Single and Repeated Exposure to Cannabidiol Differently Modulate BDNF Expression and Signaling in the Cortico-Striatal Brain Network
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Drug Preparation
2.3. Experiments
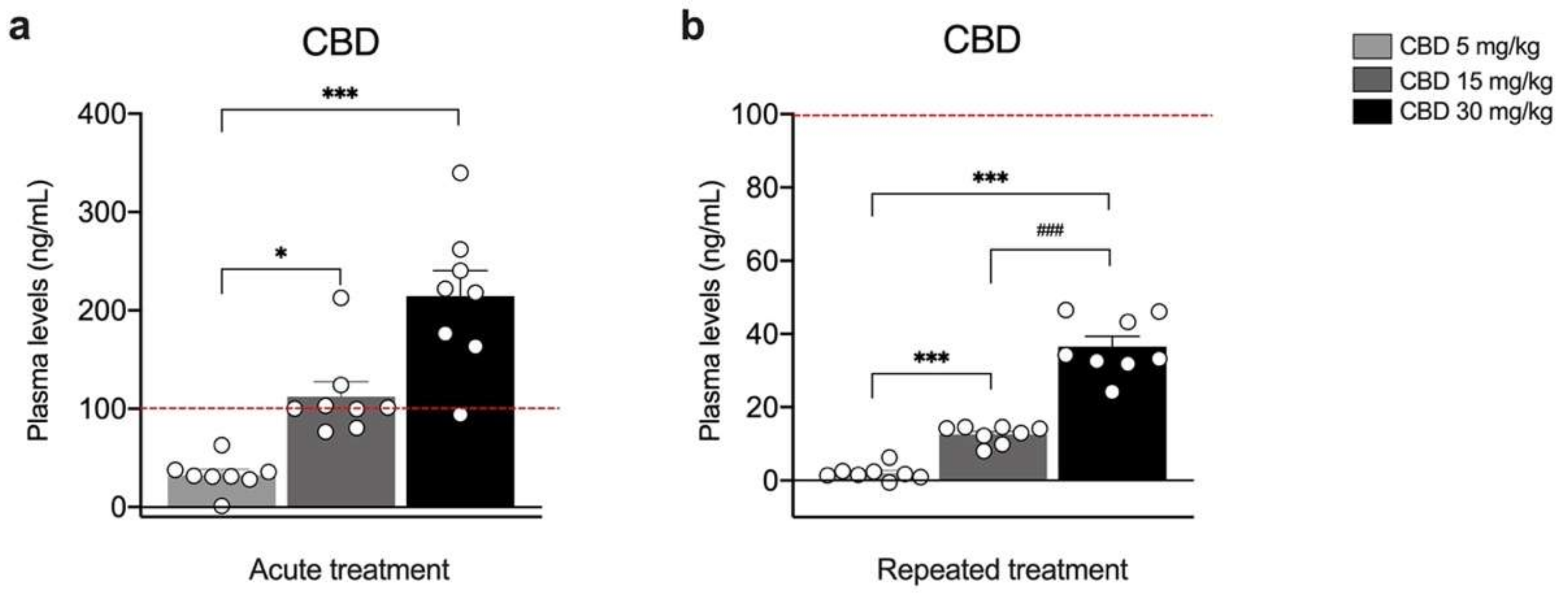
2.4. Plasma Preparation for CBD Measurements
2.5. Brain Tissue Preparation for CBD Measurements
2.6. LC–MS/MS Analysis
2.7. RNA Preparation and Real-Time Polymerase Chain Reaction
- total Bdnf: forward primer 5′-AAGTCTGCATTACATTCCTCGA-3′, reverse primer 5′-GTTTTCTGAAAGAGGGACAGTTTAT-3′, probe 5′- TGTGGTTTGTTGCCGTTGCCAAG-3′;
- 36B4: forward primer 5′-TTCCCACTGGCTGAAAAGGT-3′, reverse primer 5′-CGCAGCCGCAAATGC-3′, probe 5′-AAGGCCTTCCTGGCC GATCCATC-3′.
2.8. Protein Extracts Preparation and Western Blot Analyses
2.9. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Russo, E.B.; Marcu, J. Cannabis Pharmacology: The Usual Suspects and a Few Promising Leads. Adv. Pharmacol. 2017, 80, 67–134. [Google Scholar] [CrossRef] [PubMed]
- Zuardi, A.W.; Shirakawa, I.; Finkelfarb, E.; Karniol, I.G. Action of cannabidiol on the anxiety and other effects produced by delta 9-THC in normal subjects. Psychopharmacology 1982, 76, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Zuardi, A.W.; Morais, S.L.; Guimaraes, F.S.; Mechoulam, R. Antipsychotic effect of cannabidiol. J. Clin. Psychiatry 1995, 56, 485–486. [Google Scholar] [PubMed]
- Izzo, A.A.; Borrelli, F.; Capasso, R.; Di Marzo, V.; Mechoulam, R. Non-psychotropic plant cannabinoids: New therapeutic opportunities from an ancient herb. Trends Pharmacol. Sci. 2009, 30, 515–527. [Google Scholar] [CrossRef]
- Campos, A.C.; Moreira, F.A.; Gomes, F.V.; Del Bel, E.A.; Guimaraes, F.S. Multiple mechanisms involved in the large-spectrum therapeutic potential of cannabidiol in psychiatric disorders. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 3364–3378. [Google Scholar] [CrossRef]
- Thomas, B.F.; Gilliam, A.F.; Burch, D.F.; Roche, M.J.; Seltzman, H.H. Comparative receptor binding analyses of cannabinoid agonists and antagonists. J. Pharmacol. Exp. Ther. 1998, 285, 285–292. [Google Scholar]
- Laprairie, R.B.; Bagher, A.M.; Kelly, M.E.; Denovan-Wright, E.M. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br. J. Pharmacol. 2015, 172, 4790–4805. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Pinilla, E.; Varani, K.; Reyes-Resina, I.; Angelats, E.; Vincenzi, F.; Ferreiro-Vera, C.; Oyarzabal, J.; Canela, E.I.; Lanciego, J.L.; Nadal, X.; et al. Binding and Signaling Studies Disclose a Potential Allosteric Site for Cannabidiol in Cannabinoid CB2 Receptors. Front. Pharmacol. 2017, 8, 744. [Google Scholar] [CrossRef]
- Bisogno, T.; Hanus, L.; De Petrocellis, L.; Tchilibon, S.; Ponde, D.E.; Brandi, I.; Moriello, A.S.; Davis, J.B.; Mechoulam, R.; Di Marzo, V. Molecular targets for cannabidiol and its synthetic analogues: Effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br. J. Pharmacol. 2001, 134, 845–852. [Google Scholar] [CrossRef]
- Russo, E.B.; Burnett, A.; Hall, B.; Parker, K.K. Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem. Res. 2005, 30, 1037–1043. [Google Scholar] [CrossRef]
- O’Sullivan, S.E. An update on PPAR activation by cannabinoids. Br. J. Pharmacol. 2016, 173, 1899–1910. [Google Scholar] [CrossRef] [Green Version]
- Friedman, D.; French, J.A.; Maccarrone, M. Safety, efficacy, and mechanisms of action of cannabinoids in neurological disorders. Lancet Neurol. 2019, 18, 504–512. [Google Scholar] [CrossRef]
- Gaston, T.E.; Friedman, D. Pharmacology of cannabinoids in the treatment of epilepsy. Epilepsy Behav. 2017, 70, 313–318. [Google Scholar] [CrossRef]
- Bramham, C.R.; Messaoudi, E. BDNF function in adult synaptic plasticity: The synaptic consolidation hypothesis. Prog. Neurobiol. 2005, 76, 99–125. [Google Scholar] [CrossRef]
- Nagappan, G.; Lu, B. Activity-dependent modulation of the BDNF receptor TrkB: Mechanisms and implications. Trends Neurosci. 2005, 28, 464–471. [Google Scholar] [CrossRef]
- Wang, C.S.; Kavalali, E.T.; Monteggia, L.M. BDNF signaling in context: From synaptic regulation to psychiatric disorders. Cell 2022, 185, 62–76. [Google Scholar] [CrossRef]
- Caffino, L.; Mottarlini, F.; Fumagalli, F. Born to Protect: Leveraging BDNF Against Cognitive Deficit in Alzheimer’s Disease. CNS Drugs 2020, 34, 281–297. [Google Scholar] [CrossRef]
- Aid, T.; Kazantseva, A.; Piirsoo, M.; Palm, K.; Timmusk, T. Mouse and rat BDNF gene structure and expression revisited. J. Neurosci. Res. 2007, 85, 525–535. [Google Scholar] [CrossRef]
- Santi, S.; Cappello, S.; Riccio, M.; Bergami, M.; Aicardi, G.; Schenk, U.; Matteoli, M.; Canossa, M. Hippocampal neurons recycle BDNF for activity-dependent secretion and LTP maintenance. EMBO J. 2006, 25, 4372–4380. [Google Scholar] [CrossRef] [Green Version]
- Simonato, M.; Tongiorgi, E.; Kokaia, M. Angels and demons: Neurotrophic factors and epilepsy. Trends Pharmacol. Sci. 2006, 27, 631–638. [Google Scholar] [CrossRef]
- Altar, C.A.; Cai, N.; Bliven, T.; Juhasz, M.; Conner, J.M.; Acheson, A.L.; Lindsay, R.M.; Wiegand, S.J. Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature 1997, 389, 856–860. [Google Scholar] [CrossRef]
- Conner, J.M.; Lauterborn, J.C.; Yan, Q.; Gall, C.M.; Varon, S. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: Evidence for anterograde axonal transport. J. Neurosci. 1997, 17, 2295–2313. [Google Scholar] [CrossRef] [Green Version]
- Sangiovanni, E.; Brivio, P.; Dell’Agli, M.; Calabrese, F. Botanicals as Modulators of Neuroplasticity: Focus on BDNF. Neural Plast. 2017, 2017, 5965371. [Google Scholar] [CrossRef] [Green Version]
- Sales, A.J.; Fogaca, M.V.; Sartim, A.G.; Pereira, V.S.; Wegener, G.; Guimaraes, F.S.; Joca, S.R.L. Cannabidiol Induces Rapid and Sustained Antidepressant-Like Effects Through Increased BDNF Signaling and Synaptogenesis in the Prefrontal Cortex. Mol. Neurobiol. 2019, 56, 1070–1081. [Google Scholar] [CrossRef]
- Peres, F.F.; Diana, M.C.; Levin, R.; Suiama, M.A.; Almeida, V.; Vendramini, A.M.; Santos, C.M.; Zuardi, A.W.; Hallak, J.E.C.; Crippa, J.A.; et al. Cannabidiol Administered During Peri-Adolescence Prevents Behavioral Abnormalities in an Animal Model of Schizophrenia. Front. Pharmacol. 2018, 9, 901. [Google Scholar] [CrossRef] [Green Version]
- Galaj, E.; Xi, Z.X. Possible Receptor Mechanisms Underlying Cannabidiol Effects on Addictive-like Behaviors in Experimental Animals. Int. J. Mol. Sci. 2020, 22, 134. [Google Scholar] [CrossRef]
- Mori, M.A.; Meyer, E.; Soares, L.M.; Milani, H.; Guimaraes, F.S.; de Oliveira, R.M.W. Cannabidiol reduces neuroinflammation and promotes neuroplasticity and functional recovery after brain ischemia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2017, 75, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Watt, G.; Shang, K.; Zieba, J.; Olaya, J.; Li, H.; Garner, B.; Karl, T. Chronic Treatment with 50 mg/kg Cannabidiol Improves Cognition and Moderately Reduces Abeta40 Levels in 12-Month-Old Male AbetaPPswe/PS1DeltaE9 Transgenic Mice. J. Alzheimers Dis. 2020, 74, 937–950. [Google Scholar] [CrossRef] [PubMed]
- Chapman, R.H.; Stern, J.M. Maternal stress and pituitary-adrenal manipulations during pregnancy in rats: Effects on morphology and sexual behavior of male offspring. J. Comp. Physiol. Psychol. 1978, 92, 1074–1083. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academy of Sciences: Washington, DC, USA, 2011. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 7th ed.; Eselvier: Amsterdam, The Netherlands, 2013; p. 472. [Google Scholar]
- Ravula, A.; Chandasana, H.; Setlow, B.; Febo, M.; Bruijnzeel, A.W.; Derendorf, H. Simultaneous quantification of cannabinoids tetrahydrocannabinol, cannabidiol and CB1 receptor antagonist in rat plasma: An application to characterize pharmacokinetics after passive cannabis smoke inhalation and co-administration of rimonabant. J. Pharm. Biomed. Anal. 2018, 160, 119–125. [Google Scholar] [CrossRef]
- Caffino, L.; Giannotti, G.; Malpighi, C.; Racagni, G.; Fumagalli, F. Short-term withdrawal from developmental exposure to cocaine activates the glucocorticoid receptor and alters spine dynamics. Eur. Neuropsychopharmacol. 2015, 25, 1832–1841. [Google Scholar] [CrossRef]
- Caffino, L.; Piva, A.; Giannotti, G.; Di Chio, M.; Mottarlini, F.; Venniro, M.; Yew, D.T.; Chiamulera, C.; Fumagalli, F. Ketamine Self-Administration Reduces the Homeostasis of the Glutamate Synapse in the Rat Brain. Mol. Neurobiol. 2017, 54, 7186–7193. [Google Scholar] [CrossRef]
- Caffino, L.; Verheij, M.M.M.; Roversi, K.; Targa, G.; Mottarlini, F.; Popik, P.; Nikiforuk, A.; Golebiowska, J.; Fumagalli, F.; Homberg, J.R. Hypersensitivity to amphetamine’s psychomotor and reinforcing effects in serotonin transporter knockout rats: Glutamate in the nucleus accumbens. Br. J. Pharmacol. 2020, 177, 4532–4547. [Google Scholar] [CrossRef]
- Hlozek, T.; Uttl, L.; Kaderabek, L.; Balikova, M.; Lhotkova, E.; Horsley, R.R.; Novakova, P.; Sichova, K.; Stefkova, K.; Tyls, F.; et al. Pharmacokinetic and behavioural profile of THC, CBD, and THC+CBD combination after pulmonary, oral, and subcutaneous administration in rats and confirmation of conversion in vivo of CBD to THC. Eur. Neuropsychopharmacol. 2017, 27, 1223–1237. [Google Scholar] [CrossRef]
- Brzozowska, N.; Li, K.M.; Wang, X.S.; Booth, J.; Stuart, J.; McGregor, I.S.; Arnold, J.C. ABC transporters P-gp and Bcrp do not limit the brain uptake of the novel antipsychotic and anticonvulsant drug cannabidiol in mice. PeerJ 2016, 4, e2081. [Google Scholar] [CrossRef]
- Deiana, S.; Watanabe, A.; Yamasaki, Y.; Amada, N.; Arthur, M.; Fleming, S.; Woodcock, H.; Dorward, P.; Pigliacampo, B.; Close, S.; et al. Plasma and brain pharmacokinetic profile of cannabidiol (CBD), cannabidivarine (CBDV), Delta(9)-tetrahydrocannabivarin (THCV) and cannabigerol (CBG) in rats and mice following oral and intraperitoneal administration and CBD action on obsessive-compulsive behaviour. Psychopharmacology 2012, 219, 859–873. [Google Scholar] [CrossRef]
- De Foubert, G.; Carney, S.L.; Robinson, C.S.; Destexhe, E.J.; Tomlinson, R.; Hicks, C.A.; Murray, T.K.; Gaillard, J.P.; Deville, C.; Xhenseval, V.; et al. Fluoxetine-induced change in rat brain expression of brain-derived neurotrophic factor varies depending on length of treatment. Neuroscience 2004, 128, 597–604. [Google Scholar] [CrossRef]
- Coppell, A.L.; Pei, Q.; Zetterstrom, T.S. Bi-phasic change in BDNF gene expression following antidepressant drug treatment. Neuropharmacology 2003, 44, 903–910. [Google Scholar] [CrossRef]
- Caffino, L.; Di Chio, M.; Giannotti, G.; Venniro, M.; Mutti, A.; Padovani, L.; Cheung, D.; Fumagalli, G.F.; Yew, D.T.; Fumagalli, F.; et al. The modulation of BDNF expression and signalling dissects the antidepressant from the reinforcing properties of ketamine: Effects of single infusion vs. chronic self-administration in rats. Pharmacol. Res. 2016, 104, 22–30. [Google Scholar] [CrossRef]
- Lucas, C.J.; Galettis, P.; Schneider, J. The pharmacokinetics and the pharmacodynamics of cannabinoids. Br. J. Clin. Pharmacol. 2018, 84, 2477–2482. [Google Scholar] [CrossRef] [Green Version]
- Ujvary, I.; Hanus, L. Human Metabolites of Cannabidiol: A Review on Their Formation, Biological Activity, and Relevance in Therapy. Cannabis Cannabinoid. Res. 2016, 1, 90–101. [Google Scholar] [CrossRef] [Green Version]
- Fumagalli, F.; Caffino, L.; Racagni, G.; Riva, M.A. Repeated stress prevents cocaine-induced activation of BDNF signaling in rat prefrontal cortex. Eur. Neuropsychopharmacol. 2009, 19, 402–408. [Google Scholar] [CrossRef]
- Brivio, P.; Sbrini, G.; Riva, M.A.; Calabrese, F. Acute Stress Induces Cognitive Improvement in the Novel Object Recognition Task by Transiently Modulating Bdnf in the Prefrontal Cortex of Male Rats. Cell Mol. Neurobiol. 2020, 40, 1037–1047. [Google Scholar] [CrossRef] [Green Version]
- Grimm, O.; Loffler, M.; Kamping, S.; Hartmann, A.; Rohleder, C.; Leweke, M.; Flor, H. Probing the endocannabinoid system in healthy volunteers: Cannabidiol alters fronto-striatal resting-state connectivity. Eur. Neuropsychopharmacol. 2018, 28, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Zuccato, C.; Ciammola, A.; Rigamonti, D.; Leavitt, B.R.; Goffredo, D.; Conti, L.; MacDonald, M.E.; Friedlander, R.M.; Silani, V.; Hayden, M.R.; et al. Loss of huntingtin-mediated BDNF gene transcription in Huntington’s disease. Science 2001, 293, 493–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizuno, K.; Carnahan, J.; Nawa, H. Brain-derived neurotrophic factor promotes differentiation of striatal GABAergic neurons. Dev. Biol. 1994, 165, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, K.; Miwa, H.; Kakizaki, T.; Kaneko, R.; Mikuni, M.; Tanahira, C.; Tamamaki, N.; Yanagawa, Y. Glutamate Decarboxylase 67 Deficiency in a Subset of GABAergic Neurons Induces Schizophrenia-Related Phenotypes. Neuropsychopharmacology 2015, 40, 2475–2486. [Google Scholar] [CrossRef] [Green Version]
- Behrens, M.M.; Ali, S.S.; Dao, D.N.; Lucero, J.; Shekhtman, G.; Quick, K.L.; Dugan, L.L. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science 2007, 318, 1645–1647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, D.A.; Hashimoto, T.; Volk, D.W. Cortical inhibitory neurons and schizophrenia. Nat. Rev. Neurosci. 2005, 6, 312–324. [Google Scholar] [CrossRef] [PubMed]









| Time (min) | Flow (mL/min) | % A | % B |
|---|---|---|---|
| 0 | 0.8 | 40 | 60 |
| 1 | 0.8 | 40 | 60 |
| 10 | 0.8 | 0 | 100 |
| 12 | 0.8 | 0 | 100 |
| 12.10 | 0.8 | 40 | 60 |
| 17 | 0.8 | 40 | 60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mottarlini, F.; Fumagalli, M.; Castillo-Díaz, F.; Piazza, S.; Targa, G.; Sangiovanni, E.; Pacchetti, B.; Sodergren, M.H.; Dell’Agli, M.; Fumagalli, F.; et al. Single and Repeated Exposure to Cannabidiol Differently Modulate BDNF Expression and Signaling in the Cortico-Striatal Brain Network. Biomedicines 2022, 10, 1853. https://doi.org/10.3390/biomedicines10081853
Mottarlini F, Fumagalli M, Castillo-Díaz F, Piazza S, Targa G, Sangiovanni E, Pacchetti B, Sodergren MH, Dell’Agli M, Fumagalli F, et al. Single and Repeated Exposure to Cannabidiol Differently Modulate BDNF Expression and Signaling in the Cortico-Striatal Brain Network. Biomedicines. 2022; 10(8):1853. https://doi.org/10.3390/biomedicines10081853
Chicago/Turabian StyleMottarlini, Francesca, Marco Fumagalli, Fernando Castillo-Díaz, Stefano Piazza, Giorgia Targa, Enrico Sangiovanni, Barbara Pacchetti, Mikael H. Sodergren, Mario Dell’Agli, Fabio Fumagalli, and et al. 2022. "Single and Repeated Exposure to Cannabidiol Differently Modulate BDNF Expression and Signaling in the Cortico-Striatal Brain Network" Biomedicines 10, no. 8: 1853. https://doi.org/10.3390/biomedicines10081853
APA StyleMottarlini, F., Fumagalli, M., Castillo-Díaz, F., Piazza, S., Targa, G., Sangiovanni, E., Pacchetti, B., Sodergren, M. H., Dell’Agli, M., Fumagalli, F., & Caffino, L. (2022). Single and Repeated Exposure to Cannabidiol Differently Modulate BDNF Expression and Signaling in the Cortico-Striatal Brain Network. Biomedicines, 10(8), 1853. https://doi.org/10.3390/biomedicines10081853








