The Thioredoxin System of Mammalian Cells and Its Modulators
Abstract
:1. Introduction
2. Trx/TrxR System: Structure and Functions
2.1. Trx1 and Trx2 Isoforms: Structure and Functions
2.2. TrxR1 and TrxR2 Isoforms
2.3. Extracellular Trx/TrxR
2.4. Cytosolic and Mitochondrial Trx/TrxR. Role in Apoptosis Mechanism
2.5. Nuclear Trx Function
3. Inhibitors and Activators of the Trx/TrxR System
3.1. TrxR Inhibitors
3.1.1. Inhibition of TrxR by Metal Complexes
3.1.2. Mechanisms of Trx/TrxR Inhibition by Natural and Synthetic Compounds
3.1.3. Trx/TrxR Inhibition by Organochalcogen and Organoarsenic Compounds
3.2. Activators of the Trx/TrxR System
4. Trx/TrxR Functions in Health and Disease
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tretter, V.; Hochreiter, B.; Zach, M.L.; Krenn, K.; Klein, K. Understanding Cellular Redox Homeostasis: A Challenge for Precision Medicine. Int. J. Mol. Sci. 2022, 23, 106. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Zou, L.; Zhang, X.; Branco, V.; Wang, J.; Carvalho, C.; Holmgren, A.; Lu, J. Redox Signaling Mediated by Thioredoxin and Glutathione Systems in the Central Nervous System. Antioxid. Redox Signal. 2017, 27, 989–1010. [Google Scholar] [CrossRef] [PubMed]
- Hanschmann, E.-M.; Godoy, J.R.; Berndt, C.; Hudemann, C.; Lillig, C.H. Thioredoxins, Glutaredoxins, and Peroxiredoxins—Molecular Mechanisms and Health Significance: From Cofactors to Antioxidants to Redox Signaling. Antioxid. Redox Signal. 2013, 19, 1539–1605. [Google Scholar] [CrossRef] [PubMed]
- Jastrzab, A.; Skrzydlewska, E. Thioredoxin-dependent system. Application of inhibitors. J. Enzyme Inhib. Med. Chem. 2021, 36, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Patwardhan, R.S.; Sharma, D.; Sandur, S.K. Thioredoxin reductase: An emerging tarfet for radiosensitization of cancer. Transl. Oncol. 2022, 17, 101341. [Google Scholar] [CrossRef] [PubMed]
- Balsera, M.; Buchanan, B.B. Evolution of the thioredoxin system as a step enabling adaptation to oxidative stress. Free Radic. Biol. Med. 2019, 140, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Holmgren, A. The thioredoxin antioxidant system. Free Radic. Biol. Med. 2014, 66, 75–87. [Google Scholar] [CrossRef]
- Jia, J.J.; Geng, W.S.; Wang, Z.Q.; Chen, L.; Zeng, X.S. The role of thioredoxin system in cancer: Strategy for cancer therapy. Cancer Chemother. Pharmacol. 2019, 84, 453–470. [Google Scholar] [CrossRef]
- Scalcon, V.; Bindoli, A.; Rigobello, M.P. Significance of the mitochondrial thioredoxin reductase in cancer cells: An update on role, targets and inhibitors. Free Radic. Biol. Med. 2018, 127, 62–79. [Google Scholar] [CrossRef]
- Branco, V.; Pimentel, J.; Brito, M.A.; Carvalho, C. Thioredoxin, Glutathione and Related Molecules in Tumors of the Nervous System. Curr. Med. Chem. 2020, 27, 1878–1900. [Google Scholar] [CrossRef]
- Ningfei An, N.; Kang, Y. Thioredoxin and hematologic malignancies. Adv. Cancer Res. 2014, 122, 245–279. [Google Scholar]
- Nordberg, J.; Arner, E.S.J. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic. Biol. Med. 2001, 31, 1287–1312. [Google Scholar] [CrossRef]
- González, D.; Álamos, P.; Rivero, M.; Orellana, O.; Norambuena, J.; Chávez, R.; Levicán, G. Deciphering the Role of Multiple Thioredoxin Fold Proteins of Leptospirillum sp. in Oxidative Stress Tolerance. Int. J. Mol. Sci. 2020, 21, 1880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yodoi, J.; Matsuo, Y.; Tian, H.; Masutani, H.; Inamoto, T. Anti-Inflammatory Thioredoxin Family Proteins for Medicare, Healthcare and Aging Care. Nutrients 2017, 9, 1081. [Google Scholar] [CrossRef] [Green Version]
- Marchand, C.H.; Fermani, S.; Rossi, J.; Gurrieri, L.; Tedesco, D.; Henri, J.; Sparla, F.; Trost, P.; Lemaire, S.D.; Zaffagnini, M. Structural and Biochemical Insights into the Reactivity of Thioredoxin h1 from Chlamydomonas reinhardtii. Antioxidants 2019, 8, 10. [Google Scholar] [CrossRef] [Green Version]
- Noguera, M.E.; Vazquez, D.S.; Ferrer-Sueta, G.; Agudelo, W.A.; Howard, E.; Rasia, R.M.; Manta, B.; Cousido-Siah, A.; Mitschler, A.; Podjarny, A.; et al. Structural variability of E. coli thioredoxin captured in the crystal structures of single-point mutants. Sci. Rep. 2017, 7, 42343. [Google Scholar] [CrossRef] [Green Version]
- Buchko, G.W.; Hewitt, S.N.; Van Voorhis, W.C.; Myler, P.J. Solution NMR structures of oxidized and reduced Ehrlichia chaffeensis thioredoxin: NMR-invisible structure owing to backbone dynamics. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2018, 74, 46–56. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Kim, S.M.; Lee, R.T. Thioredoxin and Thioredoxin Target Proteins: From Molecular Mechanisms to Functional Significance. Antioxid. Redox Signal. 2013, 18, 1165–1207. [Google Scholar] [CrossRef] [Green Version]
- Awan, M.; Yan, F.; Mahmood, F.; Bai, L.; Liu, J.; Bai, J. The Functions of Thioredoxin 1 in Neurodegeneration. Antioxid. Redox Signal. 2022, 36, 1023–1036. [Google Scholar] [CrossRef]
- Collet, J.-F.; Messens, J. Structure, Function, and Mechanism of Thioredoxin Proteins. Antioxid. Redox Signal. 2010, 13, 1205–1216. [Google Scholar] [CrossRef]
- Martin, J.L. Thioredoxin —a fold for all reasons. Structure 1995, 3, 245–250. [Google Scholar] [CrossRef]
- Monteiro, H.P.; Ogata, F.T.; Stern, A. Thioredoxin promotes survival signaling events under nitrosative/oxidative stress associated with cancer development. Biomed. J. 2017, 40, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Mougiakakos, D.; Johansson, C.C.; Jitschin, R.; Böttcher, M.; Kiessling, R. Increased thioredoxin-1 production in human naturally occurring regulatory T cells confers enhanced tolerance to oxidative stress. Blood 2011, 117, 857–861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Wang, K.; Zhang, H.; Zhou, H.J.; Chen, Y.; Min, W. A Unique SUMO-Interacting Motif of Trx2 Is Critical for Its Mitochondrial Presequence Processing and Anti-oxidant Activity. Front. Physiol. 2019, 10, 1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouyang, Y.; Peng, Y.; Li, J.; Holmgren, A.; Lu, J. Modulation of thiol-dependent redox system by metal ions via thioredoxin and glutaredoxin systems. Metallomics 2018, 10, 218–228. [Google Scholar] [CrossRef]
- Lillig, C.H.; Holmgren, A. Thioredoxin and Related Molecules–From Biology to Health and Disease. Antioxid. Redox Signal. 2007, 9, 25–47. [Google Scholar] [CrossRef]
- Foloppe, N.; Sagemark, J.; Nordstrand, K.; Berndt, K.D.; Nilsson, L. Structure, dynamics and electrostatics of the active site of glutaredoxin 3 from Escherichia coli: Comparison with functionally related proteins. J. Mol. Biol. 2001, 310, 449–470. [Google Scholar] [CrossRef]
- Kouwen, T.R.H.M.; Andréll, J.; Schrijver, R.; Dubois, J.-Y.F.; Maher, M.J.; Iwata, S.; Carpenter, E.P.; van Dijl, J.M. Thioredoxin Active-Site Mutants Form Mixed Disulfide Dimers That Resemble Enzyme–Substrate Reaction Intermediates. J. Mol. Biol. 2008, 379, 520–534. [Google Scholar] [CrossRef] [Green Version]
- Du, Y.; Zhang, H.; Zhang, X.; Lu, J.; Holmgren, A. Thioredoxin 1 Is Inactivated Due to Oxidation Induced by Peroxiredoxin under Oxidative Stress and Reactivated by the Glutaredoxin System. J. Biol. Chem. 2013, 288, 32241–32247. [Google Scholar] [CrossRef] [Green Version]
- Watson, W.H.; Pohl, J.; Montfort, W.R.; Stuchlik, O.; Reed, M.S.; Powis, G.; Jones, D.P. Redox Potential of Human Thioredoxin 1 and Identification of a Second Dithiol/Disulfide Motif. J. Biol. Chem. 2003, 278, 33408–33415. [Google Scholar] [CrossRef] [Green Version]
- Haendeler, J.; Hoffmann, J.; Tischler, V.; Berk, B.C.; Zeiher, A.M.; Dimmeler, S. Redox regulatory and anti-apoptotic functions of thioredoxin depend on S-nitrosylation at cysteine 69. Nat. Cell Biol. 2002, 4, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Kern, R.; Malki, A.; Holmgren, A.; Richarme, G. Chaperone properties of Escherichia coli thioredoxin and thioredoxin reductase. Biochem. J. 2003, 371, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Barrio, R.; Fernández-San Millán, A.; Carballeda, J.; Corral-Martínez, P.; Seguí-Simarro, J.M.; Farran, I. Chaperone-like properties of tobacco plastid thioredoxins f and m. J. Exp. Bot. 2012, 63, 365–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, H.; Kim, S.; Hur, Y.-S.; Lee, M.-S.; Lee, S.-H.; Cheon, C.-I. A Cytosolic Thioredoxin Acts as a Molecular Chaperone for Peroxisome Matrix Proteins as Well as Antioxidant in Peroxisome. Mol. Cells 2015, 38, 187–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Currier, R.B.; Ulrich, K.; Leroux, A.E.; Dirdjaja, N.; Deambrosi, M.; Bonilla, M.; Ahmed, Y.L.; Adrian, L.; Antelmann, H.; Jakob, U.; et al. An essential thioredoxin-type protein of Trypanosoma brucei acts as redox-regulated mitochondrial chaperone. PLoS Pathog. 2019, 15, e1008065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.-K.; Zhao, L.; Jeong, S.; Zhang, J.; Jung, J.-H.; Seo, H.S.; Choi, J.; Lim, S. Structural and Biochemical Characterization of Thioredoxin-2 from Deinococcus radiodurans. Antioxidants 2021, 10, 1843. [Google Scholar] [CrossRef]
- Bjørklund, G.; Zou, L.; Wang, J.; Chasapis, C.T.; Peana, M. Thioredoxin reductase as a pharmacological target. Pharmacol. Res. 2021, 174, 105854. [Google Scholar] [CrossRef]
- Sandalova, T.; Zhong, L.; Lindqvist, Y.; Holmgren, A.; Schneider, G. Three-dimensional structure of a mammalian thioredoxin reductase: Implications for mechanism and evolution of a selenocysteine-dependent enzyme. Proc. Natl. Acad. Sci. USA 2001, 98, 9533–9538. [Google Scholar] [CrossRef] [Green Version]
- Gasdaska, P.Y.; Gasdaska, J.R.; Cochran, S.; Powis, G. Cloning and sequencing of a human thioredoxin reductase. FEBS Lett. 1995, 373, 5–9. [Google Scholar] [CrossRef] [Green Version]
- Waksman, G.; Krishna, T.S.; Williams, C.H.; Kuriyan, J. Crystal structure of Escherichia coli thioredoxin reductase refined at 2 A resolution. Implications for a large conformational change during catalysis. J. Mol. Biol. 1994, 236, 800–816. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Xu, Q.; Yang, H.-Y.; Yang, M.; Fang, J.; Gao, K. Inhibition of Thioredoxin Reductase by Santamarine Conferring Anticancer Effect in HeLa Cells. Front. Mol. Biosci. 2021, 8, 710676. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, D.F.D.; Abderrazak, A.; El Hadri, K.; Simmet, T.; Rouis, M. The Thioredoxin System as a Therapeutic Target in Human Health and Disease. Antioxid. Redox Signal. 2013, 19, 1266–1303. [Google Scholar] [CrossRef] [PubMed]
- Arnér, E.S.J. Focus on mammalian thioredoxin reductases—Important selenoproteins with versatile functions. Biochim. Biophys. Acta BBA-Gen. Subj. 2009, 1790, 495–526. [Google Scholar] [CrossRef] [PubMed]
- Joardar, N.; Guevara-Flores, F.; Martínez-González, J.J.; Sinha Babu, S.P. Thiol antioxidant thioredoxin reductase: A prospective biochemical crossroads between anticancer and antiparasitic treatments of the modern era. Int. J. Biol. Macromol. 2020, 165 Pt A, 249–267. [Google Scholar] [CrossRef]
- Miranda-Vizuete, A.; Damdimopoulos, A.E.; Pedrajas, J.R.; Gustafsson, J.-A.; Spyrou, G. Human mitochondrial thioredoxin reductase. cDNA cloning, expression and genomic organization. Eur. J. Biochem. 1999, 261, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-Y.; Liu, I.-C.; Lin, T.-Y. Truncated Escherichia coli thioredoxin induces proliferation of human blood mononuclear cells and production of reactive oxygen species as well as proinflammatory cytokines: Truncated E. coli Thioredoxin Induces Proliferation of PBMCs. Cell Biochem. Funct. 2016, 34, 226–232. [Google Scholar] [CrossRef]
- Pekkari, K.; Holmgren, A. Truncated Thioredoxin: Physiological Functions and Mechanism. Antioxid. Redox Signal. 2004, 6, 53–61. [Google Scholar] [CrossRef]
- Manabe, Y.; Takagi, M.; Nakamura-Yamada, M.; Goto-Inoue, N.; Taoka, M.; Isobe, T.; Fujii, N.L. Redox proteins are constitutively secreted by skeletal muscle. J. Physiol. Sci. 2014, 64, 401–409. [Google Scholar] [CrossRef]
- Léveillard, T.; Aït-Ali, N. Cell Signaling with Extracellular Thioredoxin and Thioredoxin-Like Proteins: Insight into Their Mechanisms of Action. Oxid. Med. Cell. Longev. 2017, 2017, 8475125. [Google Scholar] [CrossRef] [Green Version]
- Mourenza, Á.; Collado, C.; Bravo-Santano, N.; Gil, J.A.; Mateos, L.M.; Letek, M. The extracellular thioredoxin Etrx3 is required for macrophage infection in Rhodococcus equi. Vet. Res. 2020, 51, 38. [Google Scholar] [CrossRef] [Green Version]
- Plugis, N.M.; Weng, N.; Zhao, Q.; Palanski, B.A.; Maecker, H.T.; Habtezion, A.; Khosla, C. Interleukin 4 is inactivated via selective disulfide-bond reduction by extracellular thioredoxin. Proc. Natl. Acad. Sci. USA 2018, 15, 8781–8786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, H.; Masutani, H.; Yodoi, J. Extracellular thioredoxin and thioredoxin-binding protein 2 in control of cancer. Semin. Cancer Biol. 2006, 16, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Powis, G.; Montfort, W.R. Properties and Biological Activities of Thioredoxins. Annu. Rev. Pharmacol. Toxicol. 2001, 41, 261–295. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Zhou, F.; Katirai, F.; Li, P.-L. Lipid Raft Redox Signaling: Molecular Mechanisms in Health and Disease. Antioxidants Redox Signal. 2011, 15, 1043–1083. [Google Scholar] [CrossRef] [PubMed]
- Sorice, M.; Misasi, R.; Riitano, G.; Manganelli, V.; Martellucci, S.; Longo, A.; Garofalo, T.; Mattei, V. Targeting Lipid Rafts as a Strategy Against Coronavirus. Front. Cell Dev. Biol. 2021, 8, 618296. [Google Scholar] [CrossRef]
- Kondo, N.; Ishii, Y.; Kwon, Y.-W.; Tanito, M.; Sakakura-Nishiyama, J.; Mochizuki, M.; Maeda, M.; Suzuki, S.; Kojima, M.; Kim, Y.-C.; et al. Lipid Raft–Mediated Uptake of Cysteine-Modified Thioredoxin-1: Apoptosis Enhancement by Inhibiting the Endogenous Thioredoxin-1. Antioxid. Redox Signal. 2007, 9, 1439–1448. [Google Scholar] [CrossRef]
- Meijles, D.N.; Cull, J.J.; Markou, T.; Cooper, S.T.E.; Haines, Z.H.R.; Fuller, S.J.; O’Gara, P.; Sheppard, M.N.; Harding, S.E.; Sugden, P.H.; et al. Redox Regulation of Cardiac ASK1 (Apoptosis Signal-Regulating Kinase 1) Controls p38-MAPK (Mitogen-Activated Protein Kinase) and Orchestrates Cardiac Remodeling to Hypertension. Hypertension 2020, 76, 1208–1218. [Google Scholar] [CrossRef]
- Psenakova, K.; Hexnerova, R.; Srb, P.; Obsilova, V.; Veverka, V.; Obsil, T. The redox-active site of thioredoxin is directly involved in apoptosis signal-regulating kinase 1 binding that is modulated by oxidative stress. FEBS J. 2020, 287, 1626–1644. [Google Scholar] [CrossRef]
- Nadeau, P.J.; Charette, S.J.; Toledano, M.B.; Landry, J. Disulfide Bond-mediated Multimerization of Ask1 and Its Reduction by Thioredoxin-1 Regulate H2O2-induced c-Jun NH2-terminal Kinase Activation and Apoptosis. Mol. Biol. Cell 2007, 18, 3903–3913. [Google Scholar] [CrossRef] [Green Version]
- Shu, N.; Hägglund, P.; Cai, H.; Hawkins, C.L.; Davies, M.J. Modification of Cys residues in human thioredoxin-1 by p-benzoquinone causes inhibition of its catalytic activity and activation of the ASK1/p38-MAPK signalling pathway. Redox Biol. 2020, 29, 101400. [Google Scholar] [CrossRef]
- Kekulandara, D.N.; Nagi, S.; Seo, H.; Chow, C.S.; Ahn, Y.-H. Redox-Inactive Peptide Disrupting Trx1–Ask1 Interaction for Selective Activation of Stress Signaling. Biochemistry 2018, 57, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Al-Lamki, R.; Bai, L.; Streb, J.W.; Miano, J.M.; Bradley, J.; Min, W. Thioredoxin-2 Inhibits Mitochondria-Located ASK1-Mediated Apoptosis in a JNK-Independent Manner. Circ. Res. 2004, 94, 1483–1491. [Google Scholar] [CrossRef] [Green Version]
- Wei, B.; Li, F. Mechanisms of Trx2/ASK1-Mediated Mitochondrial Injury in Pemphigus Vulgaris. BioMed Res. Int. 2021, 2021, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, E. TXNIP/TBP-2: A Master Regulator for Glucose Homeostasis. Antioxidants 2020, 9, 765. [Google Scholar] [CrossRef]
- Hu, J.; Yu, Y. The Function of Thioredoxin-Binding Protein-2 (TBP-2) in Different Diseases. Oxid. Med. Cell. Longev. 2018, 2018, 4582130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Kandari, N.; Fadel, F.; Al-Saleh, F.; Khashab, F.; Al-Maghrebi, M. The Thioredoxin System is Regulated by the ASK-1/JNK/p38/Survivin Pathway During Germ Cell Apoptosis. Molecules 2019, 24, 3333. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.T.M.; Gillet, G.; Popgeorgiev, N. Caspases in the Developing Central Nervous System: Apoptosis and Beyond. Front. Cell Dev. Biol. 2021, 9, 702404. [Google Scholar] [CrossRef]
- Mitchell, D.A.; Morton, S.U.; Fernhoff, N.B.; Marletta, M.A. Thioredoxin is required for S-nitrosation of procaspase-3 and the inhibition of apoptosis in Jurkat cells. Proc. Natl. Acad. Sci. USA 2007, 104, 11609–11614. [Google Scholar] [CrossRef] [Green Version]
- Shelar, S.B.; Kaminska, K.K.; Reddy, S.A.; Kumar, D.; Tan, C.-T.; Yu, V.C.; Lu, J.; Holmgren, A.; Hagen, T.; Chew, E.-H. Thioredoxin-dependent regulation of AIF-mediated DNA damage. Free Radic. Biol. Med. 2015, 87, 125–136. [Google Scholar] [CrossRef]
- Shields, H.J.; Traa, A.; Van Raamsdonk, J.M. Beneficial and Detrimental Effects of Reactive Oxygen Species on Lifespan: A Comprehensive Review of Comparative and Experimental Studies. Front. Cell Dev. Biol. 2021, 9, 628157. [Google Scholar] [CrossRef]
- Ghimire, K.; Altmann, H.M.; Straub, A.C.; Isenberg, J.S. Nitric oxide: What’s new to NO? Am. J. Physiol.-Cell Physiol. 2017, 312, C254–C262. [Google Scholar] [CrossRef] [PubMed]
- Ogata, F.T.; Batista, W.L.; Sartori, A.; Gesteira, T.F.; Masutani, H.; Arai, R.J.; Yodoi, J.; Stern, A.; Monteiro, H.P. Nitrosative/Oxidative Stress Conditions Regulate Thioredoxin-Interacting Protein (TXNIP) Expression and Thioredoxin-1 (TRX-1) Nuclear Localization. PLoS ONE 2013, 8, e84588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harper, M.T. Auranofin, a thioredoxin reductase inhibitor, causes platelet death through calcium overload. Platelets 2019, 30, 98–104. [Google Scholar] [CrossRef]
- Mohammadi, F.; Soltani, A.; Ghahremanloo, A.; Javid, H.; Hashemy, S.I. The thioredoxin system and cancer therapy: A review. Cancer Chemother. Pharmacol. 2019, 84, 925–935. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Han, X.; Liu, R.; Fang, J. Targeting the Thioredoxin System for Cancer Therapy. Trends Pharmacol. Sci. 2017, 38, 794–808. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Selvaraju, K.; Saei, A.A.; D’Arcy, P.; Zubarev, R.A.; Arnér, E.S.J.; Linder, S. Repurposing of auranofin: Thioredoxin reductase remains a primary target of the drug. Biochimie 2019, 162, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.; Karge, B.; Misgeld, R.; Prokop, A.; Franke, R.; Brönstrup, M.; Ott, I. Gold(I) NHC Complexes: Antiproliferative Activity, Cellular Uptake, Inhibition of Mammalian and Bacterial Thioredoxin Reductases, and Gram-Positive Directed Antibacterial Effects. Chem.-Eur. J. 2017, 23, 1869–1880. [Google Scholar] [CrossRef] [PubMed]
- Arambula, J.F.; McCall, R.; Sidoran, K.J.; Magda, D.; Mitchell, N.A.; Bielawski, C.W.; Lynch, V.M.; Sessler, J.L.; Arumugam, K. Targeting antioxidant pathways with ferrocenylated N-heterocyclic carbene supported gold(i) complexes in A549 lung cancer cells. Chem. Sci. 2016, 7, 1245–1256. [Google Scholar] [CrossRef] [Green Version]
- Branco, V.; Canário, J.; Holmgren, A.; Carvalho, C. Inhibition of the thioredoxin system in the brain and liver of zebra-seabreams exposed to waterborne methylmercury. Toxicol. Appl. Pharmacol. 2011, 251, 95–103. [Google Scholar] [CrossRef]
- Xiao, G.-Q.; Liang, B.-X.; Chen, S.-H.; Ou, T.-M.; Bu, X.-Z.; Yan, M. 3-Nitro-2 H -chromenes as a New Class of Inhibitors against Thioredoxin Reductase and Proliferation of Cancer Cells. Arch. Pharm. 2012, 345, 767–770. [Google Scholar] [CrossRef]
- Navakoski de Oliveira, K.; Andermark, V.; von Grafenstein, S.; Onambele, L.A.; Dahl, G.; Rubbiani, R.; Wolber, G.; Gabbiani, C.; Messori, L.; Prokop, A.; et al. Butyltin(IV) Benzoates: Inhibition of Thioredoxin Reductase, Tumor Cell Growth Inhibition, and Interactions with Proteins. ChemMedChem 2013, 8, 256–264. [Google Scholar] [CrossRef]
- Oehninger, L.; Küster, L.N.; Schmidt, C.; Muñoz-Castro, A.; Prokop, A.; Ott, I. A Chemical-Biological Evaluation of Rhodium(I) N -Heterocyclic Carbene Complexes as Prospective Anticancer Drugs. Chem.-Eur. J. 2013, 19, 17871–17880. [Google Scholar] [CrossRef]
- Yan, C.; Siegel, D.; Newsome, J.; Chilloux, A.; Moody, C.J.; Ross, D. Antitumor Indolequinones Induced Apoptosis in Human Pancreatic Cancer Cells via Inhibition of Thioredoxin Reductase and Activation of Redox Signaling. Mol. Pharmacol. 2012, 81, 401–410. [Google Scholar] [CrossRef] [Green Version]
- Cotugno, R.; Gallotta, D.; Piaz, F.D.; Apicella, I.; De Falco, S.; Rosselli, S.; Bruno, M.; Belisario, M.A. Powerful tumor cell growth-inhibiting activity of a synthetic derivative of atractyligenin: Involvement of PI3K/Akt pathway and thioredoxin system. Biochim. Biophys. Acta BBA-Gen. Subj. 2014, 1840, 1135–1144. [Google Scholar] [CrossRef]
- Duan, D.; Zhang, B.; Yao, J.; Liu, Y.; Sun, J.; Ge, C.; Peng, S.; Fang, J. Gambogic acid induces apoptosis in hepatocellular carcinoma SMMC-7721 cells by targeting cytosolic thioredoxin reductase. Free Radic. Biol. Med. 2014, 69, 15–25. [Google Scholar] [CrossRef]
- Amin, A.R.M.R.; Haque, A.; Rahman, M.A.; Chen, Z.G.; Khuri, F.R.; Shin, D.M. Curcumin Induces Apoptosis of Upper Aerodigestive Tract Cancer Cells by Targeting Multiple Pathways. PLoS ONE 2015, 10, e0124218. [Google Scholar] [CrossRef]
- Clemente-Soto, A.F.; Salas-Vidal, E.; Milan-Pacheco, C.; Sánchez-Carranza, J.N.; Peralta-Zaragoza, O.; González-Maya, L. Quercetin induces G2 phase arrest and apoptosis with the activation of p53 in an E6 expression-independent manner in HPV-positive human cervical cancer-derived cells. Mol. Med. Rep. 2019, 19, 2097–2106. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.; Zhang, Y.; Xu, W.; Zhang, Y.; Yang, R.; Guo, J.; Guan, S.; Ma, Q.; Ma, K.; Xu, J. Chlorophyllin Inhibits Mammalian Thioredoxin Reductase 1 and Triggers Cancer Cell Death. Antioxidants 2021, 10, 1733. [Google Scholar] [CrossRef]
- He, J.; Li, D.; Xiong, K.; Ge, Y.; Jin, H.; Zhang, G.; Hong, M.; Tian, Y.; Yin, J.; Zeng, H. Inhibition of thioredoxin reductase by a novel series of bis-1,2-benzisoselenazol-3(2H)-ones: Organoselenium compounds for cancer therapy. Bioorg. Med. Chem. 2012, 20, 3816–3827. [Google Scholar] [CrossRef]
- Liu, Y.; Duan, D.; Yao, J.; Zhang, B.; Peng, S.; Ma, H.; Song, Y.; Fang, J. Dithiaarsanes Induce Oxidative Stress-Mediated Apoptosis in HL-60 Cells by Selectively Targeting Thioredoxin Reductase. J. Med. Chem. 2014, 57, 5203–5211. [Google Scholar] [CrossRef]
- Sun, Y.; Rowehl, L.M.; Huang, L.; Mackenzie, G.G.; Vrankova, K.; Komninou, D.; Rigas, B. Phospho-ibuprofen (MDC-917) suppresses breast cancer growth: An effect controlled by the thioredoxin system. Breast Cancer Res. 2012, 14, R20. [Google Scholar] [CrossRef] [Green Version]
- Gandin, V.; Fernandes, A. Metal- and Semimetal-Containing Inhibitors of Thioredoxin Reductase as Anticancer Agents. Molecules 2015, 20, 12732–12756. [Google Scholar] [CrossRef]
- Saccoccia, F.; Angelucci, F.; Boumis, G.; Brunori, M.; Miele, A.E.; Williams, D.L.; Bellelli, A. On the mechanism and rate of gold incorporation into thiol-dependent flavoreductases. J. Inorg. Biochem. 2012, 108, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Hwang-Bo, H.; Jeong, J.-W.; Han, M.H.; Park, C.; Hong, S.-H.; Kim, G.-Y.; Moon, S.-K.; Cheong, J.; Kim, W.-J.; Yoo, Y.H.; et al. Auranofin, an inhibitor of thioredoxin reductase, induces apoptosis in hepatocellular carcinoma Hep3B cells by generation of reactive oxygen species. Gen. Physiol. Biophys. 2017, 36, 117–128. [Google Scholar] [CrossRef] [Green Version]
- Cheng, P.; Liu, H.; Li, Y.; Pi, P.; Jiang, Y.; Zang, S.; Li, X.; Fu, A.; Ren, X.; Xu, J.; et al. Inhibition of thioredoxin reductase 1 correlates with platinum-based chemotherapeutic induced tissue injury. Biochem. Pharmacol. 2020, 175, 113873. [Google Scholar] [CrossRef]
- Prast-Nielsen, S.; Cebula, M.; Pader, I.; Arnér, E.S.J. Noble metal targeting of thioredoxin reductase—covalent complexes with thioredoxin and thioredoxin-related protein of 14kDa triggered by cisplatin. Free Radic. Biol. Med. 2010, 49, 1765–1778. [Google Scholar] [CrossRef]
- Rubbiani, R.; Zehnder, T.N.; Mari, C.; Blacque, O.; Venkatesan, K.; Gasser, G. Anticancer Profile of a Series of Gold(III) (2-phenyl)pyridine Complexes. ChemMedChem 2014, 9, 2781–2790. [Google Scholar] [CrossRef]
- Ojo, O.; Mphahlele, M.P.; Oladeji, O.S.; Mmutlane, E.M.; Ndinteh, D.T. From wandering weeds to pharmacy: An insight into traditional uses, phytochemicals and pharmacology of genus Chromolaena (Asteraceae). J. Ethnopharmacol. 2022, 291, 115155. [Google Scholar] [CrossRef]
- Fang, J.; Lu, J.; Holmgren, A. Thioredoxin Reductase Is Irreversibly Modified by Curcumin. J. Biol. Chem. 2005, 280, 25284–25290. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, N.S.; Srivastava, R.A.K. 2019. Curcumin and quercetin synergistically inhibit cancer cell proliferation in multiple cancer cells and modulate Wnt/β-catenin signaling and apoptotic pathways in A375 cells. Phytomedicine 2019, 52, 117–128. [Google Scholar] [CrossRef]
- Lu, J.; Papp, L.V.; Fang, J.; Rodriguez-Nieto, S.; Zhivotovsky, B.; Holmgren, A. Inhibition of Mammalian Thioredoxin Reductase by Some Flavonoids: Implications for Myricetin and Quercetin Anticancer Activity. Cancer Res. 2006, 66, 4410–4418. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Wang, W.; Chen, J.; Cai, X.; Yang, J.; Yang, Y.; Yan, H.; Cheng, X.; Ye, J.; Lu, W.; et al. The Natural Diterpenoid Isoforretin A Inhibits Thioredoxin-1 and Triggers Potent ROS-Mediated Antitumor Effects. Cancer Res. 2017, 77, 926–936. [Google Scholar] [CrossRef] [Green Version]
- Perin, G.; Alves, D.; Jacob, R.G.; Barcellos, A.M.; Soares, L.K.; Lenardão, E.J. Synthesis of Organochalcogen Compounds using Non-Conventional Reaction Media. ChemistrySelect 2016, 1, 205–258. [Google Scholar] [CrossRef]
- Sudati, J.H.; Nogara, P.A.; Saraiva, R.A.; Wagner, C.; Alberto, E.E.; Braga, A.L.; Fachinetto, R.; Piquini, P.C.; Rocha, J.B.T. Diselenoamino acid derivatives as GPx mimics and as substrates of TrxR: in vitro and in silico studies. Org. Biomol. Chem. 2018, 16, 3777–3787. [Google Scholar] [CrossRef]
- Hirota, K.; Nakamura, H.; Masutani, H.; Yodoi, J. Thioredoxin Superfamily and Thioredoxin-Inducing Agents. Ann. N. Y. Acad. Sci. 2002, 957, 189–199. [Google Scholar] [CrossRef]
- Li, X.; Huang, M.; Yang, L.; Guo, N.; Yang, X.; Zhang, Z.; Bai, M.; Ge, L.; Zhou, X.; Li, Y.; et al. Overexpression of Thioredoxin-1 Blocks Morphine-Induced Conditioned Place Preference Through Regulating the Interaction of γ-Aminobutyric Acid and Dopamine Systems. Front. Neurol. 2018, 9, 309. [Google Scholar] [CrossRef]
- Carvalho, C.M.L.; Lu, J.; Zhang, X.; Arnér, E.S.J.; Holmgren, A. Effects of selenite and chelating agents on mammalian thioredoxin reductase inhibited by mercury: Implications for treatment of mercury poisoning. FASEB J. 2011, 25, 370–381. [Google Scholar] [CrossRef] [Green Version]
- Dharmaraja, A.T. Role of Reactive Oxygen Species (ROS) in Therapeutics and Drug Resistance in Cancer and Bacteria. J. Med. Chem. 2017, 60, 3221–3240. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
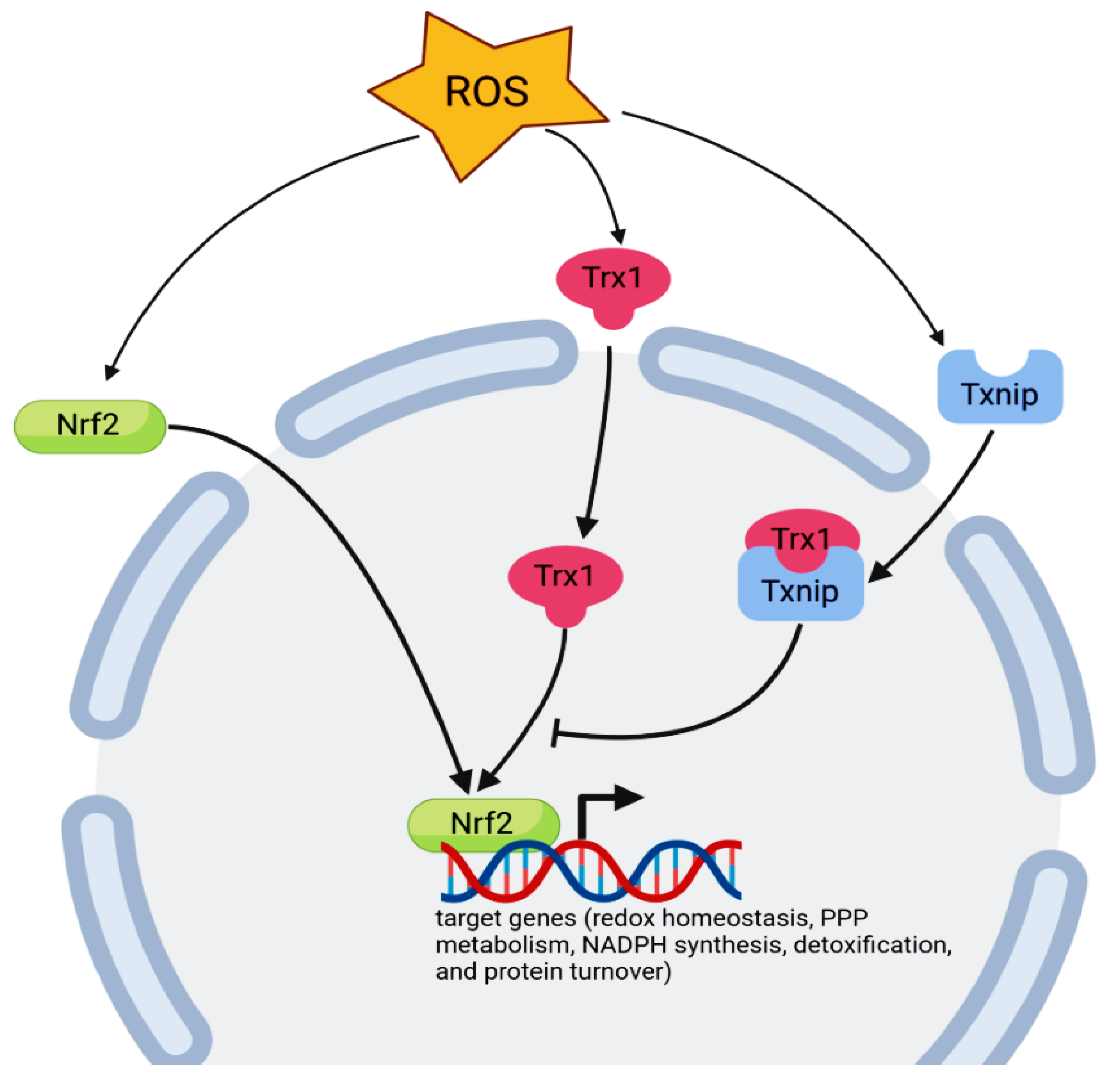
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal. 2018, 29, 1727–1745. [Google Scholar] [CrossRef] [Green Version]
- Jaganjac, M.; Milkovic, L.; Borovic Sunjic, S.; Zarkovic, N. The NRF2, Thioredoxin, and Glutathione System in Tumorigenesis and Anticancer Therapies. Antioxidants 2020, 9, 1151. [Google Scholar] [CrossRef]
- Kim, K.C.; Kang, K.A.; Zhang, R.; Piao, M.J.; Kim, G.Y.; Kang, M.Y.; Lee, S.J.; Lee, N.H.; Surh, Y.-J.; Hyun, J.W. Up-regulation of Nrf2-mediated heme oxygenase-1 expression by eckol, a phlorotannin compound, through activation of Erk and PI3K/Akt. Int. J. Biochem. Cell Biol. 2010, 42, 297–305. [Google Scholar] [CrossRef]
- Joo, M.S.; Kim, W.D.; Lee, K.Y.; Kim, J.H.; Koo, J.H.; Kim, S.G. AMPK Facilitates Nuclear Accumulation of Nrf2 by Phosphorylating at Serine 550. Mol. Cell. Biol. 2016, 36, 1931–1942. [Google Scholar] [CrossRef] [Green Version]
- Cebula, M.; Schmidt, E.E.; Arnér, E.S.J. TrxR1 as a potent regulator of the Nrf2-Keap1 response system. Antioxidants Redox Signal. 2015, 23, 823–853. [Google Scholar] [CrossRef]
- Anestal, K.; Prast-Nielsen, S.; Cenas, N.; Arner, E.S. Cell death by SecTRAPs: Thioredoxin reductase as a prooxidant killer of cells. PLoS ONE 2008, 3, e1846. [Google Scholar] [CrossRef]
- Matsuzawa, A. Thioredoxin and redox signaling: Roles of the thioredoxin system in control of cell fate. Arch. Biochem. Biophys. 2017, 617, 101–105. [Google Scholar] [CrossRef]
- Lincoln, D.T.; Ali Emadi, E.M.; Tonissen, K.F.; Clarke, F.M. The thioredoxin-thioredoxin reductase system: Over-expression in human cancer. Anticancer Res. 2003, 23, 2425–2433. [Google Scholar]
- Jovanović, M.; Podolski-Renić, A.; Krasavin, M.; Pešić, M. The Role of the Thioredoxin Detoxification System in Cancer Progression and Resistance. Front. Mol. Biosci. 2022, 9, 883297. [Google Scholar] [CrossRef]
- Powis, G.; Kirkpatrick, D.L.; Angulo, M.; Baker, A. Thioredoxin redox control of cell growth and death and the effects of inhibitors. Chem. Biol. Interact. 1998, 111–112, 23–34. [Google Scholar] [CrossRef]
- Yamawaki, H.; Haendeler, J.; Berk, B.C. Thioredoxin: A Key Regulator of Cardiovascular Homeostasis. Circ. Res. 2003, 93, 1029–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saad, I.; Fournier, C.T.; Wilson, R.L.; Lakshmanan, R.; Selvaraju, V.; Thirunavukkarasu, M.; Palesty, A.J.; McFadden, D.W.; Maulik, N. Thioredoxin-1 augments wound healing and promote angiogenesis in a murine ischemic full-thickness wound model. Surgery 2018, 164, 1077–1086. [Google Scholar] [CrossRef] [PubMed]
- Welsh, S.J.; Bellamy, W.T.; Briehl, M.M.; Powis, G. The redox protein thioredoxin-1 (Trx-1) increases hypoxia-inducible factor 1alpha protein expression: Trx-1 overexpression results in increased vascular endothelial growth factor production and enhanced tumor angiogenesis. Cancer Res. 2002, 62, 5089–5095. [Google Scholar] [PubMed]
- Du, Y.; Zhang, H.; Lu, J.; Holmgren, A. Glutathione and Glutaredoxin Act as a Backup of Human Thioredoxin Reductase 1 to Reduce Thioredoxin 1 Preventing Cell Death by Aurothioglucose. J. Biol. Chem. 2012, 287, 38210–38219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benhar, M.; Shytaj, I.L.; Stamler, J.S.; Savarino, A. Dual targeting of the thioredoxin and glutathione systems in cancer and HIV. J. Clin. Investig. 2016, 126, 1630–1639. [Google Scholar] [CrossRef] [Green Version]
- Avval, F.Z.; Holmgren, A. Molecular Mechanisms of Thioredoxin and Glutaredoxin as Hydrogen Donors for Mammalian S Phase Ribonucleotide Reductase. J. Biol. Chem. 2009, 284, 8233–8240. [Google Scholar] [CrossRef] [Green Version]
- Sengupta, R.; Coppo, L.; Mishra, P.; Holmgren, A. Glutathione-glutaredoxin is an efficient electron donor system for mammalian p53R2–R1-dependent ribonucleotide reductase. J. Biol. Chem. 2019, 294, 12708–12716. [Google Scholar] [CrossRef]
- Rhee, S.G.; Chae, H.Z.; Kim, K. Peroxiredoxins: A historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic. Biol. Med. 2005, 38, 1543–1552. [Google Scholar] [CrossRef]
- Wu, M.; Deng, C.; Lo, T.H.; Chan, K.Y.; Li, X.; Wong, C.M. Peroxiredoxin, Senescence, and Cancer. Cells 2022, 11, 1772. [Google Scholar] [CrossRef]
- Pannala, V.R.; Dash, R.K. Mechanistic characterization of the thioredoxin system in the removal of hydrogen peroxide. Free Radic. Biol. Med. 2015, 78, 42–55. [Google Scholar] [CrossRef] [Green Version]
- Hanschmann, E.-M.; Lönn, M.E.; Schütte, L.D.; Funke, M.; Godoy, J.R.; Eitner, S.; Hudemann, C.; Lillig, C.H. Both Thioredoxin 2 and Glutaredoxin 2 Contribute to the Reduction of the Mitochondrial 2-Cys Peroxiredoxin Prx3. J. Biol. Chem. 2010, 285, 40699–40705. [Google Scholar] [CrossRef] [Green Version]
| Compound | Structure | IC50, μM |
|---|---|---|
| 2,3,4,6-tetra-o-acetyll-thio-b-D-glucopyrano-sato-S-(triethyl-phosphine) gold (auranofin) | 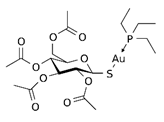 | 0.016 |
| Chlorido(4-bromo-1,3-diethyl-imidazol-2-ylidene)gold(I) |  | 0.038 |
| Chlorido(5-bromo-1,3-diethyl benzimidazol-2-ylidene)gold(I) | 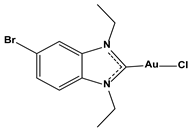 | 0.042 |
| Chlorido (1,3-diethyl-5-fluoro-benzimidazol-2-ylidene)gold(I) |  | 0.043 |
| Chlorido [4-(4-bromophenyl)-1,3-diethyl-imidazol-2-ylidene]gold(I) | 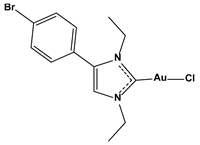 | 0.098 |
| Chlorido [1,3-diethyl-4-(4-fluorophenyl)imidazol-2-ylidene]gold(I) |  | 0.174 |
| Chlorido(1,3-diethyl-imidazol-2-ylidene)gold(I) | 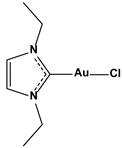 | 0.209 |
| Chlorido(1,3-diethyl-4-phenylimidazol-2-ylidene)gold(I) | 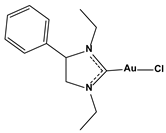 | 0.244 |
| Chemical Class | Compound | Structure | IC50, μM | Cell line | Reference | |
|---|---|---|---|---|---|---|
| 1. Metal-containing inhibitors | 1.1.Gold-N-heterocyclic carbene complexes | Bis(1,3-di(ferrocenylmethyl)imidazol-2-ylidene)-gold(I) chloride | 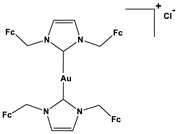 Fc = Ferrocene | 0.14 0.19 0.12 0.48 | A549 A2780 2780CP PC-3 | [78] |
| 1.2. Gold-containing thiosugar and triethylphosphine | 2,3,4,6-tetra-o-acetyll- thio-b-D-glucopyrano-sato-S-(triethyl-phosphine) gold (auranofin) | 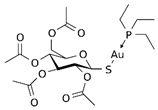 | 1.67 0.12 3.17 | A549 HeLa MRC-5 | [78] [79] | |
| 1.3. Platinum compounds | trans-Dichlorodiamine platinum(II) (cisplatin) |  | 11.5 7.9 | HeLa MRC-5 | [80] | |
| 1.3. Organotin-containing inhibitors | Tri-n-butyltin(IV) carboxylate |  | 0.97 0.13 | HT-29 MCF-7 | [81] | |
| 1.4. Rhodium(I) N-heterocyclic carbene complexes inhibitors | Chlorido(η2, η2-cycloocta-1,5-diene)(1,3-dimethylbenzimidazol-2-ylidene)-rhodium(I) | 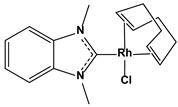 | 0.6 0.9 | MCF-7 HT-29 | [82] | |
| 2. Natural products and their synthetic analogues | 2.1. Quinone compounds (Indolequinones) | 5-methoxy-1-methyl-3-[(2,4,6-trifluorophenoxy)methyl]indole-4,7-dione | 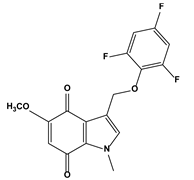 | 0.035 0.018 | MIA PaCa-2 | [83] |
| 2.2. Terpenoids (Atractyligenin derivative) | 15-ketoatractyligenin methyl ester (SC2017) | 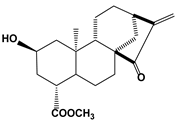 | 1.68 | Jurkat | [84] | |
| 2.3. Nitrosoureas and chromenes (3-nitro-2H-chromene derivative) | 6-fluoro-3-nitro-2H-chromene |  | 1.42 | A549 | [80] | |
| 2.4. Gambogic acid | Gambogic acid |  | 0.7 | SMMC-7721 | [85] | |
| 2.5. Diketone compounds | Curcumin | 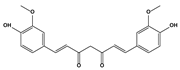 | 11.2 6.03 11.6 5.5 6.4 | A549 H1299 H292 Tu212 Tu686 | [86] | |
| 2.6. Polyphenolic flavonoid | Quercetin |  | 125 140 | HeLa SiHa | [87] | |
| 2.7. Colorants | Chlorophyllin | 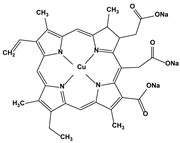 | 10.3 0.4 10.2 6.9 20.2 | A549 HeLa HepG2 MCF7 HCT116 | [88] | |
| 3. Organoselenium compounds | 3.1. Organo- selenium compounds 3.2. Phenylarsenic oxide derivatives (dithiarsanes) | 1,2-(5,5′-Dimethoxy(bis-1,2-benzisoselenazol-3(2H)-one))ethane 2-(4-Aminophenyl)-1,3,2-dithiarsenane (PAO−PDT) | 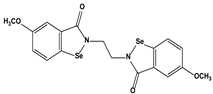 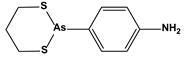 | 1.64 0.6 | U-87MG ATCC HL-60 | [89] [90] |
| 3.3. Ibuprofen Derivative | Phospho-ibuprofen (MDC-917) |  | 79 | MCF-7 | [91] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasan, A.A.; Kalinina, E.; Tatarskiy, V.; Shtil, A. The Thioredoxin System of Mammalian Cells and Its Modulators. Biomedicines 2022, 10, 1757. https://doi.org/10.3390/biomedicines10071757
Hasan AA, Kalinina E, Tatarskiy V, Shtil A. The Thioredoxin System of Mammalian Cells and Its Modulators. Biomedicines. 2022; 10(7):1757. https://doi.org/10.3390/biomedicines10071757
Chicago/Turabian StyleHasan, Aseel Ali, Elena Kalinina, Victor Tatarskiy, and Alexander Shtil. 2022. "The Thioredoxin System of Mammalian Cells and Its Modulators" Biomedicines 10, no. 7: 1757. https://doi.org/10.3390/biomedicines10071757
APA StyleHasan, A. A., Kalinina, E., Tatarskiy, V., & Shtil, A. (2022). The Thioredoxin System of Mammalian Cells and Its Modulators. Biomedicines, 10(7), 1757. https://doi.org/10.3390/biomedicines10071757






