Oral Microbiota, Its Equilibrium and Implications in the Pathophysiology of Human Diseases: A Systematic Review
Abstract
1. Introduction
2. Oral Microbiome and Systemic Diseases
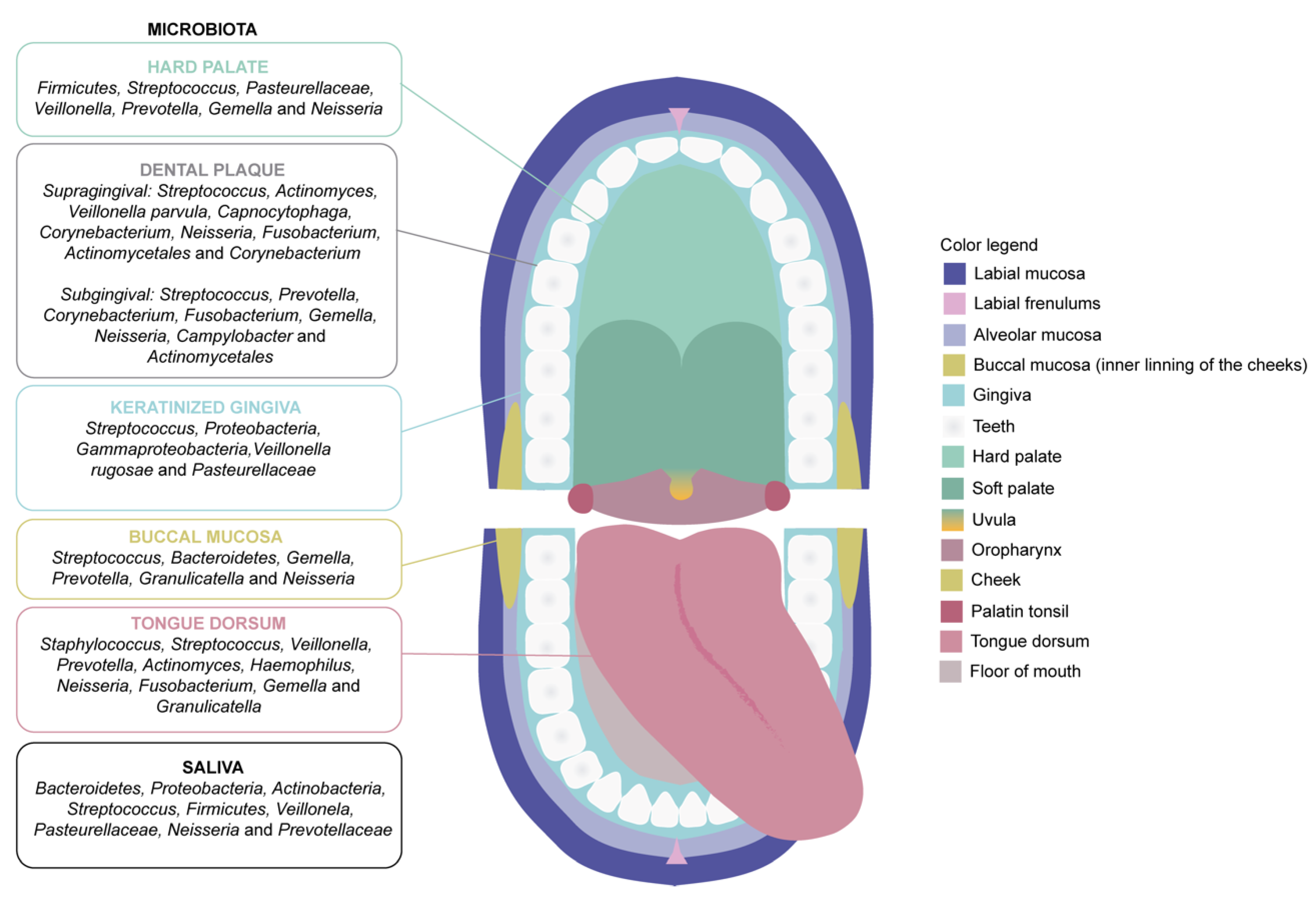
2.1. The Microbiota of the Oral Cavity
2.2. Diseases Related to the Oral Microbiota
2.2.1. Oral Diseases
Dental Caries and Tooth Decay
Periodontal Disease
2.2.2. Systemic Diseases
Cardiovascular Diseases
Neurodegenerative Diseases
3. Materials and Methods
3.1. Study Design and Protocol Registration
3.1.1. Inclusion and Exclusion Criteria
3.1.2. Search Strategy, Risk of Bias and Limitations
4. Results and Discussion
4.1. Oral Microbiota, Oral Diseases and Cancer
4.2. Oral Microbiota and Cardiovascular Diseases
4.3. Oral Microbiota and AD Neurodegeneration
4.4. Oral and Gut Microbiota in Chronic Human Diseases
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arponen, S. Microbiota Oral y Estilo de Vida Como Base Para La Salud Oral y Sistémica. El Dent. Mod. 2019, 44, 18–30. [Google Scholar]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The Human Microbiome Project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Gevers, D.; Knight, R.; Petrosino, J.F.; Huang, K.; McGuire, A.L.; Birren, B.W.; Nelson, K.E.; White, O.; Methé, B.A.; Huttenhower, C. The Human Microbiome Project: A Community Resource for the Healthy Human Microbiome. PLoS Biol. 2012, 10, e1001377. [Google Scholar] [CrossRef] [PubMed]
- Radaic, A.; Kapila, Y.L. The Oralome and Its Dysbiosis: New Insights into Oral Microbiome-Host Interactions. Comput. Struct. Biotechnol. J. 2021, 19, 1335–1360. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, S.F.; Sapp, J.; Tauber, A.I. A Symbiotic View of Life: We Have Never Been Individuals. Q. Rev. Biol. 2012, 87, 325–341. [Google Scholar] [CrossRef]
- Miller, W.B. The Eukaryotic Microbiome: Origins and Implications for Fetal and Neonatal Life. Front. Pediatr. 2016, 4, 96. [Google Scholar] [CrossRef]
- Dietert, R.R. Safety and Risk Assessment for the Human Superorganism. Hum. Ecol. Risk Assess. 2017, 23, 1819–1829. [Google Scholar] [CrossRef][Green Version]
- Pace, L.A.; Crowe, S.E. Complex Relationships Between Food, Diet and the Microbiome. Gastroenterol. Clin. N. Am. 2016, 45, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Mosca, A.; Leclerc, M.; Hugot, J.P. Gut Microbiota Diversity and Human Diseases: Should We Reintroduce Key Predators in Our Ecosystem? Front. Microbiol. 2016, 7, 455. [Google Scholar] [CrossRef]
- Chen, J.; Domingue, J.C.; Sears, C.L. Microbiota Dysbiosis in Select Human Cancers: Evidence of Association and Causality. Semin. Immunol. 2017, 32, 25–34. [Google Scholar] [CrossRef]
- Varoni, E.M.; Rimondini, L. Oral Microbiome, Oral Health and Systemic Health: A Multidirectional Link. Biomedicines 2022, 10, 186. [Google Scholar] [CrossRef]
- Chen, Z.; Han, S.; Zhou, D.; Zhou, S.; Jia, G. Effects of Oral Exposure to Titanium Dioxide Nanoparticles on Gut Microbiota and Gut-Associated Metabolism: In Vivo. Nanoscale 2019, 11, 22398–22412. [Google Scholar] [CrossRef]
- D’Argenio, V.; Salvatore, F. The Role of the Gut Microbiome in the Healthy Adult Status. Clin. Chim. Acta 2015, 451, 97–102. [Google Scholar] [CrossRef]
- Ghaisas, S.; Maher, J.; Kanthasamy, A. Gut Microbiome in Health and Disease: Linking the Microbiome-Gut-Brain Axis and Environmental Factors in the Pathogenesis of Systemic and Neurodegenerative Diseases. Pharmacol. Ther. 2016, 158, 52–62. [Google Scholar] [CrossRef]
- Kau, A.L.; Ahern, P.P.; Griffin, N.W.; Goodman, A.L.; Gordon, J.I. Human Nutrition, the Gut Microbiome and the Immune System. Nature 2011, 474, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, B.; Koren, O.; Goodrich, J.K.; Poole, A.C.; Srinivasan, S.; Ley, R.E.; Gewirtz, A.T. Dietary Emulsifiers Impact the Mouse Gut Microbiota Promoting Colitis and Metabolic Syndrome. Nature 2015, 519, 92–96. [Google Scholar] [CrossRef]
- Narengaowa; Kong, W.; Lan, F.; Awan, U.F.; Qing, H.; Ni, J. The Oral-Gut-Brain AXIS: The Influence of Microbes in Alzheimer’s Disease. Front. Cell. Neurosci. 2021, 15, 633735. [Google Scholar] [CrossRef]
- Logsdon, A.F.; Erickson, M.A.; Rhea, E.M.; Salameh, T.S.; Banks, W.A. Gut Reactions: How the Blood–Brain Barrier Connects the Microbiome and the Brain. Exp. Biol. Med. 2018, 243, 159–165. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Wang, Y.; Li, F.; Jia, J.; Song, X.; Qin, S.; Wang, R.; Jin, F.; Kitazato, K.; et al. The Gut-Microglia Connection: Implications for Central Nervous System Diseases. Front. Immunol. 2018, 9, 2325. [Google Scholar] [CrossRef]
- Sharon, G.; Sampson, T.R.; Geschwind, D.H.; Mazmanian, S.K. The Central Nervous System and the Gut Microbiome. Cell 2016, 167, 915–932. [Google Scholar] [CrossRef]
- Raimondi, I.; Izzo, L.; Tunesi, M.; Comar, M.; Albani, D.; Giordano, C. Organ-On-A-Chip in Vitro Models of the Brain and the Blood-Brain Barrier and Their Value to Study the Microbiota-Gut-Brain Axis in Neurodegeneration. Front. Bioeng. Biotechnol. 2020, 7, 435. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Zhu, H.; Feng, Y.; Guo, R.; Wan, D. The Impact of Gut Microbiota Disorders on the Blood–Brain Barrier. Infect. Drug Resist. 2020, 13, 3351–3363. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.; Fonseca, S.; Carding, S.R. Gut Microbes and Metabolites as Modulators of Blood-Brain Barrier Integrity and Brain Health. Gut Microbes 2020, 11, 135–157. [Google Scholar] [CrossRef]
- Belkaid, Y.; Hand, T. Role of the Microbiota in Immunity and Inflammation. Cell 2015, 157, 121–141. [Google Scholar] [CrossRef]
- Hernandez, C.J.; Guss, J.D.; Luna, M.; Goldring, S.R. Links Between the Microbiome and Bone. J. Bone Miner. Res. 2016, 31, 1638–1646. [Google Scholar] [CrossRef]
- Deo, P.N.; Deshmukh, R. Oral Microbiome: Unveiling the Fundamentals. J. Oral Maxillofac. Pathol. 2019, 23, 122–128. [Google Scholar] [CrossRef]
- Caselli, E.; Fabbri, C.; D’Accolti, M.; Soffritti, I.; Bassi, C.; Mazzacane, S.; Franchi, M. Defining the Oral Microbiome by Whole-Genome Sequencing and Resistome Analysis: The Complexity of the Healthy Picture. BMC Microbiol. 2020, 20, 120. [Google Scholar] [CrossRef]
- Wu, Y.; Lee, W.; Salamanca, E.; Yao, W.; Su, J.; Wang, S.; Hu, C.; Chang, W. Oral Microbiota Changes in Elderly Patients, an Indicator of Alzheimer’s Disease. Int J. Environ. Res. Public Health 2021, 18, 4211. [Google Scholar] [CrossRef]
- Suárez, L.J.; Arboleda, S.; Angelov, N.; Arce, R.M. Oral Versus Gastrointestinal Mucosal Immune Niches in Homeostasis and Allostasis. Front. Immunol. 2021, 12, 705206. [Google Scholar] [CrossRef]
- Belibasakis, G.N.; Bostanci, N.; Marsh, P.D.; Zaura, E. Applications of the Oral Microbiome in Personalized Dentistry. Arch. Oral Biol. 2019, 104, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.L.; Edlund, A. Exploiting the Oral Microbiome to Prevent Tooth Decay: Has Evolution Already Provided the Best Tools? Front. Microbiol. 2019, 9, 3323. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Chung, S.W.; Auh, Q.S.; Hong, S.J.; Lee, Y.A.; Jung, J.; Lee, G.J.; Park, H.J.; Shin, S.I.; Hong, J.Y. Progress in Oral Microbiome Related to Oral and Systemic Diseases: An Update. Diagnostics 2021, 11, 1283. [Google Scholar] [CrossRef] [PubMed]
- Sedghi, L.; DiMassa, V.; Harrington, A.; Lynch, S.V.; Kapila, Y.L. The Oral Microbiome: Role of Key Organisms and Complex Networks in Oral Health and Disease. Periodontol. 2000 2021, 87, 107–131. [Google Scholar] [CrossRef]
- Lassalle, F.; Spagnoletti, M.; Fumagalli, M.; Shaw, L.; Dyble, M.; Walker, C.; Thomas, M.G.; Bamberg Migliano, A.; Balloux, F. Oral Microbiomes from Hunter-Gatherers and Traditional Farmers Reveal Shifts in Commensal Balance and Pathogen Load Linked to Diet. Mol. Ecol. 2018, 27, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Takeshita, T. The Oral Microbiome and Human Health. J. Oral Sci. 2017, 59, 201–206. [Google Scholar] [CrossRef]
- Sampaio-Maia, B.; Caldas, I.M.; Pereira, M.L.; Pérez-Mongiovi, D.; Araujo, R. The Oral Microbiome in Health and Its Implication in Oral and Systemic Diseases. In Advances in Applied Microbiology; Academic Press Inc.: Cambridge, MA, USA, 2016; Volume 97, pp. 171–210. [Google Scholar]
- Lynge Pedersen, A.M.; Belstrøm, D. The Role of Natural Salivary Defences in Maintaining a Healthy Oral Microbiota. J. Dent. 2019, 80, S3–S12. [Google Scholar] [CrossRef]
- De la Rosa Garcia, E.; Anaya Saavedra, G.; Godoy Rivera, L.M. Manual Para La Detección de Alteraciones de La Mucosa Bucal Potencialmente Malignas. D. Of. Fed. 2003, 1, 8–10. [Google Scholar]
- Angarita-Díaz, M.P.; Díaz, J.A.; Tupaz, H.A.; López-López, A.; Forero, D.; Mira, A.; Dávila, F.; Cerón, X.A.; Ochoa-Acosta, E.M.; Goméz, O.L.; et al. Presence of Streptococcus Dentisani in the Dental Plaque of Children from Different Colombian Cities. Clin. Exp. Dent. Res. 2019, 5, 184–190. [Google Scholar] [CrossRef]
- Hoare, A.; Marsh, P.D.; Diaz, P.I. Ecological Therapeutic Opportunities for Oral Diseases. Microbiol. Spectr. 2017, 5, 235–265. [Google Scholar] [CrossRef]
- Kilian, M. The Oral Microbiome–Friend or Foe? Eur. J. Oral Sci. 2018, 126, 5–12. [Google Scholar] [CrossRef]
- Konkel, J.E.; O’Boyle, C.; Krishnan, S. Distal Consequences of Oral Inflammation. Front. Immunol. 2019, 10, 1403. [Google Scholar] [CrossRef] [PubMed]
- Zarco, M.F.; Vess, T.J.; Ginsburg, G.S. The Oral Microbiome in Health and Disease and the Potential Impact on Personalized Dental Medicine. Oral Dis. 2012, 18, 109–120. [Google Scholar] [CrossRef]
- Kilian, M.; Chapple, I.L.C.; Hannig, M.; Marsh, P.D.; Meuric, V.; Pedersen, A.M.L.; Tonetti, M.S.; Wade, W.G.; Zaura, E. The Oral Microbiome—An Update for Oral Healthcare Professionals. Br. Dent. J. 2016, 221, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.C.; Rothballer, M.; Altenburger, M.J.; Woelber, J.P.; Karygianni, L.; Vach, K.; Hellwig, E.; Al-Ahmad, A. Long-Term Fluctuation of Oral Biofilm Microbiota Following Different Dietary Phases. Appl. Environ. Microbiol. 2020, 86, e01421-20. [Google Scholar] [CrossRef]
- Lu, M.; Xuan, S.; Wang, Z. Oral Microbiota: A New View of Body Health. Food Sci. Hum. Wellness 2019, 8, 8–15. [Google Scholar] [CrossRef]
- Ren, L.; Shen, D.; Liu, C.; Ding, Y. Protein Tyrosine and Serine/Threonine Phosphorylation in Oral Bacterial Dysbiosis and Bacteria-Host Interaction. Front. Cell. Infect. Microbiol. 2022, 11, 1367. [Google Scholar] [CrossRef]
- Benahmed, A.G.; Gasmi, A.; Doşa, A.; Chirumbolo, S.; Mujawdiya, P.K.; Aaseth, J.; Dadar, M.; Bjørklund, G. Association between the Gut and Oral Microbiome with Obesity. Anaerobe 2020, 70, 102248. [Google Scholar] [CrossRef]
- Aleti, G.; Baker, J.L.; Tang, X.; Alvarez, R.; Dinis, M.; Tran, N.C.; Melnik, A.V.; Zhong, C.; Ernst, M.; Dorrestein, P.C.; et al. Identification of the Bacterial Biosynthetic Gene Clusters of the Oral Microbiome Illuminates the Unexplored Social Language of Bacteria during Health and Disease. mBio 2019, 10, e00321-19. [Google Scholar] [CrossRef]
- Gondivkar, S.M.; Gadbail, A.R.; Gondivkar, R.S.; Sarode, S.C.; Sarode, G.S.; Patil, S.; Awan, K.H. Nutrition and Oral Health. Disease-a-Month 2019, 65, 147–154. [Google Scholar] [CrossRef]
- Sheiham, A. Dietary Effects on Dental Diseases. Public Health Nutr. 2001, 4, 569–591. [Google Scholar] [CrossRef]
- Rocha, F.R.; Regis, W.F.M.; Duarte, S.; Muniz, F.W.M.G.; Rodrigues, L.K.A. Effect of Bioactive Compounds on the Regulation of Quorum Sensing Network-Associated Genes and Virulence in Streptococcus Mutans—A Systematic Review. Arch. Oral Biol. 2020, 119, 104893. [Google Scholar] [CrossRef] [PubMed]
- Peres, M.A.; Macpherson, L.M.D.; Weyant, R.J.; Daly, B.; Venturelli, R.; Mathur, M.R.; Listl, S.; Celeste, R.K.; Guarnizo-Herreño, C.C.; Kearns, C.; et al. Oral Diseases: A Global Public Health Challenge. Lancet 2019, 394, 249–260. [Google Scholar] [CrossRef]
- World Health Organization. Guideline: Sugars Intake for Adults and Children; World Health Organization: Geneva, Switzerland, 2018; Volume 57, pp. 1716–1722.
- Anderson, A.C.; Rothballer, M.; Altenburger, M.J.; Woelber, J.P.; Karygianni, L.; Lagkouvardos, I.; Hellwig, E.; Al-Ahmad, A. In-Vivo Shift of the Microbiota in Oral Biofilm in Response to Frequent Sucrose Consumption. Sci. Rep. 2018, 8, 14202. [Google Scholar] [CrossRef] [PubMed]
- Tennert, C.; Reinmuth, A.; Bremer, K.; Al-Ahmad, A.; Karygianni, L.; Hellwig, E.; Vach, K.; Ratka-Krüger, P.; Wittmer, A.; Woelber, J.P. An Oral Health Optimized Diet Reduces the Load of Potential Cariogenic and Periodontal Bacterial Species in the Supragingival Oral Plaque: A Randomized Controlled Pilot Study. Microbiologyopen 2020, 9, e1056. [Google Scholar] [CrossRef] [PubMed]
- Touger-Decker, R.; Mobley, C. Position of the Academy of Nutrition and Dietetics: Oral Health and Nutrition. J. Acad. Nutr. Diet. 2013, 113, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Serra, A.; Gallart-Palau, X.; Koh, W.Y.; Chua, Z.J.Y.; Guo, X.; Chow, C.J.J.; Chen, W.M.; Park, J.E.; Li, T.; Tam, J.P.; et al. Prooxidant Modifications in the Cryptome of Beef Jerky, the Deleterious Post-Digestion Composition of Processed Meat Snacks. Food Res. Int. 2019, 125, 108569. [Google Scholar] [CrossRef]
- Jin, L.J.; Lamster, I.B.; Greenspan, J.S.; Pitts, N.B.; Scully, C.; Warnakulasuriya, S. Global Burden of Oral Diseases: Emerging Concepts, Management and Interplay with Systemic Health. Oral Dis. 2016, 22, 609–619. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Chavakis, T. Local and Systemic Mechanisms Linking Periodontal Disease and Inflammatory Comorbidities. Nat. Rev. Immunol. 2021, 21, 426–440. [Google Scholar] [CrossRef]
- Maitre, Y.; Mahalli, R.; Micheneau, P.; Delpierre, A.; Amador, G.; Denis, F. Evidence and Therapeutic Perspectives in the Relationship between the Oral Microbiome and Alzheimer’s Disease: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 11157. [Google Scholar] [CrossRef]
- Labrecque, J.; Bodet, C.; Chandad, F.; Grenier, D. Effects of a High-Molecular-Weight Cranberry Fraction on Growth, Biofilm Formation and Adherence of Porphyromonas Gingivalis. J. Antimicrob. Chemother. 2006, 58, 439–443. [Google Scholar] [CrossRef]
- Poole, S.; Singhrao, S.K.; Kesavalu, L.; Curtis, M.A.; Crean, S. Determining the Presence of Periodontopathic Virulence Factors in Short-Term Postmortem Alzheimer’s Disease Brain Tissue. J. Alzheimer’s Dis. 2013, 36, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Zardawi, F.; Gul, S.; Abdulkareem, A.; Sha, A.; Yates, J. Association Between Periodontal Disease and Atherosclerotic Cardiovascular Diseases: Revisited. Front. Cardiovasc. Med. 2021, 7, 625579. [Google Scholar] [CrossRef]
- Petersen, P.E.; Ogawa, H. The Global Burden of Periodontal Disease: Towards Integration with Chronic Disease Prevention and Control. Periodontol. 2000 2012, 60, 15–39. [Google Scholar] [CrossRef] [PubMed]
- Paul, O.; Arora, P.; Mayer, M.; Chatterjee, S. Inflammation in Periodontal Disease: Possible Link to Vascular Disease. Front. Physiol. 2021, 11, 609614. [Google Scholar] [CrossRef] [PubMed]
- Sudhakara, P.; Gupta, A.; Bhardwaj, A.; Wilson, A. Oral Dysbiotic Communities and Their Implications in Systemic Diseases. Dent. J. 2018, 6, 10. [Google Scholar] [CrossRef]
- Kapila, Y.L. Oral Health’s Inextricable Connection to Systemic Health: Special Populations Bring to Bear Multimodal Relationships and Factors Connecting Periodontal Disease to Systemic Diseases and Conditions. Periodontol. 2000 2021, 87, 11–16. [Google Scholar] [CrossRef]
- Kerr, J.; Tribble, G. Salivary Diagnostics and the Oral Microbiome. In Advances in Salivary Diagnostics; Streckfus, C.F., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–204. ISBN 9783662453995. [Google Scholar]
- Bartova, J.; Sommerova, P.; Lyuya-Mi, Y.; Mysak, J.; Prochazkova, J.; Duskova, J.; Janatova, T.; Podzimek, S. Periodontitis as a Risk Factor of Atherosclerosis. J. Immunol. Res. 2014, 2014, 636893. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Sommerlad, A.; Orgeta, V.; Costafreda, S.G.; Huntley, J.; Ames, D.; Ballard, C.; Banerjee, S.; Burns, A.; Cohen-Mansfield, J.; et al. Dementia Prevention, Intervention, and Care. Lancet 2017, 390, 2673–2734. [Google Scholar] [CrossRef]
- Marsh, P.D.; Zaura, E. Dental Biofilm: Ecological Interactions in Health and Disease. J. Clin. Periodontol. 2017, 44, 12–22. [Google Scholar] [CrossRef]
- Bergot, A.S.; Giri, R.; Thomas, R. The Microbiome and Rheumatoid Arthritis. Best Pract. Res. Clin. Rheumatol. 2019, 33, 101497. [Google Scholar] [CrossRef]
- Chen, X.; Daliri, E.B.M.; Chelliah, R.; Oh, D.H. Isolation and Identification of Potentially Pathogenic Microorganisms Associated with Dental Caries in Human Teeth Biofilms. Microorganisms 2020, 8, 1596. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Molinero, J.; Migueláñez-Medrán, B.D.C.; Puente-Gutiérrez, C.; Delgado-Somolinos, E.; Carreras-Presas, C.M.; Fernández-Farhall, J.; López-Sánchez, A.F. Association between Oral Cancer and Diet: An Update. Nutrients 2021, 13, 1299. [Google Scholar] [CrossRef] [PubMed]
- Mensah, G.A.; Roth, G.A.; Fuster, V. The Global Burden of Cardiovascular Diseases and Risk Factors 2020 and Beyond. J. Am. Coll. Cardiol. 2019, 74, 2529–2532. [Google Scholar] [CrossRef] [PubMed]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Van Dyke, T.E. Periodontitis and Atherosclerotic Cardiovascular Disease: Consensus Report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J. Clin. Periodontol. 2013, 40, 24–29. [Google Scholar] [CrossRef]
- Kholy, K.E.; Genco, R.J.; Van Dyke, T.E. Oral Infections and Cardiovascular Disease. Trends Endocrinol. Metab. 2015, 26, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, T.; Webb, I.; Stenhouse, L.; Pattni, A.; Ready, D.; Wanyonyi, K.L.; White, S.; Gallagher, J.E. Evidence Summary: The Relationship between Oral and Cardiovascular Disease. Br. Dent. J. 2017, 222, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Lorca, C.; Laparra, M.; Céspedes, M.V.; Casaní, L.; Florit, S.; Jové, M.; Mota-Martorell, N.; Vilella, E.; Gallart-Palau, X.; Serra, A. Industrial By-Products As a Novel Circular Source of Biocompatible Extracellular Vesicles. Adv. Funct. Mater. 2022, 32, 2202700. [Google Scholar] [CrossRef]
- Belstrøm, D. The Salivary Microbiota in Health and Disease. J. Oral Microbiol. 2020, 12, 1723975. [Google Scholar] [CrossRef]
- Cotti, E.; Dessì, C.; Piras, A.; Mercuro, G. Can a Chronic Dental Infection Be Considered a Cause of Cardiovascular Disease? A Review of the Literature. Int. J. Cardiol. 2011, 148, 4–10. [Google Scholar] [CrossRef]
- Li, Y.; Cui, J.; Liu, Y.; Chen, K.; Huang, L.; Liu, Y. Oral, Tongue-Coating Microbiota, and Metabolic Disorders: A Novel Area of Interactive Research. Front. Cardiovasc. Med. 2021, 8, 922. [Google Scholar] [CrossRef] [PubMed]
- Lorca, C.; Mulet, M.; Arévalo-Caro, C.; Sanchez, M.Á.; Perez, A.; Perrino, M.; Bach-Faig, A.; Aguilar-Martínez, A.; Vilella, E.; Gallart-Palau, X.; et al. Plant-Derived Nootropics and Human Cognition: A Systematic Review. Crit. Rev. Food Sci. Nutr. 2022, 3, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Gallart-Palau, X.; Tan, L.M.; Serra, A.; Gao, Y.; Ho, H.H.; Richards, A.M.; Kandiah, N.; Chen, C.P.; Kalaria, R.N.; Sze, S.K. Degenerative Protein Modifications in the Aging Vasculature and Central Nervous System: A Problem Shared Is Not Always Halved. Ageing Res. Rev. 2019, 53, 100909. [Google Scholar] [CrossRef] [PubMed]
- Hase, Y.; Polvikoski, T.M.; Ihara, M.; Hase, M.; Zafar, R.; Stevenson, W.; Allan, L.M.; Ennaceur, A.; Horsburgh, K.; Gallart-Palau, X.; et al. Carotid Artery Disease in Post-Stroke Survivors and Effects of Enriched Environment on Stroke Pathology in a Mouse Model of Carotid Artery Stenosis. Neuropathol. Appl. Neurobiol. 2019, 45, 681–697. [Google Scholar] [CrossRef] [PubMed]
- Umegaki, H. Neurodegeneration in Diabetes Mellitus. Adv. Exp. Med. Biol. 2012, 724, 258–265. [Google Scholar] [CrossRef]
- Jiang, T.; Yu, J.T.; Tian, Y.; Tan, L. Epidemiology and Etiology of Alzheimer’s Disease: From Genetic to Non-Genetic Factors. Curr. Alzheimer Res. 2013, 10, 852–867. [Google Scholar] [CrossRef]
- Gallart-Palau, X.; Guo, X.; Serra, A.; Sze, S.K. Alzheimer’s Disease Progression Characterized by Alterations in the Molecular Profiles and Biogenesis of Brain Extracellular Vesicles. Alzheimer’s Res. Ther. 2020, 12, 54. [Google Scholar] [CrossRef]
- Serra, A.; Gallart-Palau, X.; Park, J.E.; Lim, G.G.Y.; Lim, K.L.; Ho, H.H.; Tam, J.P.; Sze, S.K. Vascular Bed Molecular Profiling by Differential Systemic Decellularization In Vivo. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2396–2409. [Google Scholar] [CrossRef]
- Drucker, A.M.; Fleming, P.; Chan, A.-W. Research Techniques Made Simple: Assessing Risk of Bias in Systematic Reviews. J. Investig. Dermatol. 2016, 136, e109–e114. [Google Scholar] [CrossRef] [PubMed]
- Kleinstein, S.E.; Nelson, K.E.; Freire, M. Inflammatory Networks Linking Oral Microbiome with Systemic Health and Disease. J. Dent. Res. 2020, 99, 1131–1139. [Google Scholar] [CrossRef]
- Freire, M.; Nelson, K.E.; Edlund, A. The Oral Host–Microbial Interactome: An Ecological Chronometer of Health? Trends Microbiol. 2021, 29, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Michaud, D.S.; Fu, Z.; Shi, J.; Chung, M. Periodontal Disease, Tooth Loss, and Cancer Risk. Epidemiol. Rev. 2017, 39, 49–58. [Google Scholar] [CrossRef]
- Irfan, M.; Delgado, R.Z.R.; Frias-Lopez, J. The Oral Microbiome and Cancer. Front. Immunol. 2020, 11, 591088. [Google Scholar] [CrossRef] [PubMed]
- Peters, B.A.; Wu, J.; Pei, Z.; Yang, L.; Purdue, M.P.; Freedman, N.D.; Jacobs, E.J.; Gapstur, S.M.; Hayes, R.B.; Ahn, J. Oral Microbiome Composition Reflects Prospective Risk for Esophageal Cancers. Cancer Res. 2017, 77, 6777–6787. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Alekseyenko, A.V.; Wu, J.; Peters, B.A.; Jacobs, E.J.; Gapstur, S.M.; Purdue, M.P.; Abnet, C.C.; Stolzenberg-Solomon, R.; Miller, G.; et al. Human Oral Microbiome and Prospective Risk for Pancreatic Cancer: A Population Based, Nested Case Control Study. Gut 2018, 67, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Sinha, R.; Pei, Z.; Dominianni, C.; Wu, J.; Shi, J.; Goedert, J.J.; Hayes, R.B.; Yang, L. Human Gut Microbiome and Risk for Colorectal Cancer. J. Natl. Cancer Inst. 2013, 105, 1907–1911. [Google Scholar] [CrossRef] [PubMed]
- Schwabe, R.F.; Jobin, C. The Microbiome and Cancer. Nat. Rev. Cancer 2013, 13, 800–812. [Google Scholar] [CrossRef]
- Abusleme, L.; Dupuy, A.K.; Dutzan, N.; Silva, N.; Burleson, J.A.; Strausbaugh, L.D.; Gamonal, J.; Diaz, P.I. The Subgingival Microbiome in Health and Periodontitis and Its Relationship with Community Biomass and Inflammation. ISME J. 2013, 7, 1016–1025. [Google Scholar] [CrossRef]
- Kim, D.; Barraza, J.P.; Arthur, R.A.; Hara, A.; Lewis, K.; Liu, Y.; Scisci, E.L.; Hajishengallis, E.; Whiteley, M.; Koo, H. Spatial Mapping of Polymicrobial Communities Reveals a Precise Biogeography Associated with Human Dental Caries. Proc. Natl. Acad. Sci. USA 2020, 117, 12375–12386. [Google Scholar] [CrossRef]
- Gross, E.L.; Beall, C.J.; Kutsch, S.R.; Firestone, N.D.; Leys, E.J.; Griffen, A.L. Beyond Streptococcus Mutans: Dental Caries Onset Linked to Multiple Species by 16S RRNA Community Analysis. PLoS ONE 2012, 7, e47722. [Google Scholar] [CrossRef]
- Aas, J.A.; Griffen, A.L.; Dardis, S.R.; Lee, A.M.; Olsen, I.; Dewhirst, F.E.; Leys, E.J.; Paster, B.J. Bacteria of Dental Caries in Primary and Permanent Teeth in Children and Young Adults. J. Clin. Microbiol. 2008, 46, 1407–1417. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Lu, M.; Liu, R.; Tian, Y.; Vu, V.H.; Li, Y.; Liu, B.; Kushmaro, A.; Li, Y.; Sun, Q. Inhibition of Streptococcus Mutans Biofilm Formation and Virulence by Lactobacillus Plantarum K41 Isolated From Traditional Sichuan Pickles Bacterial Strains and Growth Conditions. Front. Microbiol. 2020, 11, 774. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, D.J.; Lynch, R.J.M. Diet and the Microbial Aetiology of Dental Caries: New Paradigms. Int. Dent. J. 2013, 63 (Suppl. S2), 64–72. [Google Scholar] [CrossRef] [PubMed]
- Gasmi Benahmed, A.; Gasmi, A.; Dadar, M.; Arshad, M.; Bjørklund, G. The Role of Sugar-Rich Diet and Salivary Proteins in Dental Plaque Formation and Oral Health. J. Oral Biosci. 2021, 63, 134–141. [Google Scholar] [CrossRef]
- Moynihan, P. Foods and Dietary Factors That Prevent Dental Caries. Quintessence Int. 2007, 38, 320–324. [Google Scholar]
- Akman, S.; Canakci, V.; Kara, A.; Tozoglu, U.; Arabaci, T.; Dagsuyu, I.M. Therapeutic Effects of Alpha Lipoic Acid and Vitamin C on Alveolar Bone Resorption After Experimental Periodontitis in Rats: A Biochemical, Histochemical, and Stereologic Study. J. Periodontol. 2013, 84, 666–674. [Google Scholar] [CrossRef]
- Lee, J.; Park, J.C.; Jung, U.W.; Choi, S.H.; Cho, K.S.; Park, Y.K.; Kim, C.S. Improvement in Periodontal Healing after Periodontal Surgery Supported by Nutritional Supplement Drinks. J. Periodontal Implant. Sci. 2014, 44, 109–117. [Google Scholar] [CrossRef]
- Varela-López, A.; Battino, M.; Bullón, P.; Quiles, J.L. Dietary Antioxidants for Chronic Periodontitis Prevention and Its Treatment: A Review on Current Evidences from Animal and Human Studies. Ars. Pharmaceutica. 2015, 56, 131–140. [Google Scholar] [CrossRef]
- Adegboye, A.R.A.; Boucher, B.J.; Kongstad, J.; Fiehn, N.E.; Christensen, L.B.; Heitmann, B.L. Calcium, Vitamin D, Casein and Whey Protein Intakes and Periodontitis among Danish Adults. Public Health Nutr. 2016, 19, 503–510. [Google Scholar] [CrossRef]
- Woelber, J.P.; Gärtner, M.; Breuninger, L.; Anderson, A.; König, D.; Hellwig, E.; Al-Ahmad, A.; Vach, K.; Dötsch, A.; Ratka-Krüger, P.; et al. The Influence of an Anti-Inflammatory Diet on Gingivitis. A Randomized Controlled Trial. J. Clin. Periodontol. 2019, 46, 481–490. [Google Scholar] [CrossRef]
- Varela-López, A.; Navarro-Hortal, M.D.; Giampieri, F.; Bullón, P.; Battino, M.; Quiles, J.L. Nutraceuticals in Periodontal Health: A Systematic Review on the Role of Vitamins in Periodontal Health Maintenance. Molecules 2018, 23, 1226. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Jun, H.; Kim, H.; Lee, S.; Choi, B. Fusobacterium Nucleatum GroEL Induces Risk Factors of Atherosclerosis in Human Microvascular Endothelial Cells and ApoE(−/−) Mice. Mol. Oral Microbiol. 2012, 27, 109–123. [Google Scholar] [CrossRef]
- He, J.; Li, Y.; Cao, Y.; Xue, J.; Zhou, X. The Oral Microbiome Diversity and Its Relation to Human Diseases. Folia Microbiol. 2014, 60, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Noble, J.; Scarmeas, N.; Celenti, R.S.; Elkind, M.S.V.; Wright, C.B.; Schupf, N.; Papapanou, P.N. Serum IgG Antibody Levels to Periodontal Microbiota Are Associated with Incident Alzheimer Disease. PLoS ONE 2014, 9, e114959. [Google Scholar] [CrossRef]
- Ashworth, A.; Cutler, C.; Farnham, G.; Liddle, L.; Burleigh, M.; Rodiles, A.; Sillitti, C.; Kiernan, M.; Moore, M.; Hickson, M.; et al. Dietary Intake of Inorganic Nitrate in Vegetarians and Omnivores and Its Impact on Blood Pressure, Resting Metabolic Rate and the Oral Microbiome. Free Radic. Biol. Med. 2019, 138, 63–72. [Google Scholar] [CrossRef]
- Pignatelli, P.; Fabietti, G.; Ricci, A.; Piattelli, A.; Curia, M.C. How Periodontal Disease and Presence of Nitric Oxide Reducing Oral Bacteria Can Affect Blood Pressure. Int. J. Mol. Sci. 2020, 21, 7538. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, H.S.; Jackson, K.G.; Hobbs, D.A.; Lovegrove, J.A. The Role of Dietary Nitrate and the Oral Microbiome on Blood Pressure and Vascular Tone. Nutr. Res. Rev. 2021, 34, 222–239. [Google Scholar] [CrossRef]
- Velmurugan, S.; Gan, J.M.; Rathod, K.S.; Khambata, R.S.; Ghosh, S.M.; Hartley, A.; Van Eijl, S.; Sagi-Kiss, V.; Chowdhury, T.A.; Curtis, M.; et al. Dietary Nitrate Improves Vascular Function in Patients with Hypercholesterolemia: A Randomized, Double-Blind, Placebo-Controlled Study. Am. J. Clin. Nutr. 2016, 103, 25–38. [Google Scholar] [CrossRef]
- Rosier, B.T.; Marsh, P.D.; Mira, A. Resilience of the Oral Microbiota in Health: Mechanisms That Prevent Dysbiosis. J. Dent. Res. 2018, 97, 371–380. [Google Scholar] [CrossRef]
- Vanhatalo, A.; Blackwell, J.; L’Heureux, J.; Williams, D.; Smith, A.; Van der Giezen, M.; Winyard, P.; Kelly, J.; Jones, A. Nitrate-Responsive Oral Microbiome Modulates Nitric Oxide Homeostasis and Blood Pressure in Humans. Free Radic. Biol. Med. 2018, 124, 21–30. [Google Scholar] [CrossRef]
- Bulgart, H.R.; Neczypor, E.W.; Wold, L.E.; Mackos, A.R. Microbial Involvement in Alzheimer Disease Development and Progression. Mol. Neurodegener. 2020, 15, 42. [Google Scholar] [CrossRef] [PubMed]
- Bonfili, L.; Cecarini, V.; Gogoi, O.; Gong, C.; Cuccioloni, M.; Angeletti, M.; Rossi, G.; Eleuteri, A.M. Microbiota Modulation as Preventative and Therapeutic Approach in Alzheimer’s Disease. FEBS J. 2021, 288, 2836–2855. [Google Scholar] [CrossRef] [PubMed]
- Ries, M.; Sastre, M. Mechanisms of Aβ Clearance and Degradation by Glial Cells. Front. Aging Neurosci. 2016, 8, 160. [Google Scholar] [CrossRef] [PubMed]
- Allen, H.B. Alzheimer’s Disease: Assessing the Role of Spirochetes, Biofilms, the Immune System, and Amyloid-β with Regard to Potential Treatment and Prevention. J. Alzheimer’s Dis. 2016, 53, 1271–1276. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Yamazaki, K.; Nakajima, M.; Date, Y.; Kikuchi, J.; Hase, K.; Ohno, H.; Yamazaki, K. Oral Administration of Porphyromonas Gingivalis Alters the Gut Microbiome and Serum Metabolome. mSphere 2018, 3, e00460-18. [Google Scholar] [CrossRef]
- Gallart-Palau, X.; Serra, A.; Sze, S.K. System-Wide Molecular Dynamics of Endothelial Dysfunction in Gram-Negative Sepsis. BMC Biol. 2020, 18, 175. [Google Scholar] [CrossRef] [PubMed]
- Ilievski, V.; Zuchowska, P.K.; Green, S.J.; Toth, P.; Ragozzino, M.E.; Le, K.; Aljewari, H.W.; O’Brien-Simpson, N.; Reynolds, E.C.; Watanabe, K. Chronic Oral Application of a Periodontal Pathogen Results in Brain Inflammation, Neurodegeneration and Amyloid Beta Production in Wild Type Mice. PLoS ONE 2018, 13, e0204941. [Google Scholar] [CrossRef]
- Miklossy, J.; Kis, A.; Radenovic, A.; Miller, L.; Forro, L.; Martins, R.; Reiss, K.; Darbinian, N.; Darekar, P.; Mihaly, L. Beta-Amyloid Deposition and Alzheimer’s Type Changes Induced by Borrelia Spirochetes. Neurobiol. Aging 2006, 27, 228–236. [Google Scholar] [CrossRef]
- Dominy, S.S.; Lynch, C.; Ermini, F.; Benedyk, M.; Marczyk, A.; Konradi, A.; Nguyen, M.; Haditsch, U.; Raha, D.; Griffin, C.; et al. Porphyromonas Gingivalis in Alzheimer’s Disease Brains: Evidence for Disease Causation and Treatment with Small-Molecule Inhibitors. Sci. Adv. 2019, 5, e0204941. [Google Scholar] [CrossRef]
- Leblhuber, F.; Huemer, J.; Steiner, K.; Gostner, J.M.; Fuchs, D. Knock-on Effect of Periodontitis to the Pathogenesis of Alzheimer’s Disease? Wien. Klin. Wochenschr. 2020, 132, 493–498. [Google Scholar] [CrossRef]
- Chi, L.; Cheng, X.; Lin, L.; Yang, T.; Sun, J.; Feng, Y.; Liang, F.; Pei, Z.; Teng, W. Porphyromonas Gingivalis-Induced Cognitive Impairment Is Associated With Gut Dysbiosis, Neuroinflammation, and Glymphatic Dysfunction. Front. Cell. Infect. Microbiol. 2021, 11, 977. [Google Scholar] [CrossRef] [PubMed]
- Borsa, L.; Dubois, M.; Sacco, G.; Lupi, L. Analysis the Link between Periodontal Diseases and Alzheimer’s Disease: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 9312. [Google Scholar] [CrossRef] [PubMed]
- Leblhuber, F.; Ehrlich, D.; Steiner, K.; Geisler, S.; Fuchs, D.; Lanser, L.; Kurz, K. The Immunopathogenesis of Alzheimer’s Disease Is Related to the Composition of Gut Microbiota. Nutrients 2021, 13, 361. [Google Scholar] [CrossRef] [PubMed]
- Olsen, I. Can Porphyromonas Gingivalis Contribute to Alzheimer’s Disease Already at the Stage of Gingivitis? J. Alzheimer’s Dis. Rep. 2021, 5, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Leszek, J.; Mikhaylenko, E.V.; Belousov, D.M.; Koutsouraki, E.; Szczechowiak, K.; Kobusiak-Prokopowicz, M.; Mysiak, A.; Diniz, B.S.; Somasundaram, S.G.; Kirkland, C.E.; et al. The Links between Cardiovascular Diseases and Alzheimer’s Disease. Curr. Neuropharmacol. 2020, 19, 152–169. [Google Scholar] [CrossRef]
- Dibello, V.; Lozupone, M.; Manfredini, D.; Dibello, A.; Zupo, R.; Sardone, R.; Daniele, A.; Lobbezoo, F.; Panza, F. Oral Frailty and Neurodegeneration in Alzheimer’s Disease. Neural Regen. Res. 2021, 16, 2149–2153. [Google Scholar]
- Simas, A.M.; Kramer, C.D.; Weinberg, E.O.; Genco, C.A. Oral Infection with a Periodontal Pathogen Alters Oral and Gut Microbiomes. Anaerobe 2021, 71, 102399. [Google Scholar] [CrossRef]
- Boeri, L.; Perottoni, S.; Izzo, L.; Giordano, C.; Albani, D. Microbiota-Host Immunity Communication in Neurodegenerative Disorders: Bioengineering Challenges for In Vitro Modeling. Adv. Healthc. Mater. 2021, 10, 2002043. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Evans, D.A.; Bienias, J.L.; Tangney, C.C.; Bennett, D.A.; Wilson, R.S.; Aggarwal, N.; Schneider, J. Consumption of Fish and N-3 Fatty Acids and Risk of Incident Alzheimer Disease. Arch. Neurol. 2003, 60, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Yurko-Mauro, K.; McCarthy, D.; Rom, D.; Nelson, E.B.; Ryan, A.S.; Blackwell, A.; Salem, N.; Stedman, M.; MIDAS Investigators. Beneficial Effects of Docosahexaenoic Acid on Cognition in Age-Related Cognitive Decline. Alzheimer’s Dement. 2010, 6, 456–464. [Google Scholar] [CrossRef]
- Pomponi, M.F.L.; Gambassi, G.; Pomponi, M.; Masullo, C. Alzheimer’s Disease: Fatty Acids We Eat May Be Linked to a Specific Protection via Low-Dose Aspirin. Aging Dis. 2010, 1, 37–59. [Google Scholar] [PubMed]
- Caracciolo, B.; Xu, W.; Collins, S.; Fratiglioni, L. Cognitive Decline, Dietary Factors and Gut-Brain Interactions. Mech. Ageing Dev. 2014, 136–137, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Huhn, S.; Masouleh, S.K.; Stumvoll, M.; Villringer, A.; Witte, A.V. Components of a Mediterranean Diet and Their Impact on Cognitive Functions in Aging. Front. Aging Neurosci. 2015, 7, 132. [Google Scholar] [CrossRef]
- Ribeiro-Vidal, H.; Sánchez, M.C.; Alonso-Español, A.; Figuero, E.; Ciudad, M.J.; Collado, L.; Herrera, D.; Sanz, M. Antimicrobial Activity of Epa and Dha against Oral Pathogenic Bacteria Using an in Vitro Multi-Species Subgingival Biofilm Model. Nutrients 2020, 12, 2812. [Google Scholar] [CrossRef]
- Khalifa, K.; Bergland, A.K.; Soennesyn, H.; Oppedal, K.; Oesterhus, R.; Dalen, I.; Larsen, A.I.; Fladby, T.; Brooker, H.; Wesnes, K.A.; et al. Effects of Purified Anthocyanins in People at Risk for Dementia: Study Protocol for a Phase II Randomized Controlled Trial. Front. Neurol. 2020, 11, 916. [Google Scholar] [CrossRef] [PubMed]
- Ullah, R.; Khan, M.; Shah, S.A.; Saeed, K.; Kim, M.O. Natural Antioxidant Anthocyanins—A Hidden Therapeutic Candidate in Metabolic Disorders with Major Focus in Neurodegeneration. Nutrients 2019, 11, 1195. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Ikram, M.; Park, J.S.; Park, T.J.; Kim, M.O. Gut Microbiota, Its Role in Induction of Alzheimer’s Disease Pathology, and Possible Therapeutic Interventions: Special Focus on Anthocyanins. Cells 2020, 9, 853. [Google Scholar] [CrossRef]
- Sukprasansap, M.; Chanvorachote, P.; Tencomnao, T. Cyanidin-3-Glucoside Activates Nrf2-Antioxidant Response Element and Protects against Glutamate-Induced Oxidative and Endoplasmic Reticulum Stress in HT22 Hippocampal Neuronal Cells. BMC Complement. Med. Ther. 2020, 20, 46. [Google Scholar] [CrossRef]
- Panza, F.; Lozupone, M.; Solfrizzi, V.; Watling, M.; Imbimbo, B.P. Time to Test Antibacterial Therapy in Alzheimer’s Disease. Brain 2019, 142, 2905–2929. [Google Scholar] [CrossRef] [PubMed]
- Atarashi, K.; Suda, W.; Luo, C.; Kawaguchi, T.; Motoo, I.; Narushima, S.; Kiguchi, Y.; Yasuma, K.; Watanabe, E.; Tanoue, T.; et al. Ectopic Colonization of Oral Bacteria in the Intestine Drives TH1 Cell Induction and Inflammation. Science 2017, 358, 359–365. [Google Scholar] [CrossRef]

| Age | Host and Environment | Habitat | Biofilm Maturation |
|---|---|---|---|
| Changes in the host and its habits | Genetic factors | Surface 1 | Environment |
| Microevolution | Diet and lifestyle Immune system | Oxygen | Probiotics |
| Horizontal transfer of microorganisms | Changes in host defenses | Nutritional status | Oral hygiene |
| Changes in diversity | Broad spectrum antibiotics | Oral hygiene | Microbial interactions |
| Hormonal balance | pH | Immune response | |
| Environment | Cell flaking in the mucosa | Density | |
| Salivary flow and gingival crevicular fluid |
| Autoimmune Disorders | Metabolic and Inflammatory Diseases | Cancer Diseases | Neurodegenerative Diseases |
|---|---|---|---|
| Rheumatoid arthritis [42,73] | Non-alcoholic hepatic steatosis | Colorectal cancer (F. nucleatum) | Multiple sclerosis |
| Sjögren syndrome, systemic lupus erythematosus [32] | Insulin resistance, diabetes, atherosclerosis [74] | Pancreatic cancer (P. gingivalis and A. actinomycetemcomitans) | Dementia |
| Inflammatory bowel disease [29] | Chronic kidney disease | Gastrointestinal cancer [32] | Alzheimer’s disease |
| Hypertension, stroke, obesity | Head and neck tumors | ||
| Oral cancer [75] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giordano-Kelhoffer, B.; Lorca, C.; March Llanes, J.; Rábano, A.; del Ser, T.; Serra, A.; Gallart-Palau, X. Oral Microbiota, Its Equilibrium and Implications in the Pathophysiology of Human Diseases: A Systematic Review. Biomedicines 2022, 10, 1803. https://doi.org/10.3390/biomedicines10081803
Giordano-Kelhoffer B, Lorca C, March Llanes J, Rábano A, del Ser T, Serra A, Gallart-Palau X. Oral Microbiota, Its Equilibrium and Implications in the Pathophysiology of Human Diseases: A Systematic Review. Biomedicines. 2022; 10(8):1803. https://doi.org/10.3390/biomedicines10081803
Chicago/Turabian StyleGiordano-Kelhoffer, Barbara, Cristina Lorca, Jaume March Llanes, Alberto Rábano, Teodoro del Ser, Aida Serra, and Xavier Gallart-Palau. 2022. "Oral Microbiota, Its Equilibrium and Implications in the Pathophysiology of Human Diseases: A Systematic Review" Biomedicines 10, no. 8: 1803. https://doi.org/10.3390/biomedicines10081803
APA StyleGiordano-Kelhoffer, B., Lorca, C., March Llanes, J., Rábano, A., del Ser, T., Serra, A., & Gallart-Palau, X. (2022). Oral Microbiota, Its Equilibrium and Implications in the Pathophysiology of Human Diseases: A Systematic Review. Biomedicines, 10(8), 1803. https://doi.org/10.3390/biomedicines10081803









