009, 373, 1083–1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotchen, T.A. Obesity-related hypertension: Epidemiology, pathophysiology, and clinical management. Am. J. Hypertens. 2010, 23, 1170–1178. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.E.; do Carmo, J.M.; da Silva, A.A.; Wang, Z.; Hall, M.E. Obesity-induced hypertension: Interaction of neurohumoral and renal mechanisms. Circ. Res. 2015, 116, 991–1006. [Google Scholar] [CrossRef] [Green Version]
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef] [Green Version]
- Abais-Battad, J.M.; Lund, H.; Fehrenbach, D.J.; Dasinger, J.H.; Alsheikh, A.J.; Mattson, D.L. Parental Dietary Protein Source and the Role of CMKLR1 in Determining the Severity of Dahl Salt-Sensitive Hypertension. Hypertension 2019, 73, 440–448. [Google Scholar] [CrossRef]
- Alfadda, A.A.; Sallam, R.M.; Chishti, M.A.; Moustafa, A.S.; Fatma, S.; Alomaim, W.S.; Al-Naami, M.Y.; Bassas, A.F.; Chrousos, G.P.; Jo, H. Differential patterns of serum concentration and adipose tissue expression of chemerin in obesity: Adipose depot specificity and gender dimorphism. Mol. Cells 2012, 33, 591–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Athyros, V.G.; Kakafika, A.I.; Tziomalos, K.; Karagiannis, A.; Mikhailidis, D.P. Antisense technology for the prevention or the treatment of cardiovascular disease: The next blockbuster? Expert Opin. Investig. Drugs 2008, 17, 969–972. [Google Scholar] [CrossRef]
- Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; Naghavi, M.; et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2018, 71, e127–e248. [Google Scholar] [CrossRef]
- Beyer, A.M.; Raffai, G.; Weinberg, B.; Fredrich, K.; Lombard, J.H. Dahl salt-sensitive rats are protected against vascular defects related to diet-induced obesity. Hypertension 2012, 60, 404–410. [Google Scholar] [CrossRef] [Green Version]
- Bozaoglu, K.; Bolton, K.; McMillan, J.; Zimmet, P.; Jowett, J.; Collier, G.; Walder, K.; Segal, D. Chemerin is a novel adipokine associated with obesity and metabolic syndrome. Endocrinology 2007, 148, 4687–4694. [Google Scholar] [CrossRef] [PubMed]
- Bozaoglu, K.; Segal, D.; Shields, K.A.; Cummings, N.; Curran, J.E.; Comuzzie, A.G.; Mahaney, M.C.; Rainwater, D.L.; VandeBerg, J.L.; MacCluer, J.W.; et al. Chemerin is associated with metabolic syndrome phenotypes in a Mexican-American population. J. Clin. Endocrinol. Metab. 2009, 94, 3085–3088. [Google Scholar] [CrossRef] [PubMed]
- Buechler, C.; Feder, S.; Haberl, E.M.; Aslanidis, C. Chemerin Isoforms and Activity in Obesity. Int. J. Mol. Sci. 2019, 20, 1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheon, D.Y.; Kang, J.G.; Lee, S.J.; Ihm, S.H.; Lee, E.J.; Choi, M.G.; Yoo, H.J.; Kim, C.S. Serum Chemerin Levels are Associated with Visceral Adiposity, Independent of Waist Circumference, in Newly Diagnosed Type 2 Diabetic Subjects. Yonsei Med. J. 2017, 58, 319–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chou, H.H.; Teng, M.S.; Hsu, L.A.; Er, L.K.; Wu, S.; Ko, Y.L. Circulating chemerin level is associated with metabolic, biochemical and haematological parameters-A population-based study. Clin. Endocrinol. 2021, 94, 927–939. [Google Scholar] [CrossRef] [PubMed]
- Dessein, P.H.; Tsang, L.; Woodiwiss, A.J.; Norton, G.R.; Solomon, A. Circulating concentrations of the novel adipokine chemerin are associated with cardiovascular disease risk in rheumatoid arthritis. J. Rheumatol. 2014, 41, 1746–1754. [Google Scholar] [CrossRef]
- Eichelmann, F.; Weikert, C.; di Giuseppe, R.; Biemann, R.; Isermann, B.; Schulze, M.B.; Boeing, H.; Aleksandrova, K. Methodological utility of chemerin as a novel biomarker of immunity and metabolism. Endocr. Connect. 2017, 6, 340–347. [Google Scholar] [CrossRef] [Green Version]
- Ernst, M.C.; Sinal, C.J. Chemerin: At the crossroads of inflammation and obesity. Trends Endocrinol. Metab. 2010, 21, 660–667. [Google Scholar] [CrossRef]
- Ferland, D.J.; Mullick, A.E.; Watts, S.W. Chemerin as a Driver of Hypertension: A Consideration. Am. J. Hypertens. 2020, 33, 975–986. [Google Scholar] [CrossRef]
- Ferland, D.J.; Watts, S.W. Chemerin: A comprehensive review elucidating the need for cardiovascular research. Pharmacol. Res. 2015, 99, 351–361. [Google Scholar] [CrossRef] [Green Version]
- Gu, P.; Cheng, M.; Hui, X.; Lu, B.; Jiang, W.; Shi, Z. Elevating circulation chemerin level is associated with endothelial dysfunction and early atherosclerotic changes in essential hypertensive patients. J. Hypertens. 2015, 33, 1624–1632. [Google Scholar] [CrossRef] [PubMed]
- Parlee, S.D.; Ernst, M.C.; Muruganandan, S.; Sinal, C.J.; Goralski, K.B. Serum chemerin levels vary with time of day and are modified by obesity and tumor necrosis factor-{alpha}. Endocrinology 2010, 151, 2590–2602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sell, H.; Divoux, A.; Poitou, C.; Basdevant, A.; Bouillot, J.L.; Bedossa, P.; Tordjman, J.; Eckel, J.; Clément, K. Chemerin correlates with markers for fatty liver in morbidly obese patients and strongly decreases after weight loss induced by bariatric surgery. J. Clin. Endocrinol. Metab. 2010, 95, 2892–2896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, H.Y.; Lee, D.C.; Chu, S.H.; Jeon, J.Y.; Lee, M.K.; Im, J.A.; Lee, J.W. Chemerin levels are positively correlated with abdominal visceral fat accumulation. Clin. Endocrinol 2012, 77, 47–50. [Google Scholar] [CrossRef]
- Yoo, H.J.; Choi, H.Y.; Yang, S.J.; Kim, H.Y.; Seo, J.A.; Kim, S.G.; Kim, N.H.; Choi, K.M.; Choi, D.S.; Baik, S.H. Circulating chemerin level is independently correlated with arterial stiffness. J. Atheroscler. Thromb. 2012, 19, 59–66, discussion 67–58. [Google Scholar] [CrossRef] [Green Version]
- Mattern, A.; Zellmann, T.; Beck-Sickinger, A.G. Processing, signaling, and physiological function of chemerin. IUBMB Life 2014, 66, 19–26. [Google Scholar] [CrossRef]
- Ferland, D.J.; Flood, E.D.; Garver, H.; Yeh, S.T.; Riney, S.; Mullick, A.E.; Fink, G.D.; Watts, S.W. Different blood pressure responses in hypertensive rats following chemerin mRNA inhibition in dietary high fat compared to dietary high-salt conditions. Physiol. Genom. 2019, 51, 553–561. [Google Scholar] [CrossRef]
- Huang, Y. Preclinical and Clinical Advances of GalNAc-Decorated Nucleic Acid Therapeutics. Mol. Ther. Nucleic. Acids 2017, 6, 116–132. [Google Scholar] [CrossRef] [Green Version]
- Ferland, D.J.; Seitz, B.; Darios, E.S.; Thompson, J.M.; Yeh, S.T.; Mullick, A.E.; Watts, S.W. Whole-Body but Not Hepatic Knockdown of Chemerin by Antisense Oligonucleotide Decreases Blood Pressure in Rats. J. Pharmacol. Exp. Ther. 2018, 365, 212–218. [Google Scholar] [CrossRef] [Green Version]
- Kolonin, M.G.; Saha, P.K.; Chan, L.; Pasqualini, R.; Arap, W. Reversal of obesity by targeted ablation of adipose tissue. Nat. Med. 2004, 10, 625–632. [Google Scholar] [CrossRef]
- Mishra, S.; Murphy, L.C.; Nyomba, B.L.; Murphy, L.J. Prohibitin: A potential target for new therapeutics. Trends Mol. Med. 2005, 11, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Won, Y.W.; Adhikary, P.P.; Lim, K.S.; Kim, H.J.; Kim, J.K.; Kim, Y.H. Oligopeptide complex for targeted non-viral gene delivery to adipocytes. Nat. Mater. 2014, 13, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Tatsuta, T.; Model, K.; Langer, T. Formation of membrane-bound ring complexes by prohibitins in mitochondria. Mol. Biol. Cell 2005, 16, 248–259. [Google Scholar] [CrossRef] [Green Version]
- Hossen, M.N.; Kajimoto, K.; Akita, H.; Hyodo, M.; Ishitsuka, T.; Harashima, H. Ligand-based targeted delivery of a peptide modified nanocarrier to endothelial cells in adipose tissue. J. Control Release 2010, 147, 261–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakurai, Y.; Kajimoto, K.; Harashima, H. Anti-angiogenic nanotherapy via active targeting systems to tumors and adipose tissue vasculature. Biomater. Sci. 2015, 3, 1253–1265. [Google Scholar] [CrossRef] [PubMed]
- Hiradate, R.; Khalil, I.A.; Matsuda, A.; Sasaki, M.; Hida, K.; Harashima, H. A novel dual-targeted rosiglitazone-loaded nanoparticle for the prevention of diet-induced obesity via the browning of white adipose tissue. J. Control Release 2021, 329, 665–675. [Google Scholar] [CrossRef]
- Fernandes, R.; Garver, H.; Harkema, J.R.; Galligan, J.J.; Fink, G.D.; Xu, H. Sex Differences in Renal Inflammation and Injury in High-Fat Diet-Fed Dahl Salt-Sensitive Rats. Hypertension 2018, 72, e43–e52. [Google Scholar] [CrossRef]
- Cheng, Y.; Sun, C.; Liu, R.; Yang, J.; Dai, J.; Zhai, T.; Lou, X.; Xia, F. A Multifunctional Peptide-Conjugated AIEgen for Efficient and Sequential Targeted Gene Delivery into the Nucleus. Angew. Chem. Int. Ed. Engl. 2019, 58, 5049–5053. [Google Scholar] [CrossRef]
- Chung, J.Y.; Hong, J.; Kim, H.J.; Song, Y.; Yong, S.B.; Lee, J.; Kim, Y.H. White adipocyte-targeted dual gene silencing of FABP4/5 for anti-obesity, anti-inflammation and reversal of insulin resistance: Efficacy and comparison of administration routes. Biomaterials 2021, 279, 121209. [Google Scholar] [CrossRef]
- Yong, S.B.; Song, Y.; Kim, Y.H. Visceral adipose tissue macrophage-targeted TACE silencing to treat obesity-induced type 2 diabetes. Biomaterials 2017, 148, 81–89. [Google Scholar] [CrossRef]
- Ferland, D. Chemerin as a Link between Hypertension and Obesity. Ph.D. Thesis, Michigan State University, East Lansing, MI, USA, 2020. [Google Scholar]
- Tönjes, A.; Scholz, M.; Breitfeld, J.; Marzi, C.; Grallert, H.; Gross, A.; Ladenvall, C.; Schleinitz, D.; Krause, K.; Kirsten, H.; et al. Genome wide meta-analysis highlights the role of genetic variation in RARRES2 in the regulation of circulating serum chemerin. PLoS Genet. 2014, 10, e1004854. [Google Scholar] [CrossRef]
- Stelmanska, E.; Sledzinski, T.; Turyn, J.; Presler, M.; Korczynska, J.; Swierczynski, J. Chemerin gene expression is regulated by food restriction and food restriction-refeeding in rat adipose tissue but not in liver. Regul. Pept. 2013, 181, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Wang, X.; Li, J.; Nie, Y.; Liao, G.; Yu, Y.; Li, C. Overcoming the Reticuloendothelial System Barrier to Drug Delivery with a “Don’t-Eat-Us” Strategy. ACS Nano 2019, 13, 13015–13026. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, W.; Brown, L.E.; Qiu, C.; Lajkiewicz, N.; Zhao, T.; Zhou, J.; Porco, J.A.; Wang, T.T. A Novel Class of Small Molecule Compounds that Inhibit Hepatitis C Virus Infection by Targeting the Prohibitin-CRaf Pathway. EBioMedicine 2015, 2, 1600–1606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goralski, K.B.; Sinal, C.J. Elucidation of chemerin and chemokine-like receptor-1 function in adipocytes by adenoviral-mediated shRNA knockdown of gene expression. Methods Enzymol. 2009, 460, 289–312. [Google Scholar] [CrossRef]
- Watts, S.W.; Darios, E.S.; Mullick, A.E.; Garver, H.; Saunders, T.L.; Hughes, E.D.; Filipiak, W.E.; Zeidler, M.G.; McMullen, N.; Sinal, C.J.; et al. The chemerin knockout rat reveals chemerin dependence in female, but not male, experimental hypertension. FASEB J. 2018, 32, fj201800479. [Google Scholar] [CrossRef]
- Yanan, Y.; Yi, J.; Xiaojing, L.; Jing, Q.; Xiaohui, W. Adipo-specific chemerin knockout alters the metabolomic profile of adipose tissue under normal and high-fat diet conditions: Application of an untargeted liquid chromatography-tandem mass spectrometry metabolomics method. Biomed. Chromatogr. 2021, 35, e5220. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orr, A.; Liu, K.; Mullick, A.E.; Huang, X.; Watts, S.W. Divergence of Chemerin Reduction by an ATS9R Nanoparticle Targeting Adipose Tissue In Vitro vs. In Vivo in the Rat. Biomedicines 2022, 10, 1635. https://doi.org/10.3390/biomedicines10071635
Orr A, Liu K, Mullick AE, Huang X, Watts SW. Divergence of Chemerin Reduction by an ATS9R Nanoparticle Targeting Adipose Tissue In Vitro vs. In Vivo in the Rat. Biomedicines. 2022; 10(7):1635. https://doi.org/10.3390/biomedicines10071635
Chicago/Turabian StyleOrr, Alexis, Kunli Liu, Adam E. Mullick, Xuefei Huang, and Stephanie W. Watts. 2022. "Divergence of Chemerin Reduction by an ATS9R Nanoparticle Targeting Adipose Tissue In Vitro vs. In Vivo in the Rat" Biomedicines 10, no. 7: 1635. https://doi.org/10.3390/biomedicines10071635
APA StyleOrr, A., Liu, K., Mullick, A. E., Huang, X., & Watts, S. W. (2022). Divergence of Chemerin Reduction by an ATS9R Nanoparticle Targeting Adipose Tissue In Vitro vs. In Vivo in the Rat. Biomedicines, 10(7), 1635. https://doi.org/10.3390/biomedicines10071635





