A Brominated Furanone Inhibits Pseudomonas aeruginosa Quorum Sensing and Type III Secretion, Attenuating Its Virulence in a Murine Cutaneous Abscess Model
Abstract
:1. Introduction
2. Materials and Methods
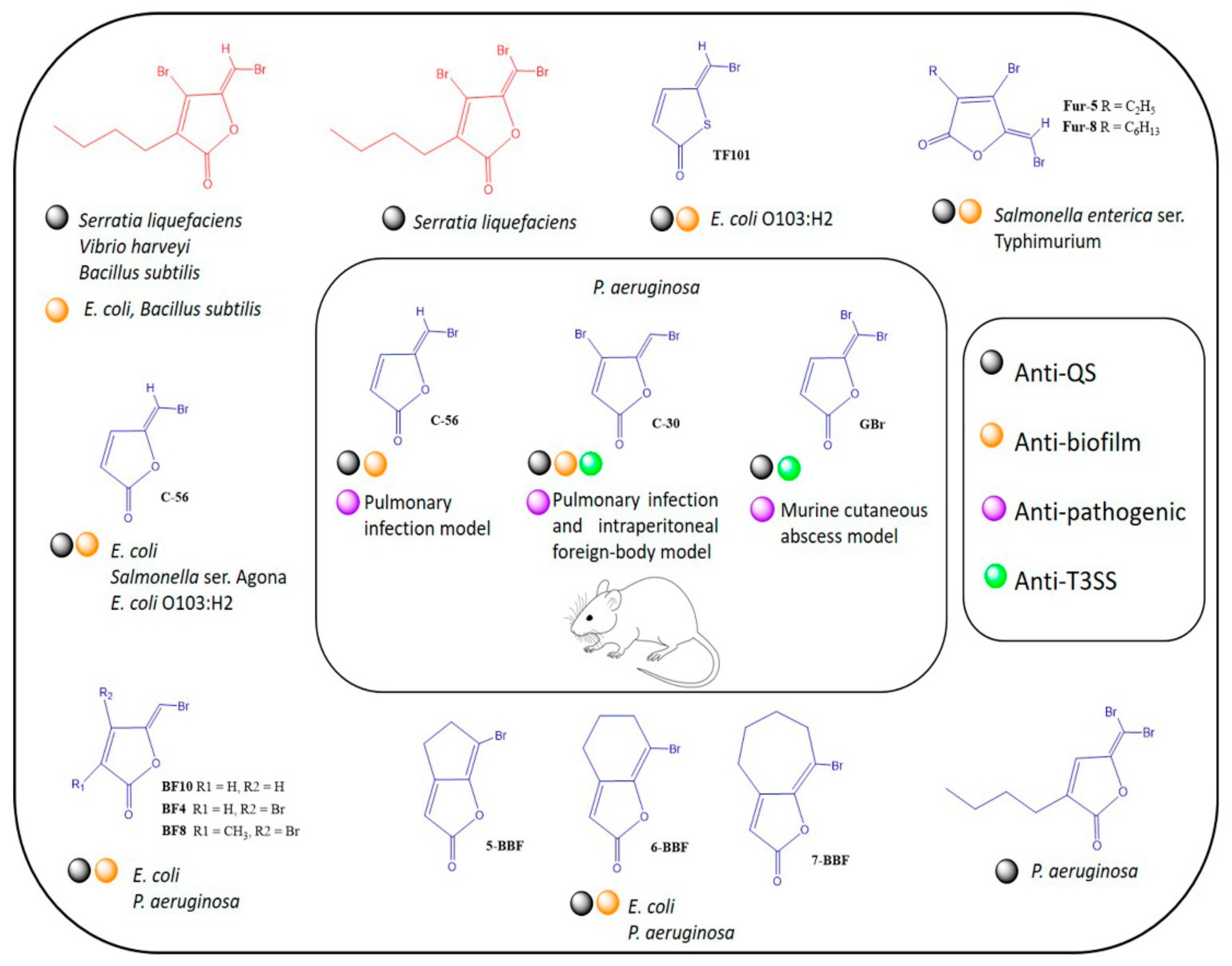
2.1. Brominated Furanones
2.2. Strains and Culture Conditions
2.3. Virulence Factor Production
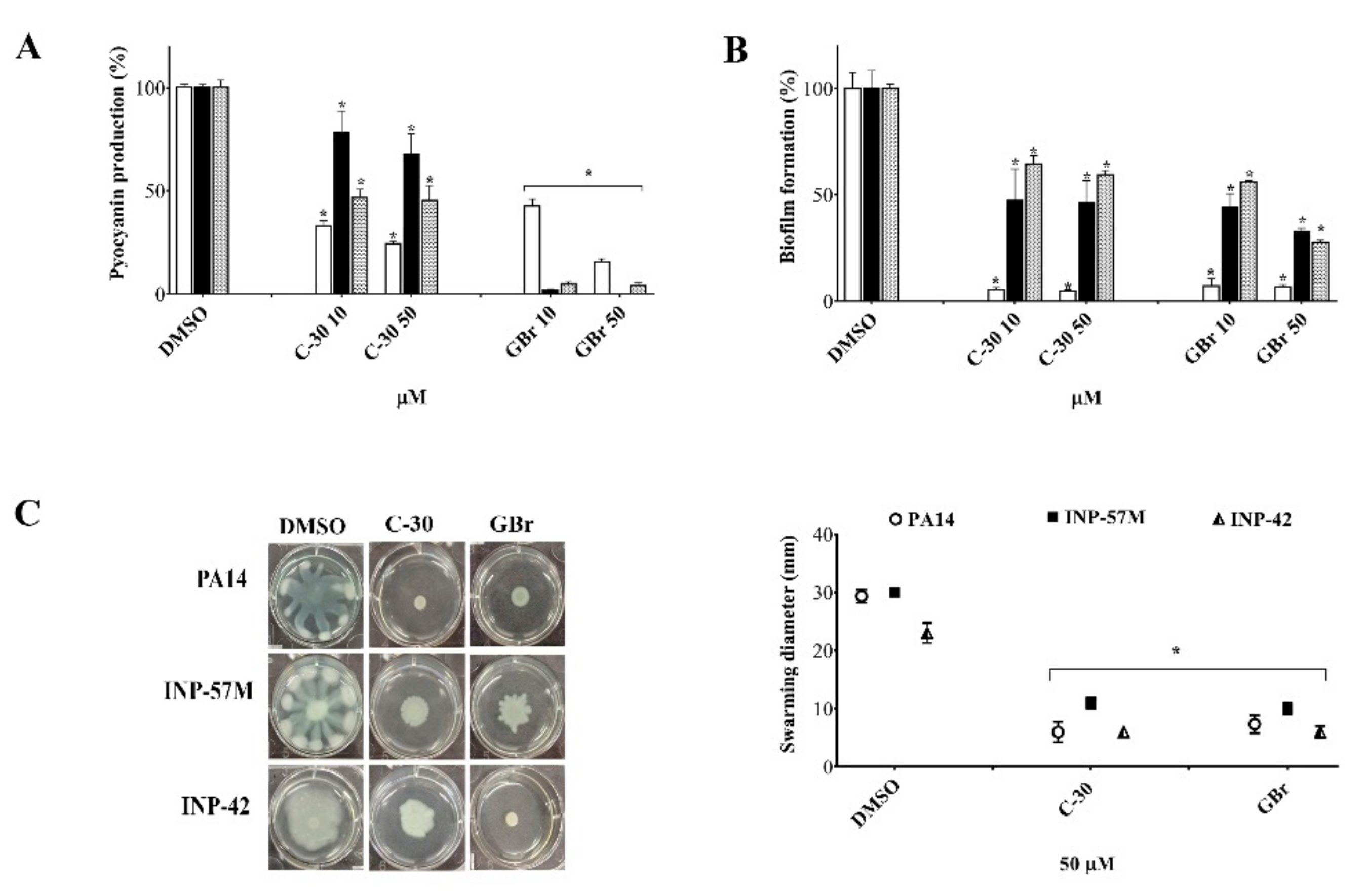
2.3.1. Pyocyanin Production
2.3.2. Biofilm Formation
2.3.3. Swarming Motility
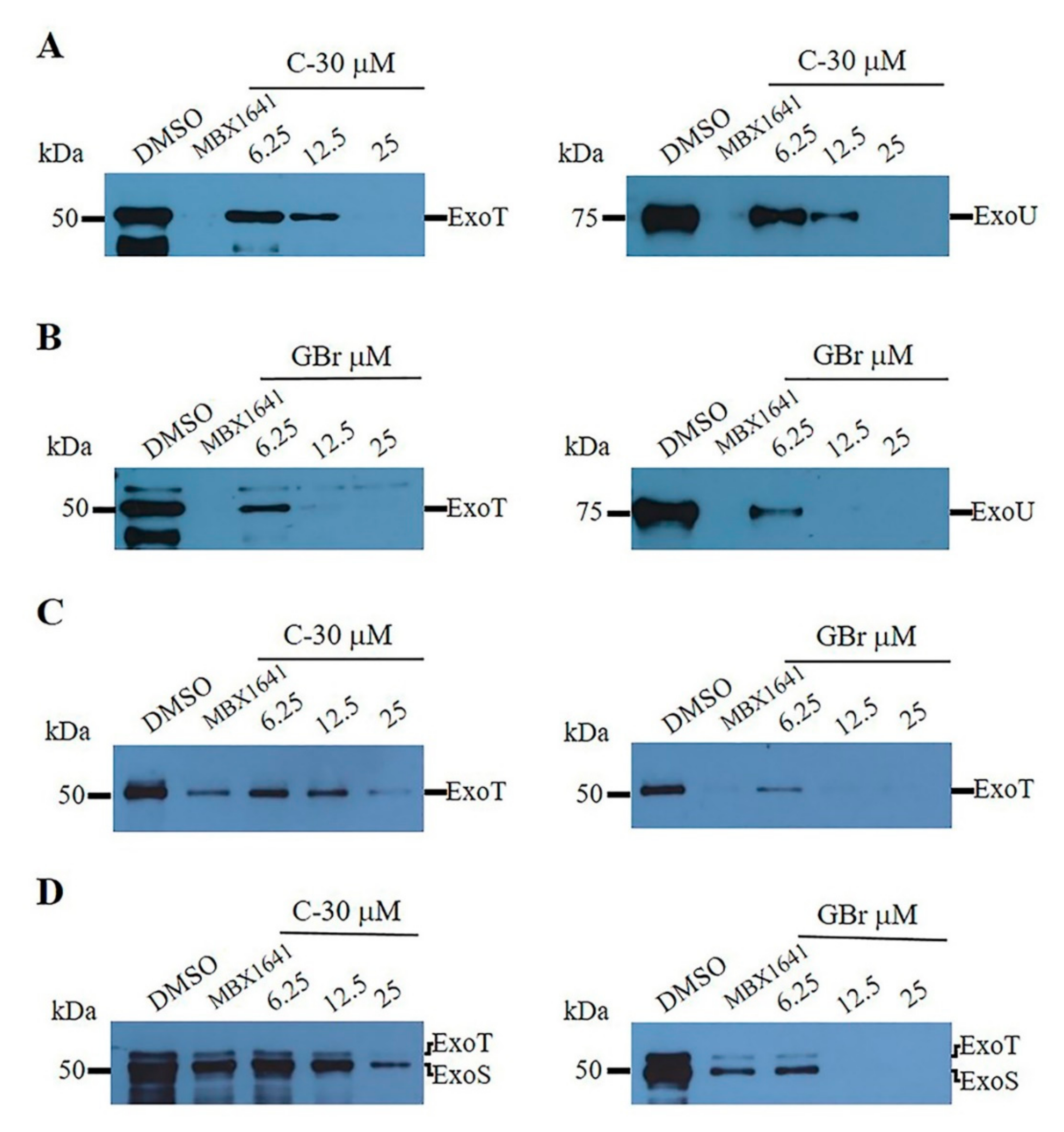
2.3.4. Type III Secretion Assay
2.4. Docking Analysis of 3-oxo-C12-HSL and Furanones C-30 and GBr on the Binding Site of LasR
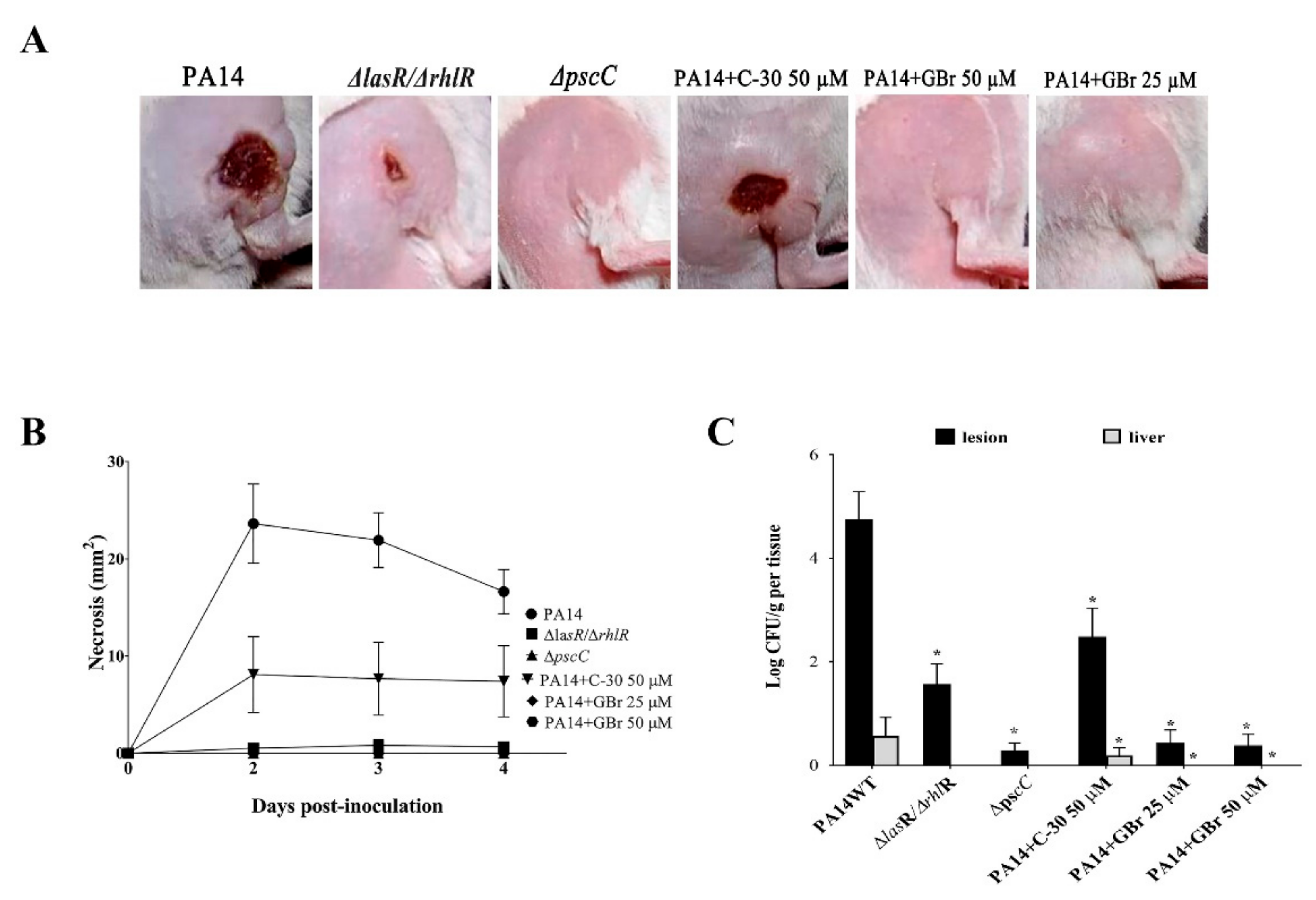
2.5. Mice Abscess Model
2.6. Statistical Analysis
3. Results
3.1. The GBr Furanone Exhibits Anti-Virulence Properties in P. aeruginosa
3.2. Furanones Inhibit the Secretion of Type III Effector Proteins
3.3. Effect of GBr on a P. aeruginosa-Mouse Infection Model
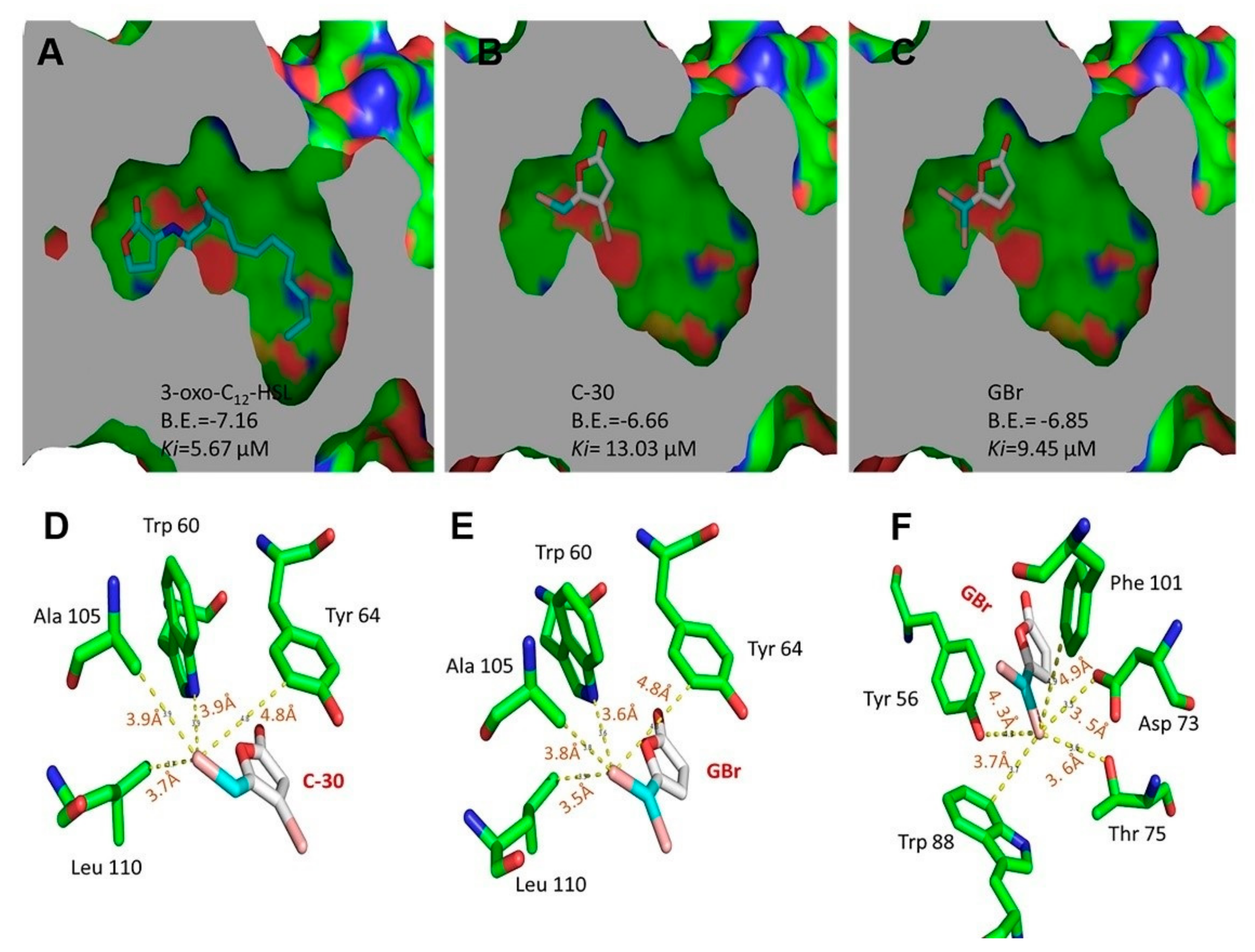
3.4. Molecular Docking Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crofts, T.S.; Gasparrini, A.J.; Dantas, G. Next-Generation Approaches to Understand and Combat the Antibiotic Resistome. Nat. Rev. Microbiol. 2017, 15, 422–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shrivastava, S.R.; Shrivastava, P.S.; Ramasamy, J. World Health Organization Releases Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. J. Med. Soc. 2018, 32, 76. [Google Scholar] [CrossRef]
- Dickey, S.W.; Cheung, G.Y.C.; Otto, M. Different Drugs for Bad Bugs: Antivirulence Strategies in the Age of Antibiotic Resistance. Nat. Rev. Drug Discov. 2017, 16, 457–471. [Google Scholar] [CrossRef] [PubMed]
- Defoirdt, T. Specific Antivirulence Activity, A New Concept for Reliable Screening of Virulence Inhibitors. Trends Biotechnol. 2016, 34, 527–529. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Cazares, N.; García-Contreras, R.; Soto-Hernández, M.; Martínez-Vázquez, M.; Castillo-Juárez, I. Natural Products With Quorum Quenching-Independent Antivirulence Properties. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 327–348. [Google Scholar]
- Deng, W.; Marshall, N.C.; Rowland, J.L.; McCoy, J.M.; Worrall, L.J.; Santos, A.S.; Strynadka, N.C.J.; Finlay, B.B. Assembly, Structure, Function and Regulation of Type III Secretion Systems. Nat. Rev. Microbiol. 2017, 15, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Gaytán, M.O.; Martínez-Santos, V.I.; Soto, E.; González-Pedrajo, B. Type Three Secretion System in Attaching and Effacing Pathogens. Front. Cell. Infect. Microbiol. 2016, 6, 129. [Google Scholar] [CrossRef] [Green Version]
- Silva, L.N.; Zimmer, K.R.; Macedo, A.J.; Trentin, D.S. Plant Natural Products Targeting Bacterial Virulence Factors. Chem. Rev. 2016, 116, 9162–9236. [Google Scholar] [CrossRef]
- Zhang, L.-H.; Dong, Y.-H. Quorum Sensing and Signal Interference: Diverse Implications. Mol. Microbiol. 2004, 53, 1563–1571. [Google Scholar] [CrossRef]
- Lee, J.; Zhang, L. The Hierarchy Quorum Sensing Network in Pseudomonas aeruginosa. Protein Cell 2015, 6, 26–41. [Google Scholar] [CrossRef] [Green Version]
- Castillo-Juarez, I.; Maeda, T.; Mandujano-Tinoco, E.A.; Tomas, M.; Perez-Eretza, B.; García-Contreras, S.J.; Wood, T.K.; García-Contreras, R. Role of Quorum Sensing in Bacterial Infections. World J. Clin. Cases 2015, 3, 575–598. [Google Scholar] [CrossRef]
- Moore, J.D.; Rossi, F.M.; Welsh, M.A.; Nyffeler, K.E.; Blackwell, H.E. A Comparative Analysis of Synthetic Quorum Sensing Modulators in Pseudomonas aeruginosa: New Insights into Mechanism, Active Efflux Susceptibility, Phenotypic Response, and Next-Generation Ligand Design. J. Am. Chem. Soc. 2015, 137, 14626–14639. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, T.B.; Givskov, M. Quorum Sensing Inhibitors: A Bargain of Effects. Microbiology 2006, 152, 895–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, K.; Zhang, X.-H. Quorum Quenching Agents: Resources for Antivirulence Therapy. Mar. Drugs 2014, 12, 3245–3282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hentzer, M.; Wu, H.; Andersen, J.B.; Riedel, K.; Rasmussen, T.B.; Bagge, N.; Kumar, N.; Schembri, M.A.; Song, Z.; Kristoffersen, P.; et al. Attenuation of Pseudomonas aeruginosa Virulence by Quorum Sensing Inhibitors. EMBO J. 2003, 22, 3803–3815. [Google Scholar] [CrossRef] [PubMed]
- Maeda, T.; Garcia-Contreras, R.; Pu, M.; Sheng, L.; Garcia, L.R.; Tomas, M.; Wood, T.K. Quorum Quenching Quandary: Resistance to Antivirulence Compounds. ISME J. 2012, 6, 493–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.; Song, Z.; Hentzer, M.; Andersen, J.B.; Molin, S.; Givskov, M.; Høiby, N. Synthetic Furanones Inhibit Quorum-Sensing and Enhance Bacterial Clearance in Pseudomonas aeruginosa Lung Infection in Mice. J. Antimicrob. Chemother. 2004, 53, 1504–1601. [Google Scholar] [CrossRef] [Green Version]
- García-Contreras, R. Is Quorum Sensing Interference a Viable Alternative to Treat Pseudomonas aeruginosa Infections? Front. Microbiol. 2016, 7, 1454. [Google Scholar] [CrossRef] [Green Version]
- García-Contreras, R.; Peréz-Eretza, B.; Jasso-Chávez, R.; Lira-Silva, E.; Roldán-Sánchez, J.A.; González-Valdez, A.; Soberón-Chávez, G.; Coria-Jiménez, R.; Martínez-Vázquez, M.; Alcaraz, L.D.; et al. High Variability in Quorum Quenching and Growth Inhibition by Furanone C-30 in Pseudomonas aeruginosa Clinical Isolates from Cystic Fibrosis Patients. Pathog. Dis. 2015, 73, ftv040. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Rahman, S.A.; El-Gohary, N.S.; El-Bendary, E.R.; El-Ashry, S.M.; Shaaban, M.I. Synthesis, Antimicrobial, Antiquorum-Sensing, Antitumor and Cytotoxic Activities of New Series of Cyclopenta(Hepta)[b]Thiophene and Fused Cyclohepta[b]Thiophene Analogs. Eur. J. Med. Chem. 2017, 140, 200–211. [Google Scholar] [CrossRef]
- Janssens, J.C.A.; De Keersmaecker, S.C.J.; De Vos, D.E.; Vanderleyden, J. Small Molecules for Interference with Cell-Cell-Communication Systems in Gram-Negative Bacteria. Curr. Med. Chem. 2008, 15, 2144–2156. [Google Scholar] [CrossRef]
- Steenackers, H.P.; Levin, J.; Janssens, J.C.; De Weerdt, A.; Balzarini, J.; Vanderleyden, J.; De Vos, D.E.; De Keersmaecker, S.C. Structure-Activity Relationship of Brominated 3-Alkyl-5-Methylene-2(5H)-Furanones and Alkylmaleic Anhydrides as Inhibitors of Salmonella Biofilm Formation and Quorum Sensing Regulated Bioluminescence in Vibrio harveyi. Bioorg. Med. Chem. 2010, 18, 5224–5233. [Google Scholar] [CrossRef] [PubMed]
- Wells, P.R. Enol Lactones of Dibromoacetylacrylic Acid. Aust. J. Chem. 1963, 16, 165–169. [Google Scholar] [CrossRef]
- Manny, A.J.; Kjelleberg, S.; Kumar, N.; De Nys, R.; Read, R.W.; Steinberg, P. Reinvestigation of the Sulfuric Acid-Catalysed Cyclisation of Brominated 2-Alkyllevulinic Acids to 3-Alkyl-5-Methylene-2(5H)-Furanones. Tetrahedron 1997, 53, 15813–15826. [Google Scholar] [CrossRef]
- Liberati, N.T.; Urbach, J.M.; Miyata, S.; Lee, D.G.; Drenkard, E.; Wu, G.; Villanueva, J.; Wei, T.; Ausubel, F.M. An Ordered, Nonredundant Library of Pseudomonas aeruginosa Strain PA14 Transposon Insertion Mutants. Proc. Natl. Acad. Sci. USA 2006, 103, 2833–2838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.-Y.; Heo, Y.-J.; Choi, Y.-S.; Déziel, E.; Cho, Y.-H. Conserved Virulence Factors of Pseudomonas aeruginosa Are Required for Killing Bacillus subtilis. J. Microbiol. 2005, 43, 443–450. [Google Scholar] [PubMed]
- Essar, D.W.; Eberly, L.; Hadero, A.; Crawford, I.P. Identification and Characterization of Genes for a Second Anthranilate Synthase in Pseudomonas aeruginosa: Interchangeability of the Two Anthranilate Synthases and Evolutionary Implications. J. Bacteriol. 1990, 172, 884–900. [Google Scholar] [CrossRef] [Green Version]
- Malešević, M.; Di Lorenzo, F.; Filipić, B.; Stanisavljević, N.; Novović, K.; Senerovic, L.; Polović, N.; Molinaro, A.; Kojić, M.; Jovčić, B. Pseudomonas aeruginosa Quorum Sensing Inhibition by Clinical Isolate Delftia Tsuruhatensis 11304: Involvement of N-Octadecanoylhomoserine Lactones. Sci. Rep. 2019, 9, 16465. [Google Scholar] [CrossRef]
- O’Toole, G.A. Microtiter Dish Biofilm Formation Assay. J. Vis. Exp. 2011, 47, e2437. [Google Scholar] [CrossRef]
- Caiazza, N.C.; Shanks, R.M.Q.; O’Toole, G.A. Rhamnolipids Modulate Swarming Motility Patterns of Pseudomonas aeruginosa. J. Bacteriol. 2005, 187, 7351. [Google Scholar] [CrossRef] [Green Version]
- García-Ulloa, M.; Ponce-Soto, G.Y.; González-Valdez, A.; González-Pedrajo, B.; Díaz-Guerrero, M.; Souza, V.; Soberón-Chávez, G. Two Pseudomonas aeruginosa Clonal Groups Belonging to the PA14 Clade Are Indigenous to the Churince System in Cuatro Ciénegas Coahuila, México. Environ. Microbiol. 2019, 21, 2964–2976. [Google Scholar] [CrossRef]
- Thompson, M.A. Molecular Docking Using ArgusLab, an Efficient Shape-Based Search Algorithm and the AScore Scoring Function. Proc. ACS Meet. Phila. 2004, 172, 42. [Google Scholar]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A Visualization System for Exploratory Research and Analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanner, M.F. Python: A Programming Language for Software Integration and Development. J. Mol. Graph. Model. 1999, 17, 57–61. [Google Scholar] [PubMed]
- Morris, G.M.; Ruth, H.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [Green Version]
- Huey, R.; Morris, G.M.; Olson, A.J.; Goodsell, D.S. A Semiempirical Free Energy Force Field with Charge-Based Desolvation. J. Comput. Chem. 2007, 28, 1145–1152. [Google Scholar] [CrossRef]
- Berube, B.J.; Murphy, K.R.; Torhan, M.C.; Bowlin, N.O.; Williams, J.D.; Bowlin, T.L.; Moir, D.T.; Hauser, A.R. Impact of Type III Secretion Effectors and of Phenoxyacetamide Inhibitors of Type III Secretion on Abscess Formation in a Mouse Model of Pseudomonas aeruginosa Infection. Antimicrob. Agents Chemother. 2017, 61, e01202-17. [Google Scholar] [CrossRef] [Green Version]
- Pletzer, D.; Mansour, S.C.; Wuerth, K.; Rahanjam, N.; Hancock, R.E.W. New Mouse Model for Chronic Infections by Gram-Negative Bacteria Enabling the Study of Anti-Infective Efficacy and Host-Microbe Interactions. MBio 2017, 8, e00140-17. [Google Scholar] [CrossRef] [Green Version]
- Markus, V.; Golberg, K.; Teralı, K.; Ozer, N.; Kramarsky-Winter, E.; Marks, R.S.; Kushmaro, A. Assessing the Molecular Targets and Mode of Action of Furanone C-30 on Pseudomonas aeruginosa Quorum Sensing. Molecules 2021, 26, 1620. [Google Scholar] [CrossRef]
- Proctor, C.R.; McCarron, P.A.; Ternan, N.G. Furanone Quorum-Sensing Inhibitors with Potential as Novel Therapeutics against Pseudomonas aeruginosa. J. Med. Microbiol. 2020, 69, 195–206. [Google Scholar] [CrossRef]
- de Nys, R.; Wright, A.D.; König, G.M.; Sticher, O. New Halogenated Furanones from the Marine Alga Delisea pulchra (Cf. Fimbriata). Tetrahedron 1993, 49, 11213–11220. [Google Scholar] [CrossRef]
- Husain, A.; Khan, S.A.; Iram, F.; Iqbal, M.A.; Asif, M. Insights into the Chemistry and Therapeutic Potential of Furanones: A Versatile Pharmacophore. Eur. J. Med. Chem. 2019, 171, 66–92. [Google Scholar] [CrossRef] [PubMed]
- Manefield, M.; Rasmussen, T.B.; Henzter, M.; Andersen, J.B.; Steinberg, P.; Kjelleberg, S.; Givskov, M. Halogenated Furanones Inhibit Quorum Sensing through Accelerated LuxR Turnover. Microbiology 2002, 148, 1119–1127. [Google Scholar] [CrossRef] [Green Version]
- Gu, L.; Zhou, S.; Zhu, L.; Liang, C.; Chen, X. Small-Molecule Inhibitors of the Type III Secretion System. Molecules 2015, 20, 17659–17674. [Google Scholar] [CrossRef] [PubMed]
- Pena, R.T.; Blasco, L.; Ambroa, A.; González-Pedrajo, B.; Fernández-García, L.; López, M.; Bleriot, I.; Bou, G.; García-Contreras, R.; Wood, T.K.; et al. Relationship Between Quorum Sensing and Secretion Systems. Front. Microbiol. 2019, 10, 1100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galle, M.; Carpentier, I.; Beyaert, R. Structure and Function of the Type III Secretion System of Pseudomonas aeruginosa. Curr. Protein Pept. Sci. 2012, 13, 831–842. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, N.; Lee, B.; Hentzer, M.; Rasmussen, T.B.; Song, Z.; Johansen, H.K.; Givskov, M.; Høiby, N. Azithromycin Blocks Quorum Sensing and Alginate Polymer Formation and Increases the Sensitivity to Serum and Stationary-Growth-Phase Killing of Pseudomonas aeruginosa and Attenuates Chronic P. aeruginosa Lung Infection in Cftr(-/-) Mice. Antimicrob. Agents Chemother. 2007, 51, 3677–3687. [Google Scholar] [CrossRef] [Green Version]
- Lesic, B.; Lépine, F.; Déziel, E.; Zhang, J.; Zhang, Q.; Padfield, K.; Castonguay, M.-H.; Milot, S.; Stachel, S.; Tzika, A.A.; et al. Inhibitors of Pathogen Intercellular Signals as Selective Anti-Infective Compounds. PLoS Pathog. 2007, 3, e126. [Google Scholar] [CrossRef]
- Kuehl, R.; Al-Bataineh, S.; Gordon, O.; Luginbuehl, R.; Otto, M.; Textor, M.; Landmann, R. Furanone at Subinhibitory Concentrations Enhances Staphylococcal Biofilm Formation by LuxS Repression. Antimicrob. Agents Chemother. 2009, 53, 4159. [Google Scholar] [CrossRef] [Green Version]
- Defoirdt, T.; Miyamoto, C.M.; Wood, T.K.; Meighen, E.A.; Sorgeloos, P.; Verstraete, W.; Bossier, P. The Natural Furanone (5Z)-4-Bromo-5-(Bromomethylene)-3-Butyl-2(5H)-Furanone Disrupts Quorum Sensing-Regulated Gene Expression in Vibrio harveyi by Decreasing the DNA-Binding Activity of the Transcriptional Regulator Protein LuxR. Environ. Microbiol. 2007, 9, 2486–2495. [Google Scholar] [CrossRef] [Green Version]
- Soto-Aceves, M.P.; Cocotl-Yañez, M.; Merino, E.; Castillo-Juárez, I.; Cortés-López, H.; González-Pedrajo, B.; Díaz-Guerrero, M.; Servín-González, L.; Soberón-Chávez, G. Inactivation of the Quorum-Sensing Transcriptional Regulators LasR or RhlR Does Not Suppress the Expression of Virulence Factors and the Virulence of Pseudomonas aeruginosa PAO1. Microbiology 2019, 165, 425–432. [Google Scholar] [CrossRef]
- Bleves, S.; Soscia, C.; Nogueira-Orlandi, P.; Lazdunski, A.; Filloux, A. Quorum Sensing Negatively Controls Type III Secretion Regulon Expression in Pseudomonas aeruginosa PAO1. J. Bacteriol. 2005, 187, 3898–3902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Givskov, M.; De Nys, R.; Manefield, M.; Gram, L.; Maximilien, R.; Eberl, L.; Molin, S.; Steinberg, P.D.; Kjelleberg, S. Eukaryotic Interference with Homoserine Lactone-Mediated Prokaryotic Signalling. J. Bacteriol. 1996, 178, 6618–6622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manefield, M.; De Nys, R.; Kumar, N.; Read, R.; Givskov, M.; Steinberg, P.; Kjelleberg, S. Evidence That Halogenated Furanones from Delisea pulchra Inhibit Acylated Homoserine Lactone (AHL)-Mediated Gene Expression by Displacing the AHL Signal from Its Receptor Protein. Microbiology 1999, 145 Pt 2, 283–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasmussen, T.B.; Manefield, M.; Andersen, J.B.; Eberl, L.; Anthoni, U.; Christophersen, C.; Steinberg, P.; Kjelleberg, S.; Givskov, M. How Delisea pulchra Furanones Affect Quorum Sensing and Swarming Motility in Serratia Liquefaciens MG1. Microbiology 2000, 146, 3237–3244. [Google Scholar] [CrossRef] [Green Version]
- Ren, D.; Sims, J.J.; Wood, T.K. Inhibition of Biofilm Formation and Swarming of Escherichia coli by (5Z)-4-Bromo-5-(Bromomethylene)-3-Butyl-2(5H)-Furanone. Environ. Microbiol. 2001, 3, 731–736. [Google Scholar] [CrossRef]
- Hentzer, M.; Riedel, K.; Rasmussen, T.B.; Heydorn, A.; Andersen, J.B.; Parsek, M.R.; Rice, S.A.; Eberl, L.; Molin, S.; Høiby, N.; et al. Inhibition of Quorum Sensing in Pseudomonas aeruginosa Biofilm Bacteria by a Halogenated Furanone Compound. Microbiology 2002, 148, 87–102. [Google Scholar] [CrossRef] [Green Version]
- Shetye, G.S.; Singh, N.; Gao, X.; Bandyopadhyay, D.; Yan, A.; Luk, Y.Y. Structures and Biofilm Inhibition Activities of Brominated Furanones for Escherichia coli and Pseudomonas aeruginosa. MedChemComm 2013, 4, 1079–1084. [Google Scholar] [CrossRef]
- Yang, S.; Abdel-Razek, O.A.; Cheng, F.; Bandyopadhyay, D.; Shetye, G.S.; Wang, G.; Luk, Y.Y. Bicyclic Brominated Furanones: A New Class of Quorum Sensing Modulators That Inhibit Bacterial Biofilm Formation. Bioorg. Med. Chem. 2014, 22, 1313–1317. [Google Scholar] [CrossRef]
- Vestby, L.K.; Johannesen, K.C.S.; Witsø, I.L.; Habimana, O.; Scheie, A.A.; Urdahl, A.M.; Benneche, T.; Langsrud, S.; Nesse, L.L. Synthetic Brominated Furanone F202 Prevents Biofilm Formation by Potentially Human Pathogenic Escherichia coli O103:H2 and Salmonella Ser. Agona on Abiotic Surfaces. J. Appl. Microbiol. 2014, 116, 258–268. [Google Scholar] [CrossRef] [Green Version]
- Witsø, I.L.; Benneche, T.; Vestby, L.K.; Nesse, L.L.; Lönn-Stensrud, J.; Scheie, A.A. Thiophenone and Furanone in Control of Escherichia coli O103:H2 Virulence. Pathog. Dis. 2014, 70, 297–306. [Google Scholar] [CrossRef] [Green Version]
- Christensen, L.D.; Moser, C.; Jensen, P.Ø.; Rasmussen, T.B.; Christophersen, L.; Kjelleberg, S.; Kumar, N.; Høiby, N.; Givskov, M.; Bjarnsholt, T. Impact of Pseudomonas aeruginosa Quorum Sensing on Biofilm Persistence in an in vivo Intraperitoneal Foreign-Body Infection Model. Microbiology 2007, 153, 2312–2320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juárez-Rodríguez, M.M.; Cortes-López, H.; García-Contreras, R.; González-Pedrajo, B.; Díaz-Guerrero, M.; Martínez-Vázquez, M.; Rivera-Chávez, J.A.; Soto-Hernández, R.M.; Castillo-Juárez, I. Tetradecanoic Acids With Anti-Virulence Properties Increase the Pathogenicity of Pseudomonas Aeruginosa in a Murine Cutaneous Infection Model. Front. Cell. Infect. Microbiol. 2021, 10, 597517. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Cázares, N.; Castillo-Juárez, I.; García-Contreras, R.; Castro-Torres, V.A.; Díaz-Guerrero, M.; Rodríguez-Zavala, J.S.; Quezada, H.; González-Pedrajo, B.; Martínez-Vázquez, M. A Brominated Furanone Inhibits Pseudomonas aeruginosa Quorum Sensing and Type III Secretion, Attenuating Its Virulence in a Murine Cutaneous Abscess Model. Biomedicines 2022, 10, 1847. https://doi.org/10.3390/biomedicines10081847
Muñoz-Cázares N, Castillo-Juárez I, García-Contreras R, Castro-Torres VA, Díaz-Guerrero M, Rodríguez-Zavala JS, Quezada H, González-Pedrajo B, Martínez-Vázquez M. A Brominated Furanone Inhibits Pseudomonas aeruginosa Quorum Sensing and Type III Secretion, Attenuating Its Virulence in a Murine Cutaneous Abscess Model. Biomedicines. 2022; 10(8):1847. https://doi.org/10.3390/biomedicines10081847
Chicago/Turabian StyleMuñoz-Cázares, Naybi, Israel Castillo-Juárez, Rodolfo García-Contreras, Víctor Alberto Castro-Torres, Miguel Díaz-Guerrero, José S. Rodríguez-Zavala, Héctor Quezada, Bertha González-Pedrajo, and Mariano Martínez-Vázquez. 2022. "A Brominated Furanone Inhibits Pseudomonas aeruginosa Quorum Sensing and Type III Secretion, Attenuating Its Virulence in a Murine Cutaneous Abscess Model" Biomedicines 10, no. 8: 1847. https://doi.org/10.3390/biomedicines10081847





