Role of Erythropoietin Receptor Signaling in Macrophages or Choroidal Endothelial Cells in Choroidal Neovascularization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Ethical Statement
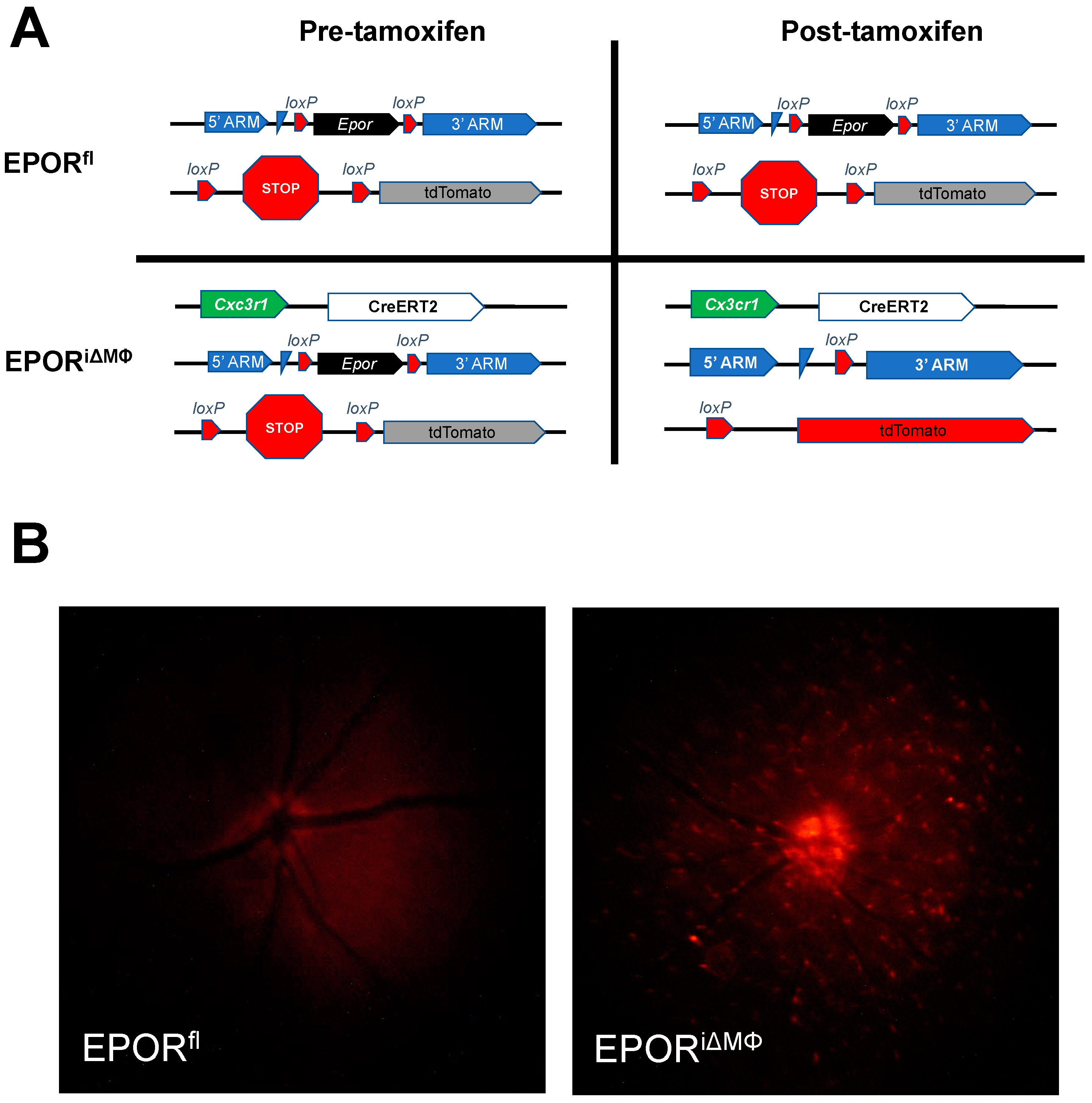
2.2. Generation of Conditional Knockout Mice and Tamoxifen Injections
2.3. Laser-Induced Choroidal Neovascularization Model
2.4. Posterior Eye Cup Flat Mounts and Immunostaining
2.5. Imaging and Quantifying Laser-Induced Lesion Volumes
2.6. Choroidal Endothelial Cell Culture Conditions
2.7. Transfection and Treatment
2.8. RNA Extraction and Quantitative RT-PCR Analysis
2.9. Protein Extraction and Western Blot Analysis
2.10. Statistical Analyses
3. Results
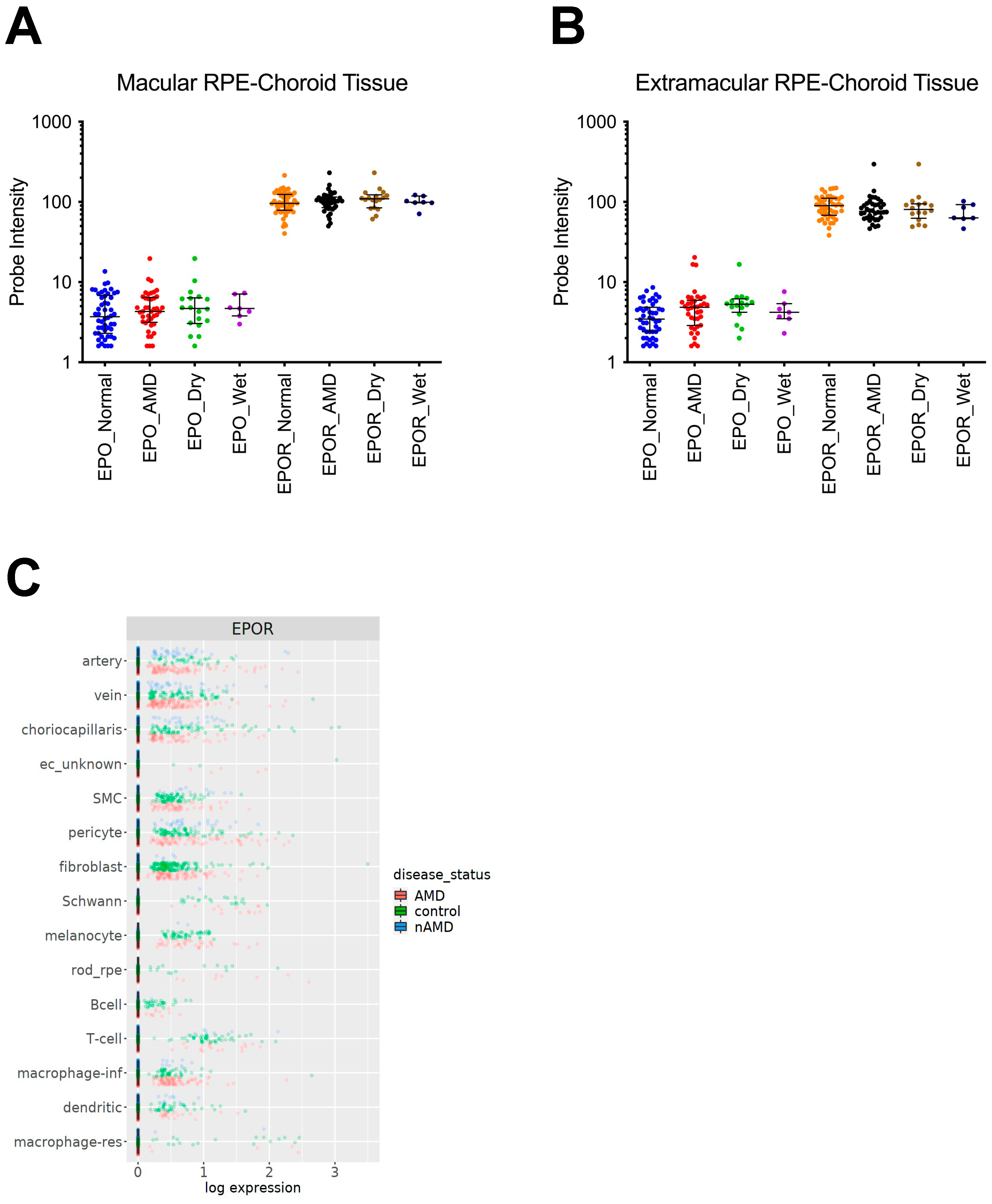
3.1. EPOR Expression in RPE/Choroids from Human Donor Eyes
3.2. The Effect of EPOR-Triggered Signaling in Macrophages on Laser-Induced Choroidal Neovascularization
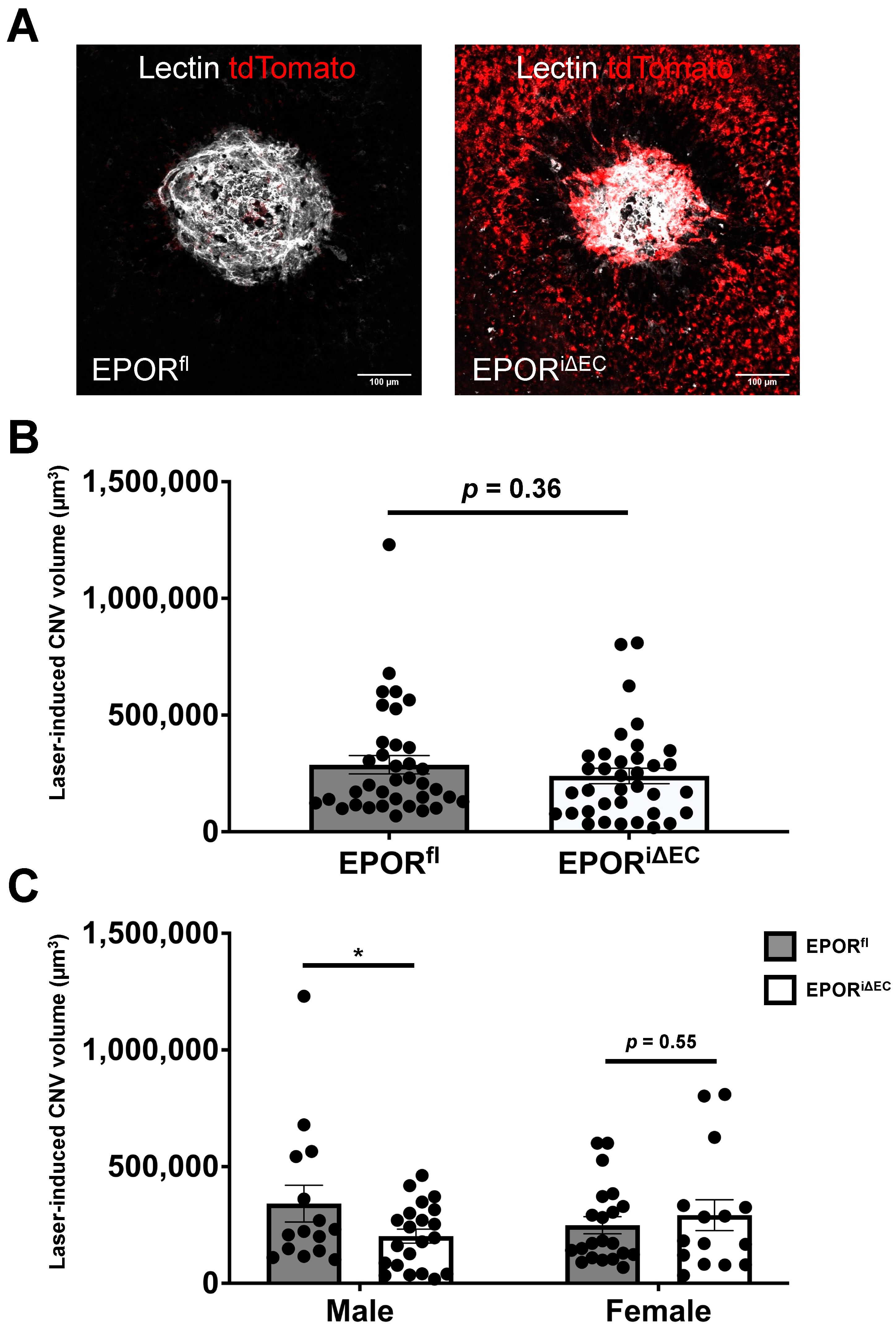
3.3. The Effect of EPOR-Triggered Signaling in Endothelial Cells on Laser-Induced Choroidal Neovascularization
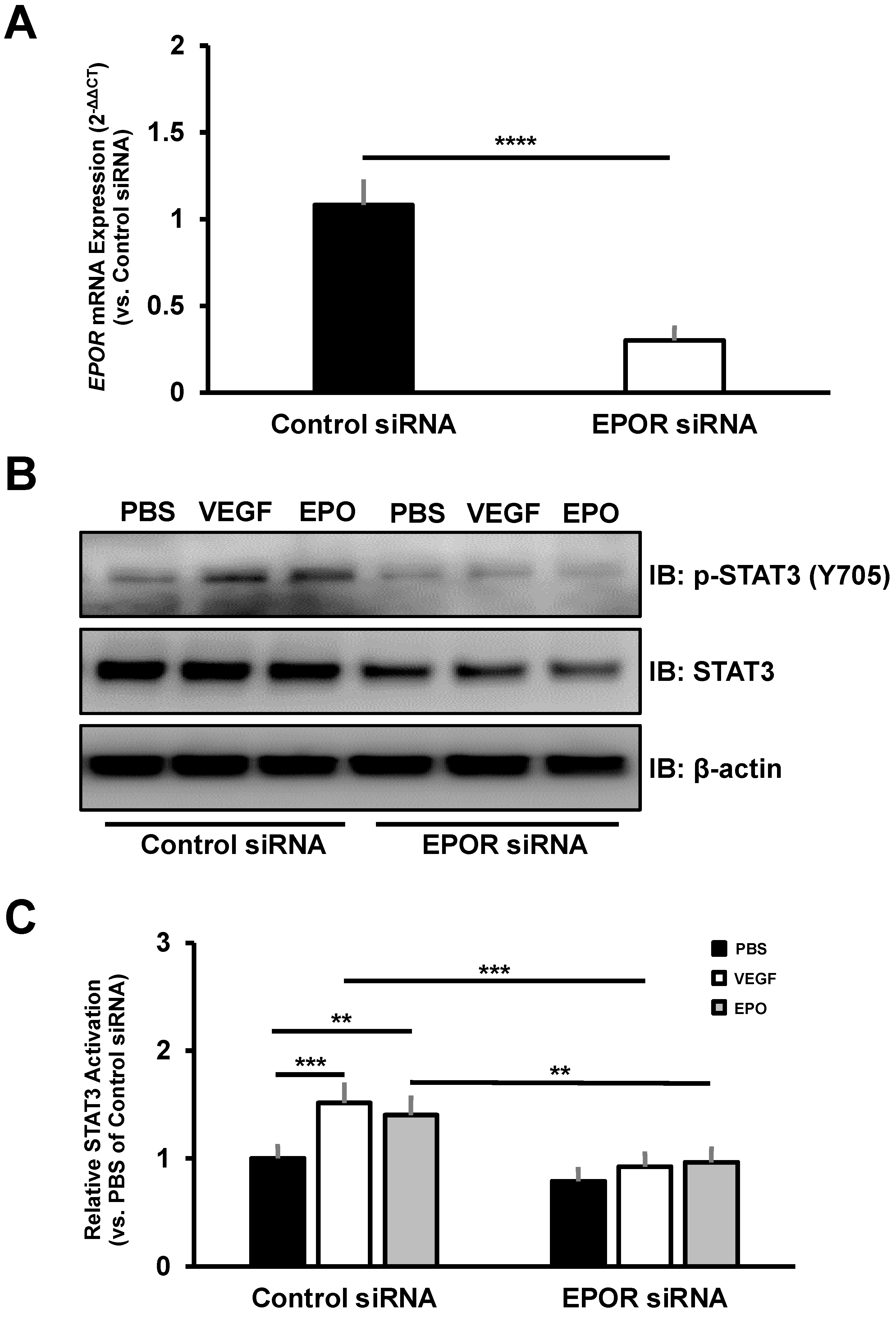
3.4. The Effect of EPOR-Triggered Signaling in Choroidal Endothelial Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [Green Version]
- Fleckenstein, M.; Mitchell, P.; Freund, K.B.; Sadda, S.; Holz, F.G.; Brittain, C.; Henry, E.C.; Ferrara, D. The Progression of Geographic Atrophy Secondary to Age-Related Macular Degeneration. Ophthalmology 2018, 125, 369–390. [Google Scholar] [CrossRef]
- Fleckenstein, M.; Keenan, T.D.L.; Guymer, R.H.; Chakravarthy, U.; Schmitz-Valckenberg, S.; Klaver, C.C.; Wong, W.T.; Chew, E.Y. Age-related macular degeneration. Nat. Rev. Dis. Primers 2021, 7, 31. [Google Scholar] [CrossRef]
- Zouache, M.A.; Bennion, A.; Hageman, J.L.; Pappas, C.; Richards, B.T.; Hageman, G.S. Macular retinal thickness differs markedly in age-related macular degeneration driven by risk polymorphisms on chromosomes 1 and 10. Sci. Rep. 2020, 10, 21093. [Google Scholar] [CrossRef]
- Scimone, C.; Donato, L.; Alibrandi, S.; Vadala, M.; Giglia, G.; Sidoti, A.; D’Angelo, R. N-retinylidene-N-retinylethanolamine adduct induces expression of chronic inflammation cytokines in retinal pigment epithelium cells. Exp. Eye Res. 2021, 209, 108641. [Google Scholar] [CrossRef]
- Jaffe, G.J.; Ying, G.S.; Toth, C.A.; Daniel, E.; Grunwald, J.E.; Martin, D.F.; Maguire, M.G. Macular Morphology and Visual Acuity in Year Five of the Comparison of Age-related Macular Degeneration Treatments Trials. Ophthalmology 2019, 126, 252–260. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Zhao, K.K.; Song, Z.M.; Shen, L.J.; Qu, J. Erythropoietin as a novel therapeutic agent for atrophic age-related macular degeneration. Med. Hypotheses 2009, 72, 448–450. [Google Scholar] [CrossRef]
- Becerra, S.P.; Amaral, J. Erythropoietin—An Endogenous Retinal Survival Factor. N. Engl. J. Med. 2002, 347, 1968–1970. [Google Scholar] [CrossRef]
- Yodoi, Y.; Sasahara, M.; Kameda, T.; Yoshimura, N.; Otani, A. Circulating hematopoietic stem cells in patients with neovascular age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2007, 48, 5464–5472. [Google Scholar] [CrossRef] [Green Version]
- Bretz, C.A.; Divoky, V.; Prchal, J.; Kunz, E.; Simmons, A.B.; Wang, H.; Hartnett, M.E. Erythropoietin Signaling Increases Choroidal Macrophages and Cytokine Expression, and Exacerbates Choroidal Neovascularization. Sci. Rep. 2018, 8, 2161. [Google Scholar] [CrossRef] [Green Version]
- Brines, M.; Cerami, A. Discovering erythropoietin’s extra-hematopoietic functions: Biology and clinical promise. Kidney Int. 2006, 70, 246–250. [Google Scholar] [CrossRef] [Green Version]
- Constantinescu, S.N.; Ghaffari, S.; Lodish, H.F. The Erythropoietin Receptor: Structure, Activation and Intracellular Signal Transduction. Trends Endocrinol. Metab. 1999, 10, 18–23. [Google Scholar] [CrossRef]
- Shirley Ding, S.L.; Leow, S.N.; Munisvaradass, R.; Koh, E.H.; Bastion, M.L.; Then, K.Y.; Kumar, S.; Mok, P.L. Revisiting the role of erythropoietin for treatment of ocular disorders. Eye 2016, 30, 1293–1309. [Google Scholar] [CrossRef] [Green Version]
- Grimm, C.; Wenzel, A.; Groszer, M.; Mayser, H.; Seeliger, M.; Samardzija, M.; Bauer, C.; Gassmann, M.; Reme, C.E. HIF-1-induced erythropoietin in the hypoxic retina protects against light-induced retinal degeneration. Nat. Med. 2002, 8, 718–724. [Google Scholar] [CrossRef]
- Shah, S.S.; Tsang, S.H.; Mahajan, V.B. Erythropoetin receptor expression in the human diabetic retina. BMC Res. Notes 2009, 2, 234. [Google Scholar] [CrossRef] [Green Version]
- Bond, W.S.; Rex, T.S. Evidence That Erythropoietin Modulates Neuroinflammation through Differential Action on Neurons, Astrocytes, and Microglia. Front. Immunol. 2014, 5, 523. [Google Scholar] [CrossRef]
- Wu, H.; Liu, X.; Jaenisch, R.; Lodish, H.F. Generation of committed erythroid BFU-E and CFU-E progenitors does not require erythropoietin or the erythropoietin receptor. Cell 1995, 83, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Kieran, M.W.; Perkins, A.C.; Orkin, S.H.; Zon, L.I. Thrombopoietin rescues in vitro erythroid colony formation from mouse embryos lacking the erythropoietin receptor. Proc. Natl. Acad. Sci. USA 1996, 93, 9126–9131. [Google Scholar] [CrossRef] [Green Version]
- Divoky, V.; Liu, Z.; Ryan, T.M.; Prchal, J.F.; Townes, T.M.; Prchal, J.T. Mouse model of congenital polycythemia: Homologous replacement of murine gene by mutant human erythropoietin receptor gene. Proc. Natl. Acad. Sci. USA 2001, 98, 986–991. [Google Scholar] [CrossRef] [Green Version]
- Bretz, C.A.; Ramshekar, A.; Kunz, E.; Wang, H.; Hartnett, M.E. Signaling Through the Erythropoietin Receptor Affects Angiogenesis in Retinovascular Disease. Investig. Ophthalmol. Vis. Sci. 2020, 61, 23. [Google Scholar] [CrossRef]
- Bretz, C.A.; Simmons, A.B.; Kunz, E.; Ramshekar, A.; Kennedy, C.; Cardenas, I.; Hartnett, M.E. Erythropoietin Receptor Signaling Supports Retinal Function after Vascular Injury. Am. J. Pathol. 2020, 190, 630–641. [Google Scholar] [CrossRef]
- Chang, B.; Hurd, R.; Wang, J.; Nishina, P. Survey of common eye diseases in laboratory mouse strains. Investig. Ophthalmol. Vis. Sci. 2013, 54, 4974–4981. [Google Scholar] [CrossRef]
- Lambert, V.; Lecomte, J.; Hansen, S.; Blacher, S.; Gonzalez, M.L.; Struman, I.; Sounni, N.E.; Rozet, E.; de Tullio, P.; Foidart, J.M.; et al. Laser-induced choroidal neovascularization model to study age-related macular degeneration in mice. Nat. Protoc. 2013, 8, 2197–2211. [Google Scholar] [CrossRef]
- Ramshekar, A.; Wang, H.; Kunz, E.; Pappas, C.; Hageman, G.S.; Chaqour, B.; Sacks, D.B.; Hartnett, M.E. Active Rap1-mediated inhibition of choroidal neovascularization requires interactions with IQGAP1 in choroidal endothelial cells. FASEB J. 2021, 35, e21642. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Newman, A.M.; Gallo, N.B.; Hancox, L.S.; Miller, N.J.; Radeke, C.M.; Maloney, M.A.; Cooper, J.B.; Hageman, G.S.; Anderson, D.H.; Johnson, L.V.; et al. Systems-level analysis of age-related macular degeneration reveals global biomarkers and phenotype-specific functional networks. Genome Med. 2012, 4, 16. [Google Scholar] [CrossRef]
- Voigt, A.P.; Mulfaul, K.; Mullin, N.K.; Flamme-Wiese, M.J.; Giacalone, J.C.; Stone, E.M.; Tucker, B.A.; Scheetz, T.E.; Mullins, R.F. Single-cell transcriptomics of the human retinal pigment epithelium and choroid in health and macular degeneration. Proc. Natl. Acad. Sci. USA 2019, 116, 24100–24107. [Google Scholar] [CrossRef] [Green Version]
- Voigt, A.P.; Whitmore, S.S.; Lessing, N.D.; DeLuca, A.P.; Tucker, B.A.; Stone, E.M.; Mullins, R.F.; Scheetz, T.E. Spectacle: An interactive resource for ocular single-cell RNA sequencing data analysis. Exp. Eye Res. 2020, 200, 108204. [Google Scholar] [CrossRef]
- Voigt, A.P.; Mullin, N.K.; Mulfaul, K.; Lozano, L.P.; Wiley, L.A.; Flamme-Wiese, M.J.; Boese, E.A.; Han, I.C.; Scheetz, T.E.; Stone, E.M.; et al. Choroidal Endothelial and Macrophage Gene Expression in Atrophic and Neovascular Macular Degeneration. Hum. Mol. Genet. 2022. [Google Scholar] [CrossRef]
- Sinclair, A.M.; Coxon, A.; McCaffery, I.; Kaufman, S.; Paweletz, K.; Liu, L.; Busse, L.; Swift, S.; Elliott, S.; Begley, C.G. Functional erythropoietin receptor is undetectable in endothelial, cardiac, neuronal, and renal cells. Blood 2010, 115, 4264–4272. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Wang, H.; Jiang, Y.; Hartnett, M.E. VEGFA activates erythropoietin receptor and enhances VEGFR2-mediated pathological angiogenesis. Am. J. Pathol. 2014, 184, 1230–1239. [Google Scholar] [CrossRef] [Green Version]
- Fasler-Kan, E.; Wunderlich, K.; Hildebrand, P.; Flammer, J.; Meyer, P. Activated STAT3 in choroidal neovascular membranes of patients with age-related macular degeneration. Ophthalmologica 2005, 219, 214–221. [Google Scholar] [CrossRef]
- Christakis, P.G.; Agrón, E.; Klein, M.L.; Clemons, T.E.; Campbell, J.P.; Ferris, F.L.; Chew, E.Y.; Keenan, T.D. Incidence of Macular Atrophy after Untreated Neovascular Age-Related Macular Degeneration: Age-Related Eye Disease Study Report 40. Ophthalmology 2020, 127, 784–792. [Google Scholar] [CrossRef]
- Daniel, E.; Maguire, M.G.; Grunwald, J.E.; Toth, C.A.; Jaffe, G.J.; Martin, D.F.; Ying, G.S. Incidence and Progression of Nongeographic Atrophy in the Comparison of Age-Related Macular Degeneration Treatments Trials (CATT) Clinical Trial. JAMA Ophthalmol. 2020, 138, 510–518. [Google Scholar] [CrossRef]
- Maguire, M.G.; Martin, D.F.; Ying, G.S.; Jaffe, G.J.; Daniel, E.; Grunwald, J.E.; Toth, C.A.; Ferris, F.L., 3rd; Fine, S.L. Five-Year Outcomes with Anti-Vascular Endothelial Growth Factor Treatment of Neovascular Age-Related Macular Degeneration: The Comparison of Age-Related Macular Degeneration Treatments Trials. Ophthalmology 2016, 123, 1751–1761. [Google Scholar] [CrossRef] [Green Version]
- Evans, R.N.; Reeves, B.C.; Phillips, D.; Muldrew, K.A.; Rogers, C.; Harding, S.P.; Chakravarthy, U. Long-term Visual Outcomes after Release from Protocol in Patients who Participated in the Inhibition of VEGF in Age-related Choroidal Neovascularisation (IVAN) Trial. Ophthalmology 2020, 127, 1191–1200. [Google Scholar] [CrossRef]
- Fishbane, S.; Pollock, C.A.; El-Shahawy, M.; Escudero, E.T.; Rastogi, A.; Van, B.P.; Frison, L.; Houser, M.; Pola, M.; Little, D.J.; et al. Roxadustat Versus Epoetin Alfa for Treating Anemia in Patients with Chronic Kidney Disease on Dialysis: Results from the Randomized Phase 3 ROCKIES Study. J. Am. Soc. Nephrol. 2022, 33, 850–866. [Google Scholar] [CrossRef]
- Lifshitz, L.; Tabak, G.; Gassmann, M.; Mittelman, M.; Neumann, D. Macrophages as novel target cells for erythropoietin. Haematologica 2010, 95, 1823–1831. [Google Scholar] [CrossRef] [Green Version]
- Haroon, Z.A.; Amin, K.; Jiang, X.; Arcasoy, M.O. A novel role for erythropoietin during fibrin-induced wound-healing response. Am. J. Pathol. 2003, 163, 993–1000. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Wang, S.; Liu, D.; Gao, C.; Han, Y.; Guo, X.; Qu, X.; Li, W.; Zhang, S.; Geng, J.; et al. EpoR-tdTomato-Cre mice enable identification of EpoR expression in subsets of tissue macrophages and hematopoietic cells. Blood 2021, 138, 1986–1997. [Google Scholar] [CrossRef]
- Wood, M.A.; Goldman, N.; DePierri, K.; Somerville, J.; Riggs, J.E. Erythropoietin increases macrophage-mediated T cell suppression. Cell. Immunol. 2016, 306–307, 17–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, J.; Yun, J.Y.; Hur, J.; Kang, J.A.; Choi, J.I.; Ko, S.B.; Lee, J.; Kim, J.Y.; Hwang, I.C.; Park, Y.B.; et al. Erythropoietin priming improves the vasculogenic potential of G-CSF mobilized human peripheral blood mononuclear cells. Cardiovasc. Res. 2014, 104, 171–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anagnostou, A.; Liu, Z.; Steiner, M.; Chin, K.; Lee, E.S.; Kessimian, N.; Noguchi, C.T. Erythropoietin receptor mRNA expression in human endothelial cells. Proc. Natl. Acad. Sci. USA 1994, 91, 3974–3978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimakova, P.; Solar, P.; Solarova, Z.; Komel, R.; Debeljak, N. Erythropoietin and Its Angiogenic Activity. Int. J. Mol. Sci. 2017, 18, 1519. [Google Scholar] [CrossRef]
- Izumi-Nagai, K.; Nagai, N.; Ozawa, Y.; Mihara, M.; Ohsugi, Y.; Kurihara, T.; Koto, T.; Satofuka, S.; Inoue, M.; Tsubota, K.; et al. Interleukin-6 receptor-mediated activation of signal transducer and activator of transcription-3 (STAT3) promotes choroidal neovascularization. Am. J. Pathol. 2007, 170, 2149–2158. [Google Scholar] [CrossRef] [Green Version]
- Biswal, M.R.; Wang, Z.; Paulson, R.J.; Uddin, R.R.; Tong, Y.; Zhu, P.; Li, H.; Lewin, A.S. Erythropoietin Gene Therapy Delays Retinal Degeneration Resulting from Oxidative Stress in the Retinal Pigment Epithelium. Antioxidants 2021, 10, 842. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramshekar, A.; Bretz, C.A.; Kunz, E.; Cung, T.; Richards, B.T.; Stoddard, G.J.; Hageman, G.S.; Chaqour, B.; Hartnett, M.E. Role of Erythropoietin Receptor Signaling in Macrophages or Choroidal Endothelial Cells in Choroidal Neovascularization. Biomedicines 2022, 10, 1655. https://doi.org/10.3390/biomedicines10071655
Ramshekar A, Bretz CA, Kunz E, Cung T, Richards BT, Stoddard GJ, Hageman GS, Chaqour B, Hartnett ME. Role of Erythropoietin Receptor Signaling in Macrophages or Choroidal Endothelial Cells in Choroidal Neovascularization. Biomedicines. 2022; 10(7):1655. https://doi.org/10.3390/biomedicines10071655
Chicago/Turabian StyleRamshekar, Aniket, Colin A. Bretz, Eric Kunz, Thaonhi Cung, Burt T. Richards, Gregory J. Stoddard, Gregory S. Hageman, Brahim Chaqour, and M. Elizabeth Hartnett. 2022. "Role of Erythropoietin Receptor Signaling in Macrophages or Choroidal Endothelial Cells in Choroidal Neovascularization" Biomedicines 10, no. 7: 1655. https://doi.org/10.3390/biomedicines10071655
APA StyleRamshekar, A., Bretz, C. A., Kunz, E., Cung, T., Richards, B. T., Stoddard, G. J., Hageman, G. S., Chaqour, B., & Hartnett, M. E. (2022). Role of Erythropoietin Receptor Signaling in Macrophages or Choroidal Endothelial Cells in Choroidal Neovascularization. Biomedicines, 10(7), 1655. https://doi.org/10.3390/biomedicines10071655





