G-Quadruplex Formed by the Promoter Region of the hTERT Gene: Structure-Driven Effects on DNA Mismatch Repair Functions
Abstract
1. Introduction
2. Materials and Methods
2.1. DNA Oligonucleotides
2.2. Preparation of Intramolecular G4 Structures and DNA Duplexes
2.3. Purification of Recombinant Proteins
2.4. A Chemical Probing Assay
2.5. UV Melting Experiments
2.6. Circular Dichroism Measurements
2.7. DNA-Binding Activity of ecMutS
2.8. DNA-Binding Activity of ecMutL and ngMutL
2.9. Hydrolysis of DNA by ngMutL
2.10. Bioinformatic Analysis of MutS and MutL Sequence and Structure
3. Results
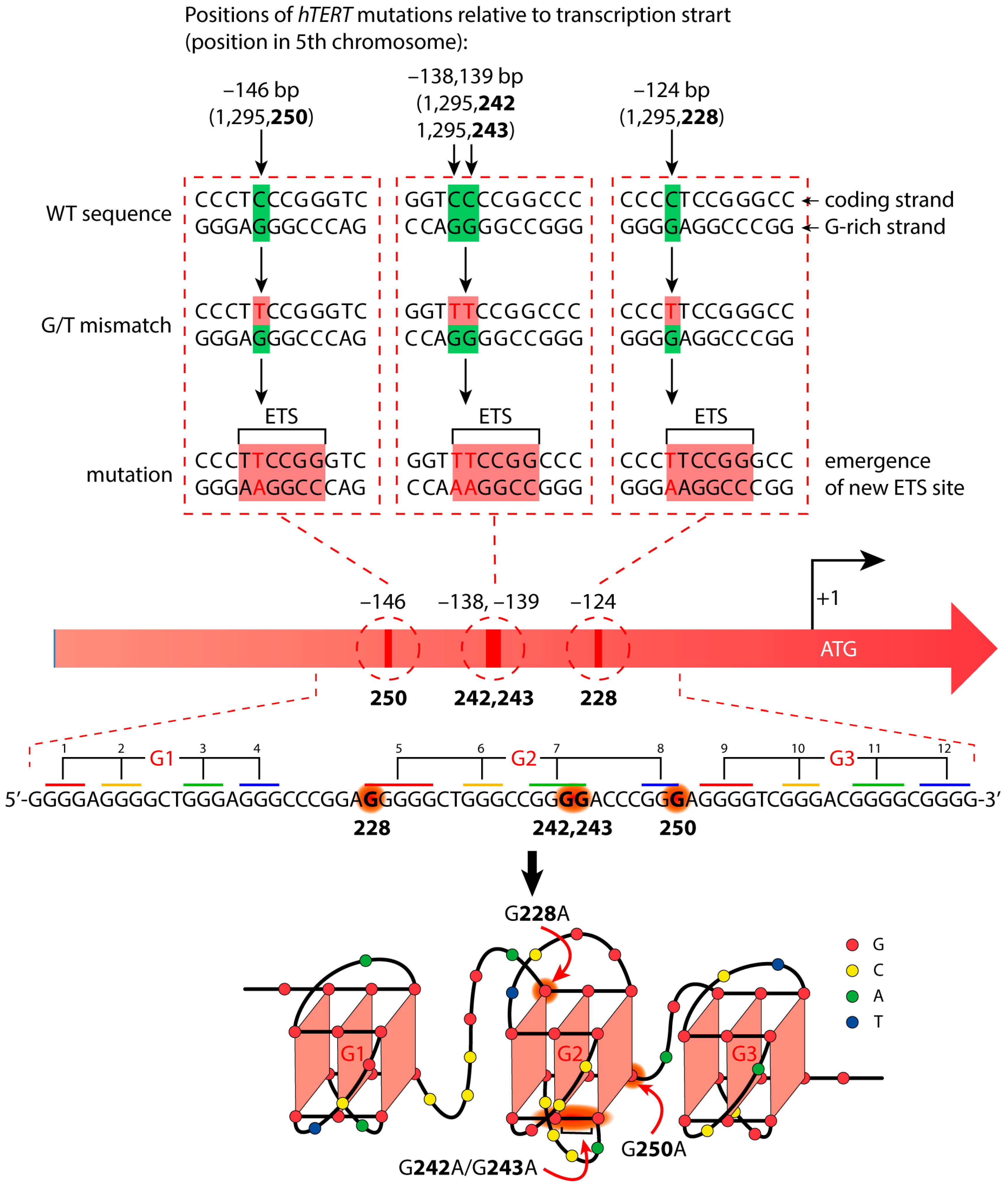
3.1. Design of the DNA Models
3.2. Thermal Stability and Topology of the G4 Structure Formed by the WT hTERT Promoter Region
3.3. Chemical Probing Assays of WT and Altered hTERT G4 Structures
3.4. The Structural Similarity of Prokaryotic MutS and MutL with Eukaryotic Homologs
3.5. Interaction of ecMutS and ecMutL with the WT hTERT G4 and Its G>A Substituted Analogs
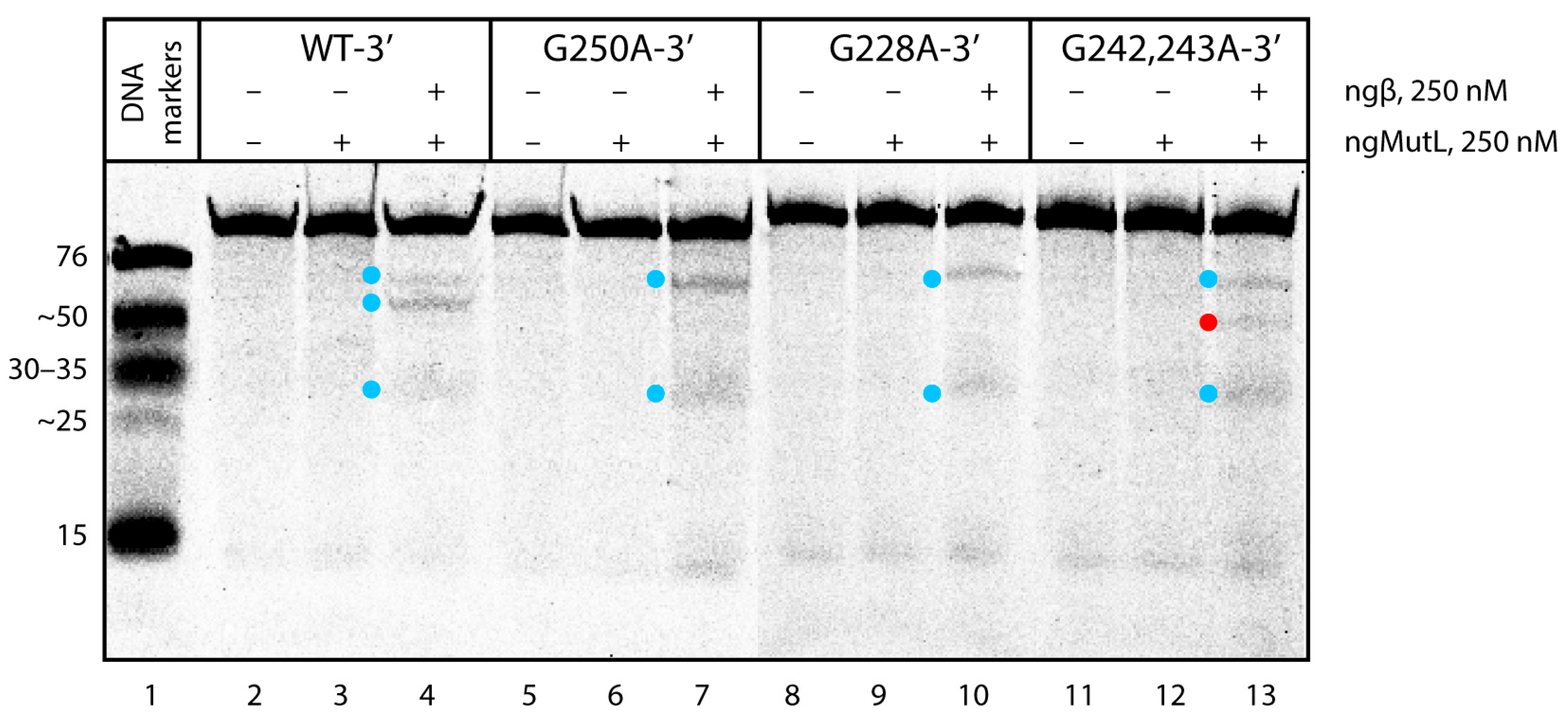
3.6. Binding Affinity of ngMutL to the WT hTERT G4 and Its Altered Variants; ngMutL-Mediated Processing of DNA Substrates Containing Multiquadruplex Structures
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jafri, M.A.; Ansari, S.A.; Alqahtani, M.H.; Shay, J.W. Roles of Telomeres and Telomerase in Cancer, and Advances in Telomerase-Targeted Therapies. Genome Med. 2016, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Roizen, M.F. Hallmarks of Cancer: The Next Generation. Yearb. Anesthesiol. Pain Manag. 2012, 2012, 13. [Google Scholar] [CrossRef]
- Kim, N.W.; Piatyszek, M.A.; Prowse, K.R.; Harley, C.B.; West, M.D.; Ho, P.L.C.; Coviello, G.M.; Wright, W.E.; Weinrich, S.L.; Shay, J.W. Specific Association of Human Telomerase Activity with Immortal Cells and Cancer. Science 1994, 266, 2011–2015. [Google Scholar] [CrossRef]
- Hiyama, E.; Hiyama, K. Telomere and Telomerase in Stem Cells. Br. J. Cancer 2007, 96, 1020–1024. [Google Scholar] [CrossRef] [PubMed]
- Vinagre, J.; Almeida, A.; Pópulo, H.; Batista, R.; Lyra, J.; Pinto, V.; Coelho, R.; Celestino, R.; Prazeres, H.; Lima, L.; et al. Frequency of TERT Promoter Mutations in Human Cancers. Nat. Commun. 2013, 4, 2185. [Google Scholar] [CrossRef] [PubMed]
- Rachakonda, S.; Hoheisel, J.D.; Kumar, R. Occurrence, Functionality and Abundance of the TERT Promoter Mutations. Int. J. Cancer 2021, 149, 1852–1862. [Google Scholar] [CrossRef] [PubMed]
- Borah, S.; Xi, L.; Zaug, A.J.; Powell, N.M.; Dancik, G.M.; Cohen, S.B.; Costello, J.C.; Theodorescu, D.; Cech, T.R. TERT Promoter Mutations and Telomerase Reactivation in Urothelial Cancer. Science 2015, 347, 1006–1010. [Google Scholar] [CrossRef]
- Huang, F.W.; Hodis, E.; Xu, M.J.; Kryukov, G.V.; Chin, L.; Garraway, L.A. Highly Recurrent TERT Promoter Mutations in Human Melanoma. Science 2013, 339, 957–959. [Google Scholar] [CrossRef]
- Killela, P.J.; Reitman, Z.J.; Jiao, Y.; Bettegowda, C.; Agrawal, N.; Diaz, L.A.; Friedman, A.H.; Friedman, H.; Gallia, G.L.; Giovanella, B.C.; et al. TERT Promoter Mutations Occur Frequently in Gliomas and a Subset of Tumors Derived from Cells with Low Rates of Self-Renewal. Proc. Natl. Acad. Sci. USA 2013, 110, 6021–6026. [Google Scholar] [CrossRef] [PubMed]
- Schofield, M.J.; Hsieh, P. DNA Mismatch Repair: Molecular Mechanisms and Biological Function. Annu. Rev. Microbiol. 2003, 57, 579–608. [Google Scholar] [CrossRef]
- Bell, R.J.A.; Rube, H.T.; Kreig, A.; Mancini, A.; Fouse, S.D.; Nagarajan, R.P.; Choi, S.; Hong, C.; He, D.; Pekmezci, M.; et al. The Transcription Factor GABP Selectively Binds and Activates the Mutant TERT Promoter in Cancer. Science 2015, 348, 1036–1039. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Hurley, L.H. Structures, folding patterns, and functions of intramolecular DNA G-quadruplexes found in eukaryotic promoter regions. Biochimie 2008, 90, 1149–1171. [Google Scholar] [CrossRef] [PubMed]
- Monsen, R.C.; DeLeeuw, L.W.; Dean, W.L.; Gray, R.D.; Chakravarthy, S.; Hopkins, J.B.; Chaires, J.B.; Trent, J.O. Long Promoter Sequences Form Higher-Order G-Quadruplexes: An Integrative Structural Biology Study of c-Myc, k-Ras and c-Kit Promoter Sequences. Nucleic Acids Res. 2022, 50, 4127–4147. [Google Scholar] [CrossRef] [PubMed]
- Henderson, A.; Wu, Y.; Huang, Y.C.; Chavez, E.A.; Platt, J.; Johnson, F.B.; Brosh, R.M.; Sen, D.; Lansdorp, P.M. Detection of G-Quadruplex DNA in Mammalian Cells. Nucleic Acids Res. 2014, 42, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Tippana, R.; Xiao, W.; Myong, S. G-Quadruplex Conformation and Dynamics are Determined by Loop Length and Sequence. Nucleic Acids Res. 2014, 42, 8106–8114. [Google Scholar] [CrossRef]
- Palumbo, S.L.; Ebbinghaus, S.W.; Hurley, L.H. Formation of a Unique End-to-End Stacked Pair of G-Quadruplexes in the hTERT Core Promoter with Implications for Inhibition of Telomerase by G-Quadruplex-Interactive Ligands. J. Am. Chem. Soc. 2009, 131, 10878–10891. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.W.; Lacroix, L.; Yue, D.J.E.; Lim, J.K.C.; Lim, J.M.W.; Phan, A.T. Coexistence of Two Distinct G-Quadruplex Conformations in the hTERT Promoter. J. Am. Chem. Soc. 2010, 132, 12331–12342. [Google Scholar] [CrossRef] [PubMed]
- Selvam, S.; Yu, Z.; Mao, H. Exploded View of Higher Order G-Quadruplex Structures Through Click-Chemistry Assisted Single-Molecule aMechanical Unfolding. Nucleic Acids Res. 2016, 44, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Chaires, J.B.; Trent, J.O.; Gray, R.D.; Dean, W.L.; Buscaglia, R.; Thomas, S.D.; Miller, D.M. An Improved Model for the hTERT Promoter Quadruplex. PLoS ONE 2014, 9, e115580. [Google Scholar] [CrossRef] [PubMed]
- Sissi, C.; Gatto, B.; Palumbo, M. The Evolving World of Protein-G-Quadruplex Recognition: A Medicinal Chemist’s Perspective. Biochimie 2011, 93, 1219–1230. [Google Scholar] [CrossRef]
- Rhodes, D.; Lipps, H.J. G-quadruplexes and Their Regulatory Roles in Biology. Nucleic Acids Res. 2015, 43, 8627–8637. [Google Scholar] [CrossRef] [PubMed]
- Dolinnaya, N.G.; Ogloblina, A.M.; Yakubovskaya, M.G. Structure, Properties, and Biological Relevance of the DNA and RNA G-Quadruplexes: Overview 50 Years After Their Discovery. Biochemistry 2016, 81, 1602–1649. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, A.V.; Kubareva, E.A.; Monakhova, M.V.; Zvereva, M.I.; Dolinnaya, N.G. Impact of G-Quadruplexes on the Regulation of Genome Integrity, DNA Damage and Repair. Biomolecules 2021, 11, 1284. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, S.; Hurley, L.H.; Neidle, S. Targeting G-Quadruplexes in Gene Promoters: A Novel Anticancer Strategy? Nat. Rev. Drug Discov. 2011, 10, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Larson, E.D.; Duquette, M.L.; Cummings, W.J.; Streiff, R.J.; Maizels, N. MutSα Binds to and Promotes Synapsis of Transcriptionally Activated Immunoglobulin Switch Regions. Curr. Biol. 2005, 15, 470–474. [Google Scholar] [CrossRef]
- Ehrat, E.A.; Johnson, B.R.; Williams, J.D.; Borchert, G.M.; Larson, E.D. G-Quadruplex Recognition Activities of E. Coli MutS. BMC Mol. Biol. 2012, 13, 23. [Google Scholar] [CrossRef]
- Pavlova, A.V.; Monakhova, M.V.; Ogloblina, A.M.; Andreeva, N.A.; Laptev, G.Y.; Polshakov, V.I.; Gromova, E.S.; Zvereva, M.I.; Yakubovskaya, M.G.; Oretskaya, T.S.; et al. Responses of DNA Mismatch Repair Proteins to a Stable G-Quadruplex Embedded into a DNA Duplex Structure. Int. J. Mol. Sci. 2020, 21, 8773. [Google Scholar] [CrossRef]
- Fukui, K. DNA Mismatch Repair in Eukaryotes and Bacteria. J. Nucleic Acids 2010, 2010, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Augusto-Pinto, L.; Regis Da Silva, C.G.; De Oliveira Lopes, D.; Machado-Silva, A.; Machado, C.R. Escherichia coli as a Model System to Study DNA Repair Genes of Eukaryotic Organisms. Genet. Mol. Res. 2003, 31, 77–91. [Google Scholar]
- Monakhova, M.V.; Penkina, A.I.; Pavlova, A.V.; Lyaschuk, A.M.; Kucherenko, V.V.; Alexeevski, A.V.; Lunin, V.G.; Friedhoff, P.; Klug, G.; Oretskaya, T.S.; et al. Endonuclease Activity of MutL Protein of the Rhodobacter sphaeroides Mismatch Repair System. Biochemistry 2018, 83, 281–293. [Google Scholar] [CrossRef]
- Putnam, C.D. Strand Discrimination in DNA Mismatch Repair. DNA Repair 2021, 105, 103161. [Google Scholar] [CrossRef] [PubMed]
- OligoAnalyzerTM Tool. Available online: https://www.idtdna.com/calc/analyzer (accessed on 1 June 2022).
- Feng, G.; Winkler, M.E. Single-Step Purifications of His6-MutH, His6-MutL and His6-MutS repair proteins of Escherichia Coli K-12. Biotechniques 1995, 19, 956–965. [Google Scholar] [PubMed]
- Duppatla, V.; Bodda, C.; Urbanke, C.; Friedhoff, P.; Rao, D.N. The C-Terminal Domain is Sufficient for Endonuclease Activity of Neisseria Gonorrhoeae MutL. Biochem. J. 2009, 423, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Pillon, M.C.; Miller, J.H.; Guarné, A. The Endonuclease Domain of MutL Interacts with the β Sliding Clamp. DNA Repair 2011, 10, 87–93. [Google Scholar] [CrossRef]
- ProtParam Tool. Available online: https://web.expasy.org/protparam (accessed on 1 June 2022).
- Pillon, M.C.; Babu, V.M.P.; Randall, J.R.; Cai, J.; Simmons, L.A.; Sutton, M.D.; Guarné, A. The Sliding Clamp Tethers the Endonuclease Domain of MutL to DNA. Nucleic Acids Res. 2015, 43, 10746–10759. [Google Scholar] [CrossRef]
- Sachadyn, P. Conservation and Diversity of MutS Proteins. Mutat. Res. Mol. Mech. Mutagen. 2010, 694, 20–30. [Google Scholar] [CrossRef]
- Banasik, M.; Sachadyn, P. Conserved Motifs of MutL Proteins. Mutat. Res. Mol. Mech. Mutagen. 2014, 769, 69–79. [Google Scholar] [CrossRef]
- EMBOSS Water Pairwise Sequence Alignment. Available online: https://www.ebi.ac.uk/Tools/psa/emboss_water/ (accessed on 1 June 2022).
- Tran, P.L.T.; Mergny, J.-L.; Alberti, P. Stability of Telomeric G-Quadruplexes. Nucleic Acids Res. 2011, 39, 3282–3294. [Google Scholar] [CrossRef]
- Sun, D.; Hurley, L.H. Biochemical Techniques for the Characterization of G-Quadruplex Structures: EMSA, DMS Footprinting, and DNA Polymerase Stop Assay. Methods Mol. Biol. 2010, 608, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Iyer, R.R.; Pluciennik, A.; Burdett, V.; Modrich, P.L. DNA Mismatch Repair: Functions and Mechanisms. Chem. Rev. 2006, 106, 302–323. [Google Scholar] [CrossRef]
- Bjornson, K.P.; Blackwell, L.J.; Sage, H.; Baitinger, C.; Allen, D.; Modrich, P. Assembly and Molecular Activities of the MutS Tetramer. J. Biol. Chem. 2003, 278, 34667–34673. [Google Scholar] [CrossRef] [PubMed]
- Modrich, P. Mechanisms in Eukaryotic Mismatch Repair. J. Biol. Chem. 2006, 281, 30305–30309. [Google Scholar] [CrossRef] [PubMed]
- Friedhoff, P.; Li, P.; Gotthardt, J. Protein-Protein interactions in DNA mismatch repair. DNA Repair 2016, 38, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Junop, M. In vitro and in vivo Studies of MutS, MutL and MutH Mutants: Correlation of Mismatch Repair and DNA Recombination. DNA Repair 2003, 2, 387–405. [Google Scholar] [CrossRef]
- Macpherson, P.; Humbert, O.; Karran, P. Frameshift Mismatch Recognition by the Human MutSα Complex. Mutat. Res. Repair 1998, 408, 55–66. [Google Scholar] [CrossRef]
- Bende, S.M.; Grafström, R.H. The DNA Binding Properties of the MutL Protein Isolated from Escherichia Coli. Nucleic Acids Res. 1991, 19, 1549–1555. [Google Scholar] [CrossRef][Green Version]
- Furman, C.M.; Elbashir, R.; Alani, E. Expanded Roles for the MutL Family of DNA Mismatch Repair Proteins. Yeast 2021, 38, 39–53. [Google Scholar] [CrossRef]
- Monakhova, M.V.; Milakina, M.A.; Savitskaia, V.Y.; Romanova, E.A.; Rao, D.N.; Kubareva, E.A. MutL Protein from the Neisseria gonorrhoeae Mismatch Repair System: Interaction with ATP and DNA. Mol. Biol. 2021, 55, 252–266. [Google Scholar] [CrossRef]
- Namadurai, S.; Jain, D.; Kulkarni, D.S.; Tabib, C.R.; Friedhoff, P.; Rao, D.N.; Nair, D.T. The C-Terminal Domain of the MutL Homolog from Neisseria gonorrhoeae Forms an Inverted Homodimer. PLoS ONE 2010, 5, e13726. [Google Scholar] [CrossRef]
- Sengupta, P.; Chattopadhyay, S.; Chatterjee, S. G-Quadruplex Surveillance in BCL-2 gene: A Promising Therapeutic Intervention in Cancer Treatment. Drug Discov. Today 2017, 22, 1165–1186. [Google Scholar] [CrossRef] [PubMed]
- Huppert, J.L.; Balasubramanian, S. G-Quadruplexes in Promoters Throughout the Human Genome. Nucleic Acids Res. 2007, 35, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Monsen, R.C.; DeLeeuw, L.; Dean, W.L.; Gray, R.D.; Sabo, T.M.; Chakravarthy, S.; Chaires, J.B.; Trent, J.O. The hTERT Core Promoter Forms Three Parallel G-Quadruplexes. Nucleic Acids Res. 2020, 48, 5720–5734. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.-J.; Cui, Y.; Yin, H.; Scheid, A.; Hendricks, W.P.D.; Schmidt, J.; Sekulic, A.; Kong, D.; Trent, J.M.; Gokhale, V.; et al. A Pharmacological Chaperone Molecule Induces Cancer Cell Death by Restoring Tertiary DNA Structures in Mutant hTERT Promoters. J. Am. Chem. Soc. 2016, 138, 13673–13692. [Google Scholar] [CrossRef] [PubMed]
- Dapic, V. Biophysical and Biological Properties of Quadruplex Oligodeoxyribonucleotides. Nucleic Acids Res. 2003, 31, 2097–2107. [Google Scholar] [CrossRef] [PubMed]
- Oliviero, G.; D’Errico, S.; Pinto, B.; Nici, F.; Dardano, P.; Rea, I.; De Stefano, L.; Mayol, L.; Piccialli, G.; Borbone, N. Self-Assembly of G-Rich Oligonucleotides Incorporating a 3′-3′ Inversion of Polarity Site: A New Route Towards G-Wire DNA Nanostructures. ChemistryOpen 2017, 6, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Varizhuk, A.M.; Protopopova, A.D.; Tsvetkov, V.B.; Barinov, N.A.; Podgorsky, V.V.; Tankevich, M.V.; Vlasenok, M.A.; Severov, V.V.; Smirnov, I.P.; Dubrovin, E.V.; et al. Polymorphism of G4 Associates: From Stacks to Wires via Interlocks. Nucleic Acids Res. 2018, 46, 8978–8992. [Google Scholar] [CrossRef]
- Mendoza, O.; Bourdoncle, A.; Boulé, J.B.; Brosh, R.M.; Mergny, J.L. G-Quadruplexes and Helicases. Nucleic Acids Res. 2016, 44, 1989–2006. [Google Scholar] [CrossRef] [PubMed]
- Burra, S.; Marasco, D.; Malfatti, M.C.; Antoniali, G.; Virgilio, A.; Esposito, V.; Demple, B.; Galeone, A.; Tell, G. Human AP-endonuclease (Ape1) Activity on Telomeric G4 Structures Is Modulated by Acetylatable Lysine Residues in the N-Terminal Sequence. DNA Repair 2019, 73, 129–143. [Google Scholar] [CrossRef]
- Modrich, P. Mechanisms And Biological Effects Of Mismatch Repair. Annu. Rev. Genet. 1991, 25, 229–253. [Google Scholar] [CrossRef]
- Fernandez-Leiro, R.; Bhairosing-Kok, D.; Kunetsky, V.; Laffeber, C.; Winterwerp, H.H.; Groothuizen, F.; Fish, A.; Lebbink, J.H.G.; Friedhoff, P.; Sixma, T.K.; et al. The Selection Process of Licensing a DNA Mismatch for Repair. Nat. Struct. Mol. Biol. 2021, 28, 373–381. [Google Scholar] [CrossRef]







| Oligonucleotides | KDapp, nM | |
|---|---|---|
| ecMutS | ecMutL | |
| WT-5′ | 15 ± 6 | 115 ± 30 |
| G228A-5′ | 24 ± 6 | 170 ± 40 |
| G250A-5′ | 30 ± 7 | 130 ± 30 |
| G242,243A-5′ | 27 ± 4 | 110 ± 20 |
| 95G4 | 38 ± 5 | 120 ± 20 |
| DNAs | KDapp, nM |
|---|---|
| WT-3′ | 49 ± 3 |
| G228A-3′ | 44 ± 3 |
| G250A-3′ | 42 ± 2 |
| G242,243A-3′ | 51 ± 2 |
| 95G4 | 41 ± 1 |
| ds96/96* | 125 ± 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavlova, A.V.; Savitskaya, V.Y.; Dolinnaya, N.G.; Monakhova, M.V.; Litvinova, A.V.; Kubareva, E.A.; Zvereva, M.I. G-Quadruplex Formed by the Promoter Region of the hTERT Gene: Structure-Driven Effects on DNA Mismatch Repair Functions. Biomedicines 2022, 10, 1871. https://doi.org/10.3390/biomedicines10081871
Pavlova AV, Savitskaya VY, Dolinnaya NG, Monakhova MV, Litvinova AV, Kubareva EA, Zvereva MI. G-Quadruplex Formed by the Promoter Region of the hTERT Gene: Structure-Driven Effects on DNA Mismatch Repair Functions. Biomedicines. 2022; 10(8):1871. https://doi.org/10.3390/biomedicines10081871
Chicago/Turabian StylePavlova, Anzhela V., Victoria Yu. Savitskaya, Nina G. Dolinnaya, Mayya V. Monakhova, Anastasia V. Litvinova, Elena A. Kubareva, and Maria I. Zvereva. 2022. "G-Quadruplex Formed by the Promoter Region of the hTERT Gene: Structure-Driven Effects on DNA Mismatch Repair Functions" Biomedicines 10, no. 8: 1871. https://doi.org/10.3390/biomedicines10081871
APA StylePavlova, A. V., Savitskaya, V. Y., Dolinnaya, N. G., Monakhova, M. V., Litvinova, A. V., Kubareva, E. A., & Zvereva, M. I. (2022). G-Quadruplex Formed by the Promoter Region of the hTERT Gene: Structure-Driven Effects on DNA Mismatch Repair Functions. Biomedicines, 10(8), 1871. https://doi.org/10.3390/biomedicines10081871






