Increased Subcortical Sodium Levels in Patients with Progressive Supranuclear Palsy
Abstract
:1. Introduction
2. Methods
2.1. Demographics and Clinical Assessment
2.2. Ethics Statement
2.3. MR Data Acquisition and Analyses
2.4. Statistical Analyses
2.5. Data Availability
3. Results
3.1. Demographics and Clinical Assessment
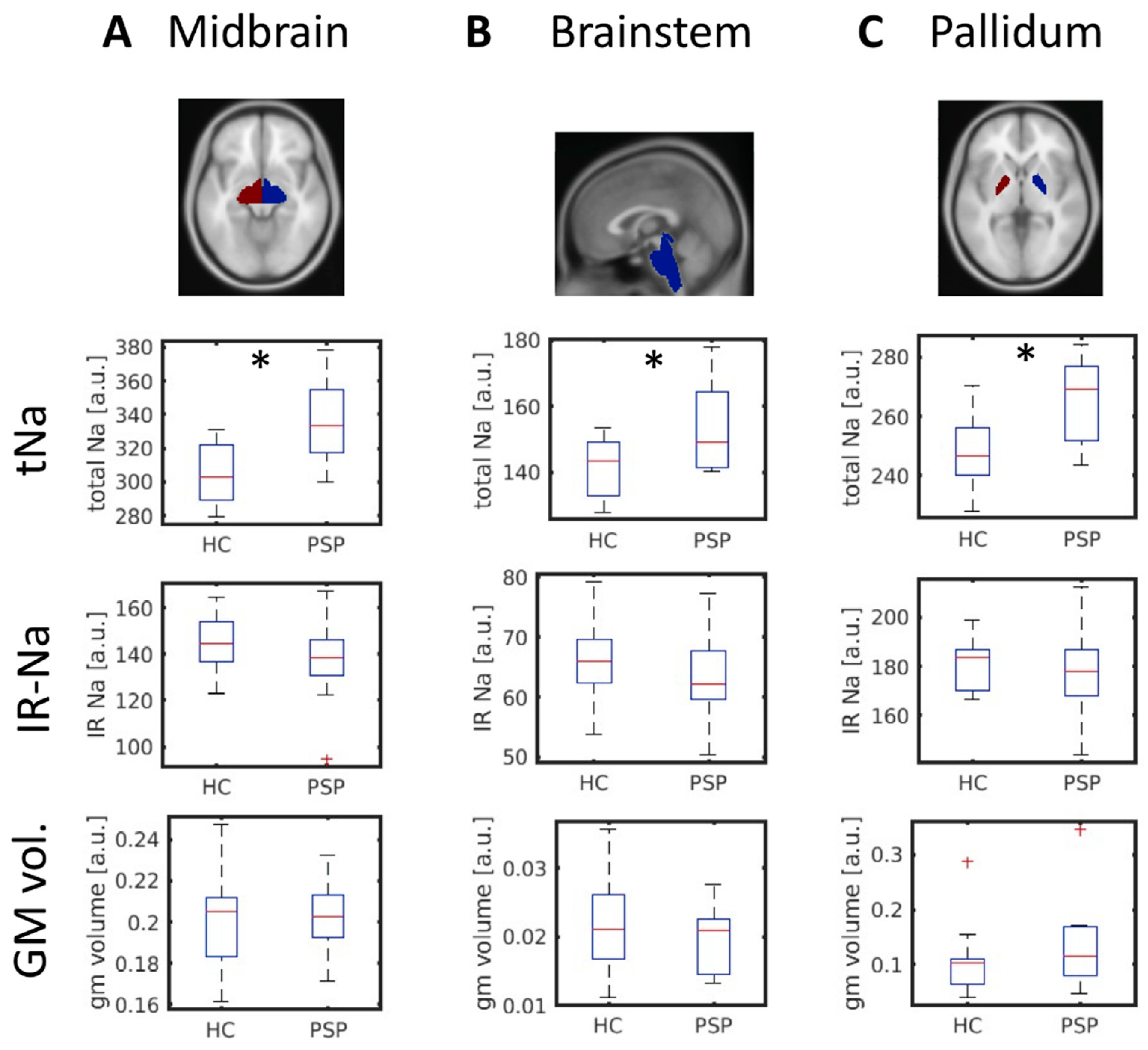
3.2. Increased Midbrain Total Sodium in PSP Patients
4. Discussion
- the neuroimaging-based differentiation of PSP subtypes,
- the applicability of 23Na-MRI in the longitudinal assessment of patients with PSP (among other atypical parkinsonian syndromes), and
- the evaluation of 23Na-MRI for the differential diagnosis of patients with idiopathic Parkinson’s disease from atypical parkinsonism.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arena, J.E.; Weigand, S.D.; Whitwell, J.L.; Hassan, A.; Eggers, S.D.; Hoglinger, G.U.; Litvan, I.; Josephs, K.A. Progressive supranuclear palsy: Progression and survival. J. Neurol. 2016, 263, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Respondek, G.; Hoglinger, G.U. The phenotypic spectrum of progressive supranuclear palsy. Parkinsonism Relat. Disord. 2016, 22 (Suppl. 1), S34–S36. [Google Scholar] [CrossRef]
- Hoglinger, G.U.; Respondek, G.; Stamelou, M.; Kurz, C.; Josephs, K.A.; Lang, A.E.; Mollenhauer, B.; Muller, U.; Nilsson, C.; Whitwell, J.L.; et al. Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria. Mov. Disord. 2017, 32, 853–864. [Google Scholar] [CrossRef]
- Massey, L.A.; Jager, H.R.; Paviour, D.C.; O’Sullivan, S.S.; Ling, H.; Williams, D.R.; Kallis, C.; Holton, J.; Revesz, T.; Burn, D.J.; et al. The midbrain to pons ratio: A simple and specific MRI sign of progressive supranuclear palsy. Neurology 2013, 80, 1856–1861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Soriano, A.; Stoessl, A.J. Tau imaging in progressive supranuclear palsy. Mov. Disord. 2017, 32, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Leuzy, A.; Chiotis, K.; Lemoine, L.; Gillberg, P.G.; Almkvist, O.; Rodriguez-Vieitez, E.; Nordberg, A. Tau PET imaging in neurodegenerative tauopathies-still a challenge. Mol. Psychiatry 2019, 24, 1112–1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shrivastava, A.N.; Redeker, V.; Pieri, L.; Bousset, L.; Renner, M.; Madiona, K.; Mailhes-Hamon, C.; Coens, A.; Buee, L.; Hantraye, P.; et al. Clustering of Tau fibrils impairs the synaptic composition of alpha3-Na(+)/K(+)-ATPase and AMPA receptors. EMBO J. 2019, 38, e99871. [Google Scholar] [CrossRef]
- Azarias, G.; Kruusmagi, M.; Connor, S.; Akkuratov, E.E.; Liu, X.L.; Lyons, D.; Brismar, H.; Broberger, C.; Aperia, A. A specific and essential role for Na,K-ATPase alpha3 in neurons co-expressing alpha1 and alpha3. J. Biol. Chem. 2013, 288, 2734–2743. [Google Scholar] [CrossRef] [Green Version]
- Huhn, K.; Engelhorn, T.; Linker, R.A.; Nagel, A.M. Potential of Sodium MRI as a Biomarker for Neurodegeneration and Neuroinflammation in Multiple Sclerosis. Front. Neurol. 2019, 10, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golbe, L.I.; Ohman-Strickland, P.A. A clinical rating scale for progressive supranuclear palsy. Brain 2007, 130, 1552–1565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasuhn, J.; Gottlich, M.; Grosser, S.S.; Reuther, K.; Ebeling, B.; Munchau, A.; Nagel, A.M.; Bruggemann, N. In Vivo Brain Sodium Disequilibrium in ATP1A3-Related Rapid-Onset Dystonia-Parkinsonism. Mov. Disord. 2022, 37, 877–879. [Google Scholar] [CrossRef] [PubMed]
- Dutt, S.; Binney, R.J.; Heuer, H.W.; Luong, P.; Attygalle, S.; Bhatt, P.; Marx, G.A.; Elofson, J.; Tartaglia, M.C.; Litvan, I.; et al. Progression of brain atrophy in PSP and CBS over 6 months and 1 year. Neurology 2016, 87, 2016–2025. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, S.; El Mendili, M.M.; Zaaraoui, W.; Ranjeva, J.P.; Azulay, J.P.; Eusebio, A.; Guye, M. Increased Sodium Concentration in Substantia Nigra in Early Parkinson’s Disease: A Preliminary Study With Ultra-High Field (7T) MRI. Front. Neurol. 2021, 12, 715618. [Google Scholar] [CrossRef] [PubMed]

| PSP | HC | p-Value | |
|---|---|---|---|
| number | 10 | 19 | |
| male/female | 5/5 | 10/9 | 0.893 1 |
| age [years] | 67.3 ± 7.9 | 68.7 ± 8.7 | 0.679 2 |
| PSPRS | 31.0 ± 16.9 3 | n.a. | |
| MDS-UPDRS-III | 36.1 ± 18.7 3 | n.a. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prasuhn, J.; Göttlich, M.; Großer, S.S.; Reuther, K.; Ebeling, B.; Bodemann, C.; Hanssen, H.; Nagel, A.M.; Brüggemann, N. Increased Subcortical Sodium Levels in Patients with Progressive Supranuclear Palsy. Biomedicines 2022, 10, 1728. https://doi.org/10.3390/biomedicines10071728
Prasuhn J, Göttlich M, Großer SS, Reuther K, Ebeling B, Bodemann C, Hanssen H, Nagel AM, Brüggemann N. Increased Subcortical Sodium Levels in Patients with Progressive Supranuclear Palsy. Biomedicines. 2022; 10(7):1728. https://doi.org/10.3390/biomedicines10071728
Chicago/Turabian StylePrasuhn, Jannik, Martin Göttlich, Sinja S. Großer, Katharina Reuther, Britt Ebeling, Christina Bodemann, Henrike Hanssen, Armin M. Nagel, and Norbert Brüggemann. 2022. "Increased Subcortical Sodium Levels in Patients with Progressive Supranuclear Palsy" Biomedicines 10, no. 7: 1728. https://doi.org/10.3390/biomedicines10071728
APA StylePrasuhn, J., Göttlich, M., Großer, S. S., Reuther, K., Ebeling, B., Bodemann, C., Hanssen, H., Nagel, A. M., & Brüggemann, N. (2022). Increased Subcortical Sodium Levels in Patients with Progressive Supranuclear Palsy. Biomedicines, 10(7), 1728. https://doi.org/10.3390/biomedicines10071728






