Increased Chondroprotective Effect of Combining Hyaluronic Acid with a Glucocorticoid Compared to Separate Administration on Cytokine-Treated Osteoarthritic Chondrocytes in a 2D Culture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Cultivation of Human Chondrocytes
2.2. Treatment of Chondrocytes
2.3. Metabolic Activity
2.4. Reactive Oxygen Species (ROS) Activity
2.5. Gene Expression
2.5.1. Total RNA Isolation and Reverse Transcription
2.5.2. Quantitative Polymerase Chain Reaction (qPCR)
2.6. Cytokine Quantification
2.7. Sulfated Glycosaminoglycans (sGAG)
2.8. Phalloidin Staining
2.9. Statistical Analysis
3. Results
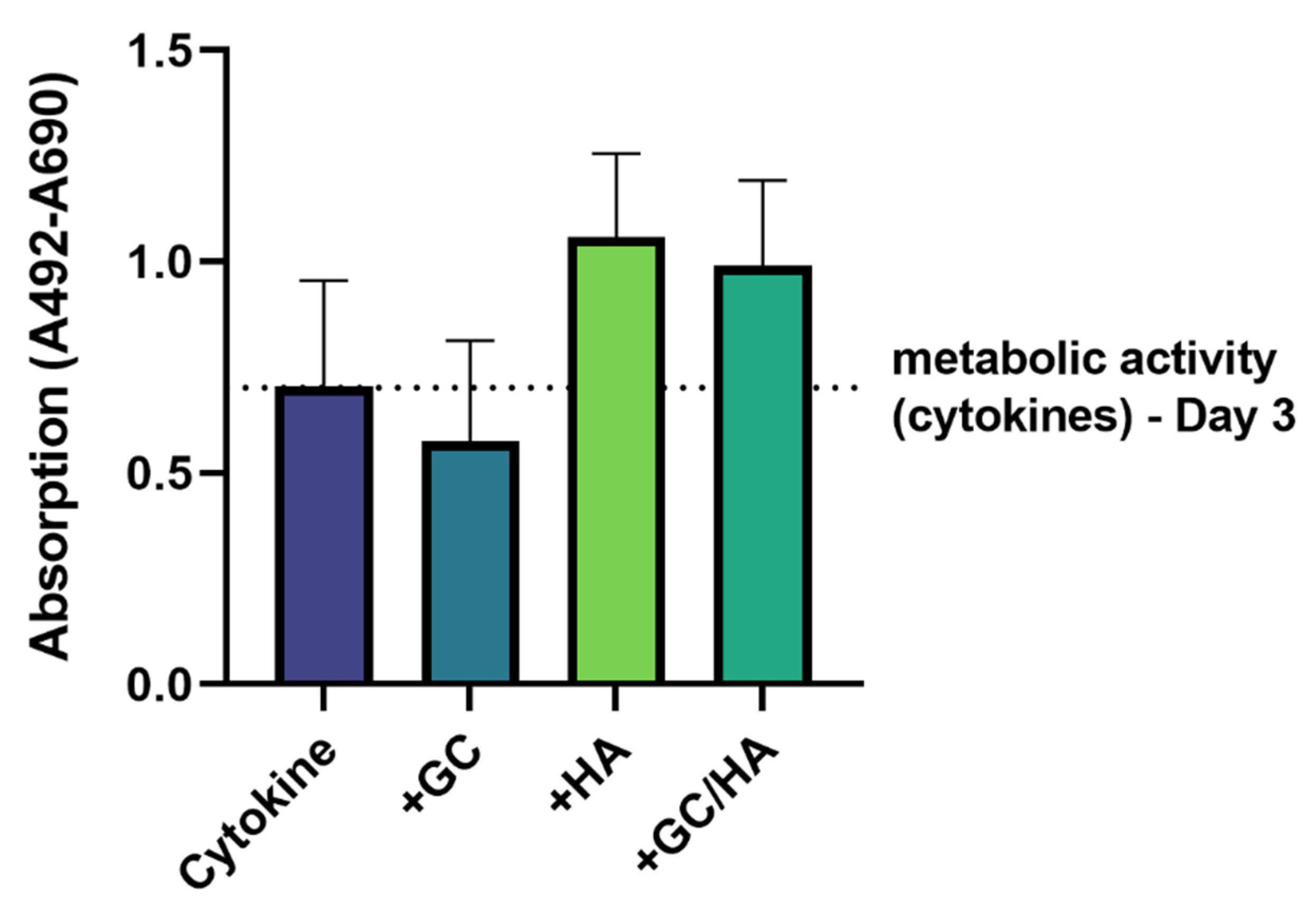
3.1. Metabolic Activity
3.2. ROS Activity
3.3. Gene Expression
3.4. Cytokine Release
3.5. Sulfated Glycosaminoglycans (sGAG)
3.6. F-Actin Staining
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goldring, M.B.; Otero, M. Inflammation in Osteoarthritis. Curr. Opin. Rheumatol. 2011, 23, 471. [Google Scholar] [CrossRef] [PubMed]
- Melrose, J.; Fuller, E.S.; Little, C.B. The Biology of Meniscal Pathology in Osteoarthritis and Its Contribution to Joint Disease: Beyond Simple Mechanics. Connect. Tissue Res. 2017, 58, 282–294. [Google Scholar] [CrossRef]
- Neogi, T. The Epidemiology and Impact of Pain in Osteoarthritis. Osteoarthr. Cartil. 2013, 21, 1145–1153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathiessen, A.; Conaghan, P.G. Synovitis in Osteoarthritis: Current Understanding with Therapeutic Implications. Arthritis Res. Ther. 2017, 19, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molnar, V.; Matišić, V.; Kodvanj, I.; Bjelica, R.; Jeleč, Ž.; Hudetz, D.; Rod, E.; Čukelj, F.; Vrdoljak, T.; Vidović, D.; et al. Cytokines and Chemokines Involved in Osteoarthritis Pathogenesis. Int. J. Mol. Sci. 2021, 22, 9208. [Google Scholar] [CrossRef] [PubMed]
- Wojdasiewicz, P.; Poniatowski, Ł.A.; Szukiewicz, D. The Role of Inflammatory and Anti-Inflammatory Cytokines in the Pathogenesis of Osteoarthritis. Mediat. Inflamm. 2014, 2014, 561459. [Google Scholar] [CrossRef] [Green Version]
- Sokolove, J.; Lepus, C.M. Role of Inflammation in the Pathogenesis of Osteoarthritis: Latest Findings and Interpretations. Ther. Adv. Musculoskelet. Dis. 2013, 5, 77–94. [Google Scholar] [CrossRef]
- Vaishya, R.; Pariyo, G.B.; Agarwal, A.K.; Vijay, V. Non-Operative Management of Osteoarthritis of the Knee Joint. J. Clin. Orthop. Trauma 2016, 7, 170–176. [Google Scholar] [CrossRef] [Green Version]
- Cross, W.W., 3rd; Saleh, K.J.; Wilt, T.J.; Kane, R.L. Agreement about Indications for Total Knee Arthroplasty. Clin. Orthop. Relat. Res. 2006, 446, 34–39. [Google Scholar] [CrossRef]
- Ethgen, O.; Bruyère, O.; Richy, F.; Dardennes, C.; Reginster, J.-Y. Health-Related Quality of Life in Total Hip and Total Knee Arthroplasty. A Qualitative and Systematic Review of the Literature. J. Bone Jt. Surg. Am. 2004, 86, 963–974. [Google Scholar] [CrossRef]
- Gerwin, N.; Hops, C.; Lucke, A. Intraarticular Drug Delivery in Osteoarthritis. Adv. Drug Deliv. Rev. 2006, 58, 226–242. [Google Scholar] [CrossRef] [PubMed]
- Creamer, P. Intra-Articular Corticosteroid Injections in Osteoarthritis: Do They Work and If so, How? Ann. Rheum. Dis. 1997, 56, 634–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piper, S.L.; Kramer, J.D.; Kim, H.T.; Feeley, B.T. Effects of Local Anesthetics on Articular Cartilage. Am. J. Sports Med. 2011, 39, 2245–2253. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, K.; Koenen, M.; Schauer, S.; Wittig-Blaich, S.; Ahmad, M.; Baschant, U.; Tuckermann, J.P. Molecular Actions of Glucocorticoids in Cartilage and Bone during Health, Disease, and Steroid Therapy. Physiol. Rev. 2016, 96, 409–447. [Google Scholar] [CrossRef] [Green Version]
- Altman, R.D.; Manjoo, A.; Fierlinger, A.; Niazi, F.; Nicholls, M. The Mechanism of Action for Hyaluronic Acid Treatment in the Osteoarthritic Knee: A Systematic Review. BMC Musculoskelet. Disord. 2015, 16, 321. [Google Scholar] [CrossRef] [Green Version]
- Akmal, M.; Singh, A.; Anand, A.; Kesani, A.; Aslam, N.; Goodship, A.; Bentley, G. The Effects of Hyaluronic Acid on Articular Chondrocytes. J. Bone Jt. Surg. Br. 2005, 87, 1143–1149. [Google Scholar] [CrossRef]
- Greenberg, D.D.; Stoker, A.; Kane, S.; Cockrell, M.; Cook, J.L. Biochemical Effects of Two Different Hyaluronic Acid Products in a Co-Culture Model of Osteoarthritis. Osteoarthr. Cartil. 2006, 14, 814–822. [Google Scholar] [CrossRef] [Green Version]
- Hangody, L.; Szody, R.; Lukasik, P.; Zgadzaj, W.; Lénárt, E.; Dokoupilova, E.; Bichovsk, D.; Berta, A.; Vasarhelyi, G.; Ficzere, A.; et al. Intraarticular Injection of a Cross-Linked Sodium Hyaluronate Combined with Triamcinolone Hexacetonide (Cingal) to Provide Symptomatic Relief of Osteoarthritis of the Knee: A Randomized, Double-Blind, Placebo-Controlled Multicenter Clinical Trial. Cartilage 2018, 9, 276–283. [Google Scholar] [CrossRef] [Green Version]
- Ozturk, C.; Atamaz, F.; Hepguler, S.; Argin, M.; Arkun, R. The Safety and Efficacy of Intraarticular Hyaluronan with/without Corticosteroid in Knee Osteoarthritis: 1-Year, Single-Blind, Randomized Study. Rheumatol. Int. 2006, 26, 314–319. [Google Scholar] [CrossRef]
- De Campos, G.C.; Rezende, M.U.; Pailo, A.F.; Frucchi, R.; Camargo, O.P. Adding Triamcinolone Improves Viscosupplementation: A Randomized Clinical Trial. Clin. Orthop. Relat. Res. 2013, 471, 613–620. [Google Scholar] [CrossRef] [Green Version]
- Caborn, D.; Rush, J.; Lanzer, W.; Parenti, D.; Murray, C. A Randomized, Single-Blind Comparison of the Efficacy and Tolerability of Hylan G-F 20 and Triamcinolone Hexacetonide in Patients with Osteoarthritis of the Knee. J. Rheumatol. 2004, 31, 333–343. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2-ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, I.; Garcia, S.; Barbier-Chassefière, V.; Caruelle, J.P.; Martelly, I.; Papy-García, D. Improved and Simple Micro Assay for Sulfated Glycosaminoglycans Quantification in Biological Extracts and Its Use in Skin and Muscle Tissue Studies. Glycobiology 2003, 13, 647–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fields, J.K.; Günther, S.; Sundberg, E.J. Structural Basis of IL-1 Family Cytokine Signaling. Front. Immunol. 2019, 10, 1412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nees, T.A.; Rosshirt, N.; Zhang, J.A.; Reiner, T.; Sorbi, R.; Tripel, E.; Walker, T.; Schiltenwolf, M.; Hagmann, S.; Moradi, B. Synovial Cytokines Significantly Correlate with Osteoarthritis-Related Knee Pain and Disability: Inflammatory Mediators of Potential Clinical Relevance. J. Clin. Med. 2019, 8, 1343. [Google Scholar] [CrossRef] [Green Version]
- Jin, W.; Dong, C. IL-17 Cytokines in Immunity and Inflammation. Emerg. Microbes Infect. 2013, 2, e60. [Google Scholar] [CrossRef] [PubMed]
- Mimpen, J.Y.; Baldwin, M.J.; Cribbs, A.P.; Philpott, M.; Carr, A.J.; Dakin, S.G.; Snelling, S.J.B. Interleukin-17A Causes Osteoarthritis-Like Transcriptional Changes in Human Osteoarthritis-Derived Chondrocytes and Synovial Fibroblasts In Vitro. Front. Immunol. 2021, 12, 676173. [Google Scholar] [CrossRef] [PubMed]
- Mimpen, J.Y.; Carr, A.J.; Dakin, S.G.; Snelling, S.J. Inhibition of Interleukin-17-Induced Effects in Osteoarthritis—An in Vitro Study. Osteoarthr. Cartil. 2018, 26, S118. [Google Scholar] [CrossRef]
- Kongdang, P.; Chokchaitaweesuk, C.; Tangyuenyong, S.; Ongchai, S. Proinflammatory Effects of IL-1β Combined with IL-17A Promoted Cartilage Degradation and Suppressed Genes Associated with Cartilage Matrix Synthesis In Vitro. Molecules 2019, 24, 3682. [Google Scholar] [CrossRef] [Green Version]
- Schaefer, E.C.; Stewart, A.A.; Durgam, S.S.; Byron, C.R.; Stewart, M.C. Effects of Sodium Hyaluronate and Triamcinolone Acetonide on Glucosaminoglycan Metabolism in Equine Articular Chondrocytes Treated with Interleukin-1. Am. J. Vet. Res. 2009, 70, 1494–1501. [Google Scholar] [CrossRef]
- Bauer, C.; Niculescu-Morzsa, E.; Jeyakumar, V.; Kern, D.; Späth, S.S.; Nehrer, S. Chondroprotective Effect of High-Molecular-Weight Hyaluronic Acid on Osteoarthritic Chondrocytes in a Co-Cultivation Inflammation Model with M1 Macrophages. J. Inflamm. 2016, 13, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siengdee, P.; Radeerom, T.; Kuanoon, S.; Euppayo, T.; Pradit, W.; Chomdej, S.; Ongchai, S.; Nganvongpanit, K. Effects of Corticosteroids and Their Combinations with Hyaluronanon on the Biochemical Properties of Porcine Cartilage Explants. BMC Vet. Res. 2015, 11, 298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayhan, E.; Kesmezacar, H.; Akgun, I. Intraarticular Injections (Corticosteroid, Hyaluronic Acid, Platelet Rich Plasma) for the Knee Osteoarthritis. World J. Orthop. 2014, 5, 351–361. [Google Scholar] [CrossRef]
- Song, Y.W.; Zhang, T.; Wang, W.B. Gluococorticoid Could Influence Extracellular Matrix Synthesis through Sox9 via P38 MAPK Pathway. Rheumatol. Int. 2012, 32, 3669–3673. [Google Scholar] [CrossRef]
- Rhee, D.K.; Marcelino, J.; Baker, M.; Gong, Y.; Smits, P.; Lefebvre, V.; Jay, G.D.; Stewart, M.; Wang, H.; Warman, M.L.; et al. The Secreted Glycoprotein Lubricin Protects Cartilage Surfaces and Inhibits Synovial Cell Overgrowth. J. Clin. Investig. 2005, 115, 622–631. [Google Scholar] [CrossRef] [Green Version]
- Richardson, D.W.; Dodge, G.R. Dose-Dependent Effects of Corticosteroids on the Expression of Matrix-Related Genes in Normal and Cytokine-Treated Articular Chondrocytes. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. 2003, 52, 39–49. [Google Scholar] [CrossRef]
- Suntiparpluacha, M.; Tammachote, N.; Tammachote, R. Triamcinolone Acetonide Reduces Viability, Induces Oxidative Stress, and Alters Gene Expressions of Human Chondrocytes. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4985–4992. [Google Scholar] [PubMed]
- Wu, P.-T.; Kuo, L.-C.; Su, F.-C.; Chen, S.-Y.; Hsu, T.-I.; Li, C.-Y.; Tsai, K.-J.; Jou, I.-M. High-Molecular-Weight Hyaluronic Acid Attenuated Matrix Metalloproteinase-1 and -3 Expression via CD44 in Tendinopathy. Sci. Rep. 2017, 7, 40840. [Google Scholar] [CrossRef]
- Coleman, J.W. Nitric Oxide in Immunity and Inflammation. Int. Immunopharmacol. 2001, 1, 1397–1406. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-ΚB Signaling in Inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [Green Version]
- Aktan, F. INOS-Mediated Nitric Oxide Production and Its Regulation. Life Sci. 2004, 75, 639–653. [Google Scholar] [CrossRef] [PubMed]
- Campos, S.B.; Ashworth, S.L.; Wean, S.; Hosford, M.; Sandoval, R.M.; Hallett, M.A.; Atkinson, S.J.; Molitoris, B.A. Cytokine-Induced F-Actin Reorganization in Endothelial Cells Involves RhoA Activation. Am. J. Physiol. Ren. Physiol. 2009, 296, F487–F495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Xie, J.; Rajappa, R.; Deng, L.; Fredberg, J.; Yang, L. Interleukin-1β and Tumor Necrosis Factor-α Increase Stiffness and Impair Contractile Function of Articular Chondrocytes. Acta Biochim. Biophys. Sin. 2015, 47, 121–129. [Google Scholar] [CrossRef] [Green Version]
- Haudenschild, D.R.; Chen, J.; Steklov, N.; Lotz, M.K.; D’Lima, D.D. Characterization of the Chondrocyte Actin Cytoskeleton in Living Three-Dimensional Culture: Response to Anabolic and Catabolic Stimuli. Mol. Cell. Biomech. 2009, 6, 135–144. [Google Scholar]
- Yourek, G.; Hussain, M.A.; Mao, J.J. Cytoskeletal Changes of Mesenchymal Stem Cells during Differentiation. ASAIO J. 2007, 53, 219–228. [Google Scholar] [CrossRef] [Green Version]
- Vorselen, D.; Roos, W.H.; MacKintosh, F.C.; Wuite, G.J.L.; van Loon, J.J.W.A. The Role of the Cytoskeleton in Sensing Changes in Gravity by Nonspecialized Cells. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2014, 28, 536–547. [Google Scholar] [CrossRef] [Green Version]
- Nemirovskiy, O.V.; Radabaugh, M.R.; Aggarwal, P.; Funckes-Shippy, C.L.; Mnich, S.J.; Meyer, D.M.; Sunyer, T.; Rodney Mathews, W.; Misko, T.P. Plasma 3-Nitrotyrosine Is a Biomarker in Animal Models of Arthritis: Pharmacological Dissection of INOS’ Role in Disease. Nitric Oxide Biol. Chem. 2009, 20, 150–156. [Google Scholar] [CrossRef]
- Lepetsos, P.; Papavassiliou, A.G. ROS/Oxidative Stress Signaling in Osteoarthritis. Biochim. Biophys. Acta 2016, 1862, 576–591. [Google Scholar] [CrossRef] [PubMed]
- Zahan, O.-M.; Serban, O.; Gherman, C.; Fodor, D. The Evaluation of Oxidative Stress in Osteoarthritis. Med. Pharm. Rep. 2020, 93, 12–22. [Google Scholar] [CrossRef]
- Li, D.; Xie, G.; Wang, W. Reactive Oxygen Species: The 2-Edged Sword of Osteoarthritis. Am. J. Med. Sci. 2012, 344, 486–490. [Google Scholar] [CrossRef]
- Henrotin, Y.; Kurz, B.; Aigner, T. Oxygen and Reactive Oxygen Species in Cartilage Degradation: Friends or Foes? Osteoarthr. Cartil. 2005, 13, 643–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wernecke, C.; Braun, H.J.; Dragoo, J.L. The Effect of Intra-Articular Corticosteroids on Articular Cartilage: A Systematic Review. Orthop. J. Sport. Med. 2015, 3, 2325967115581163. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; He, X. Intra-Articular Hyaluronic Acid and Corticosteroids in the Treatment of Knee Osteoarthritis: A Meta-Analysis. Exp. Ther. Med. 2015, 9, 493–500. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.-P.; Lee, W.-C.; Hsieh, R.-L. Effects of Repeated Co-Injections of Corticosteroids and Hyaluronic Acid on Knee Osteoarthritis: A Prospective, Double-Blind Randomized Controlled Trial. Am. J. Med. 2021, 135, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.; Patel, R.; Vannabouathong, C.; Sales, B.; Rabinovich, A.; McCormack, R.; Belzile, E.L.; Bhandari, M. Combined Intra-Articular Injection of Corticosteroid and Hyaluronic Acid Reduces Pain Compared to Hyaluronic Acid Alone in the Treatment of Knee Osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 1974–1983. [Google Scholar] [CrossRef] [PubMed]
- Von der Mark, K.; Gauss, V.; von der Mark, H.; Müller, P. Relationship between Cell Shape and Type of Collagen Synthesised as Chondrocytes Lose Their Cartilage Phenotype in Culture. Nature 1977, 267, 531–532. [Google Scholar] [CrossRef]








| Group | Cytokines (IL-1β, IL-17) | Test Substance |
|---|---|---|
| Control | − | − |
| Cytokines | + | − |
| GC | + | +10% glucocorticoid (4.5 mg/mL) |
| HA | + | +10% hyaluronic acid (22 mg/mL, MW between 1 and 2.9 × 106 Da, ultra-pure and non-avian) |
| GC/HA | + | +10% glucocorticoid/hyaluronic acid |
| Gene | Identification | Sequence (3′–5′) | |
|---|---|---|---|
| Sense | Antisense | ||
| Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) | NM_002046 | ACATCGCTCAGACACCATG | TGTAGTTGAGGTCAATGAAGGG |
| Collagen type II, alpha I (COL2A1) | NM_001844 | AAGACGTGAAAGACTGCCTC | TTCTCCTTTCTGTCCCTTTGG |
| Aggrecan core protein I (ACAN) | NM_001135 | TGTGGGACTGAAGTTCTTGG | AGCGAGTTGTCATGGTCTG |
| Transcription factor SOX-9 (SOX9) | NM_000346 | ACTTGCACAACGCCGAG | CTGGTACTTGTAATCCGGGTG |
| Proteoglycan 4/Lubricin (PRG4) | NM_005807 | AGAACTGGCCTGAATCTGTG | ACCTGTGTCGTTTCTCCATAC |
| Matrix metalloproteinase-3 (MMP3) | NM_002422 | CCAGGGATTAATGGAGATGCC | AGTGTTGGCTGAGTGAAAGAG |
| Matrix metalloproteinase-13 (MMP13) | NM_002427 | GATGACGATGTACAAGGGATCC | ACTGGTAATGGCATCAAGGG |
| Nitric oxide synthase 2 (NOS2) | NM_000625 | GTTTGACCAGAGGACCCAG | ATCTCCTTTGTTACCGCTTCC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bauer, C.; Moser, L.B.; Jeyakumar, V.; Niculescu-Morzsa, E.; Kern, D.; Nehrer, S. Increased Chondroprotective Effect of Combining Hyaluronic Acid with a Glucocorticoid Compared to Separate Administration on Cytokine-Treated Osteoarthritic Chondrocytes in a 2D Culture. Biomedicines 2022, 10, 1733. https://doi.org/10.3390/biomedicines10071733
Bauer C, Moser LB, Jeyakumar V, Niculescu-Morzsa E, Kern D, Nehrer S. Increased Chondroprotective Effect of Combining Hyaluronic Acid with a Glucocorticoid Compared to Separate Administration on Cytokine-Treated Osteoarthritic Chondrocytes in a 2D Culture. Biomedicines. 2022; 10(7):1733. https://doi.org/10.3390/biomedicines10071733
Chicago/Turabian StyleBauer, Christoph, Lukas B. Moser, Vivek Jeyakumar, Eugenia Niculescu-Morzsa, Daniela Kern, and Stefan Nehrer. 2022. "Increased Chondroprotective Effect of Combining Hyaluronic Acid with a Glucocorticoid Compared to Separate Administration on Cytokine-Treated Osteoarthritic Chondrocytes in a 2D Culture" Biomedicines 10, no. 7: 1733. https://doi.org/10.3390/biomedicines10071733
APA StyleBauer, C., Moser, L. B., Jeyakumar, V., Niculescu-Morzsa, E., Kern, D., & Nehrer, S. (2022). Increased Chondroprotective Effect of Combining Hyaluronic Acid with a Glucocorticoid Compared to Separate Administration on Cytokine-Treated Osteoarthritic Chondrocytes in a 2D Culture. Biomedicines, 10(7), 1733. https://doi.org/10.3390/biomedicines10071733






