Effects of Cancer Cell-Derived Nanovesicle Vaccines Produced by the Oxidative Stress-Induced Expression of DAMP and Spontaneous Release/Filter Extrusion in the Interplay of Cancer Cells and Macrophages
Abstract
:1. Introduction
2. Methods
2.1. Cell Culture
2.2. Production of Various Oxidative Stresses
2.3. Collection of Spontaneously-Released Nanovesicles and Production of Nanovesicles via Filter Extrusion as the Vaccine
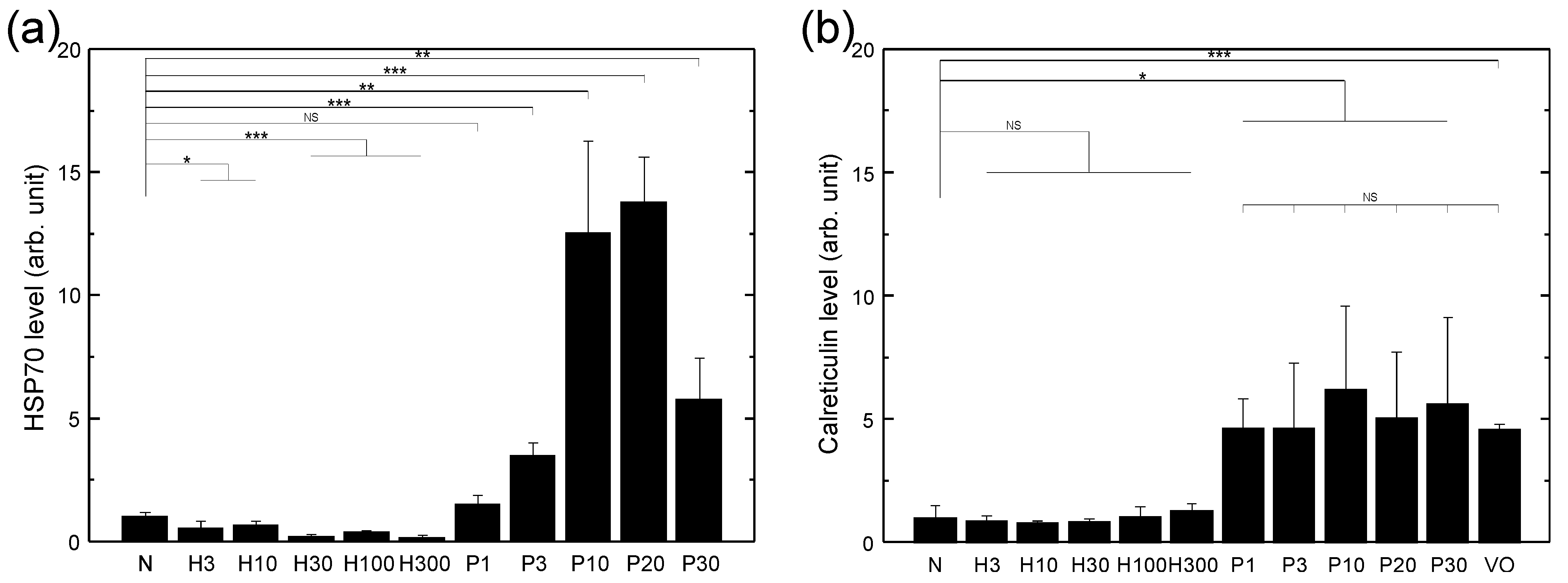
2.4. Characterization of Cell Surface Expression of HSP70 and Calreticulin
2.5. Characterization of Nanovesicles
2.6. Measurement of NO Secretion from Macrophages and Imaging of NO Expression Inside Macrophages
2.7. Characterization of the Mortality of Cancer Cells Co-Cultured with Macrophages and/or Vaccine
2.8. Statistical Analysis
3. Results
3.1. HSP70 Expression of LLC Cells Treated with Various Sources of Oxidative Stress
3.2. Nanovesicles Produced by Spontaneous Release and Filter Extrusion
3.3. NO Secretion and Morphology Change of Macrophages Stimulated by Various Vaccines
3.4. Mortality of LLC Cells Co-Cultured with Macrophages and/or Treated with Various Vaccines
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdel-Kader, M.H. (Ed.) Photodynamic Therapy-from Theory to Application; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Algorri, J.F.; Ochoa, M.; Roldan-Varona, P.; Rodriguez-Cobo, L.; Lopez-Higuera, J.M. Photodynamic therapy: A compendium of latest reviews. Cancers 2021, 13, 4447. [Google Scholar] [CrossRef]
- Garg, A.D.; Krysko, D.V.; Vandenabeele, P.; Agostinis, P. DAMPs and PDT-mediated photo-oxidative stress: Exploring the unknown. Photochem. Photobiol. Sci. 2011, 10, 670–680. [Google Scholar] [CrossRef]
- Al-Akhras, M.A.H.; Amin, A.; Mohammad, K.; AlHaddad, F.; Hamza, A. In vitro studies on the effect of phototoxicity of a new photosensitizer extracted from flowers and aerial parts of Cichorium pumilum. Am. J. Pharmacol. Toxicol. 2007, 2, 39–45. [Google Scholar] [CrossRef]
- Al-Akhras, M.A.H.; Amin, A.; Mohammad, K.; AlHaddad, F.; Hamza, A. Sensitization of photohemolysis by a new extraction from flowers and aerial parts of Cichorium Pumilum Jacq: Effects of inulin and hydrogen peroxide. Am. J. Pharmacol. Toxicol. 2007, 2, 75–79. [Google Scholar] [CrossRef]
- Al-Akhras, M.A.H.; Aljarrah, K.; Al-Khateeb, H.; Jaradat, A.; Al-omari, A.; Al-Nasser, A.; Masadeh, M.M.; Amin, A.; Hamza, A.; Mohammed, K.; et al. Introducing Cichorium Pumilum as a potential therapeutical agent against drug-induced benign breast tumor in rats. Electromagn. Biol. Med. 2012, 31, 299–309. [Google Scholar] [CrossRef]
- Korbelik, M. Cancer vaccines generated by photodynamic therapy. Photochem. Photobiol. Sci. 2011, 10, 664–669. [Google Scholar] [CrossRef]
- Korbelik, M.; Sun, J. Photodynamic therapy-generated vaccine for cancer therapy. Cancer Immunol. Immunother. 2006, 55, 900–909. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, W.; Li, Y. Generation of effective vaccines against liver cancer by using photodynamic therapy. Lasers Med. Sci. 2009, 24, 549–552. [Google Scholar] [CrossRef]
- Tanaka, M.; Kataoka, H.; Yano, S.; Sawada, T.; Akashi, H.; Inoue, M.; Suzuki, S.; Inagaki, Y.; Hayashi, N.; Nishie, H.; et al. Immunogenic cell death due to a new photodynamic therapy (PDT) with glycoconjugated chlorin (G-chlorin). Oncotarget 2016, 7, 47242–47251. [Google Scholar] [CrossRef]
- Gollnick, S.; Vaughan, L.; Henderson, B. Generation of effective antitumor vaccines using photodynamic therapy. Cancer Res. 2002, 62, 1604–1608. [Google Scholar]
- Korbelik, M.; Sun, J.; Cecic, I. Photodynamic therapy-induced cell surface expression and release of heat shock proteins: Relevance for tumor response. Cancer Res. 2005, 65, 1018–1026. [Google Scholar] [CrossRef]
- Vega, V.L.; Rodriguez-Silva, M.; Frey, T.; Gehrmann, M.; Diaz, J.C.; Steinem, C.; Multhoff, G.; Arispe, N.; De Maio, A. Hsp70 translocates into the plasma membrane after stress and is released into the extracellular environment in a membrane-associated form that activates macrophages. J. Immunol. 2008, 180, 4299–4307. [Google Scholar] [CrossRef]
- Aubertin, K.; Silva, A.K.A.; Luciani, N.; Espinosa, A.; Djemat, A.; Charue, D.; Gallet, F.; Blanc-Brude, O.; Wilhelm, C. Massive release of extracellular vesicles from cancer cells after photodynamic treatment or chemotherapy. Sci. Rep. 2016, 6, 35376. [Google Scholar] [CrossRef]
- Segal, B.; Wang, X.; Dennis, C.; Youn, R.; Repasky, E.; Manjili, M.; Subjeck, J. Heat shock proteins as vaccine adjuvants in infections and cancer. Drug Discov. Today 2006, 11, 534–540. [Google Scholar] [CrossRef]
- Xie, Y.; Bai, O.; Zhang, H.; Yuan, J.; Zong, S.; Chibbar, R.; Slattery, K.; Qureshi, M.; Wei, Y.; Deng, Y.; et al. Membrane-bound HSP70-engineered myeloma cell-derived exosomes stimulate more efficient CD8(+) CTL- and NK-mediated antitumour immunity than exosomes released from heat-shocked tumour cells expressing cytoplasmic HSP70. J. Cell. Mol. Med. 2010, 14, 2655–2666. [Google Scholar] [CrossRef]
- Morishita, M.; Takahashi, Y.; Matsumoto, A.; Nishikawa, M.; Takakura, Y. Exosome-based tumor antigens-adjuvant co-delivery utilizing genetically engineered tumor cell-derived exosomes with immunostimulatory CpG DNA. Biomaterials 2016, 111, 55–65. [Google Scholar] [CrossRef]
- Korbelik, M.; Dougherty, G. Photodynamic therapy-mediated immune response against subcutaneous mouse tumors. Cancer Res. 1999, 59, 1941–1946. [Google Scholar]
- Pollard, J. Tumour-educated macrophages promote tumour progression and metastasis. Nat. Rev. Cancer 2004, 4, 71–78. [Google Scholar] [CrossRef]
- Chanmee, T.; Ontong, P.; Konno, K.; Itano, N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers 2014, 6, 1670–1690. [Google Scholar] [CrossRef]
- Yang, M.; McKay, D.; Pollard, J.W.; Lewis, C.E. Diverse functions of macrophages in different tumor microenvironments. Cancer Res. 2018, 78, 5492–5503. [Google Scholar] [CrossRef]
- Zhou, J.; Tang, Z.; Gao, S.; Li, C.; Feng, Y.; Zhou, X. Tumor-associated macrophages: Recent Insights and therapies. Front. Oncol. 2020, 10, 188. [Google Scholar] [CrossRef]
- Coscia, M.; Quaglino, E.; Iezzi, M.; Curcio, C.; Pantaleoni, F.; Riganti, C.; Holen, I.; Monkkonen, H.; Boccadoro, M.; Forni, G.; et al. Zoledronic acid repolarizes tumour-associated macrophages and inhibits mammary carcinogenesis by targeting the mevalonate pathway. J. Cell Mol. Med. 2010, 14, 2803–2815. [Google Scholar] [CrossRef]
- Acharya, S.; Sahoo, S.K. PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect. Adv. Drug Deliv. Rev. 2011, 63, 170–183. [Google Scholar] [CrossRef]
- Da Silva, C.G.; Rueda, F.; Lowik, C.W.; Ossendorp, F.; Cruz, L.J. Combinatorial prospects of nano-targeted chemoimmunotherapy. Biomaterials 2016, 83, 308–320. [Google Scholar] [CrossRef]
- Millard, M.; Posty, S.; Piffoux, M.; Jasniewski, J.; Lassalle, H.P.; Yakavets, I.; Gazeau, F.; Wilhelm, C.; Silva, A.K.A.; Bezdetnaya, L. mTHPC-Loaded Extracellular Vesicles Significantly Improve mTHPC Diffusion and Photodynamic Activity in Preclinical Models. Pharmaceuticals 2020, 12, 676. [Google Scholar] [CrossRef]
- Pinto, A.; Marangon, I.; Mereaux, J.; Nicolas-Boluda, A.; Lavieu, G.; Wilhelm, C.; Sarda-Mantel, L.; Silva, A.K.A.; Pocard, M.; Gazeau, F. Immune reprogramming precision photodynamic therapy of peritoneal metastasis by scalable stem-cell-derived extracellular vesicles. ACS Nano 2021, 15, 3251–3263. [Google Scholar] [CrossRef]
- Milane, L.; Singh, A.; Mattheolabakis, G.; Suresh, M.; Amiji, M.M. Exosome mediated communication within the tumor microenvironment. J. Control Release 2015, 219, 278–294. [Google Scholar] [CrossRef]
- Seo, N.; Akiyoshi, K.; Shiku, H. Exosome-mediated regulation of tumor immunology. Cancer Sci. 2018, 109, 2998–3004. [Google Scholar] [CrossRef]
- Olson, F.; Hunt, C.; Szoka, F.; Vail, W.; Papahadjopoulos, D. Preparation of liposomes of defined size distribution by extrusion through polycarbonate membranes. Biochim. Biophys. Acta 1979, 557, 9–23. [Google Scholar] [CrossRef]
- Hunter, D.; Frisken, B. Effect of extrusion pressure and lipid properties on the size and polydispersity of lipid vesicles. Biophys. J. 1998, 74, 2996–3002. [Google Scholar] [CrossRef]
- Song, S.; Zhou, F.; Chen, W.R.; Xing, D. PDT-induced HSP70 externalization up-regulates NO production via TLR2 signal pathway in macrophages. FEBS Lett. 2013, 587, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.; Nathan, C.F. Molecular mechanisms in tumer-cell killing by activated macrophages. Immunol. Today 1983, 4, 166–170. [Google Scholar] [CrossRef]
- Jang, S.C.; Kim, O.Y.; Yoon, C.M.; Choi, D.S.; Roh, T.Y.; Park, J.; Nilsson, J.; Lotvall, J.; Kim, Y.K.; Gho, Y.S. Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS Nano 2013, 7, 7698–7710. [Google Scholar] [CrossRef] [PubMed]
- Parisse, P.; Rago, I.; Severino, L.U.; Perissinotto, F.; Ambrosetti, E.; Paoletti, P.; Ricci, M.; Beltrami, A.P.; Cesselli, D.; Casalis, L. Atomic force microscopy analysis of extracellular vesicles. Eur. Biophys. J. Biophy. 2017, 46, 813–820. [Google Scholar] [CrossRef]
- He, C.; Hu, X.; Weston, T.A.; Jung, R.S.; Sandhu, J.; Huang, S.; Heizer, P.; Kim, J.; Ellison, R.; Xu, J.; et al. Macrophages release plasma membrane-derived particles rich in accessible cholesterol. Proc. Natl. Acad. Sci. USA 2018, 115, E8499–E8508. [Google Scholar] [CrossRef]
- Multhoff, G.; Botzler, C.; Wiesnet, M.; Muller, E.; Meier, T.; Wilmanns, W.; Issels, R. A stress-inducible 72-kDa heat-shock protein (HSP72) is expressed on the surface of human tumor cells, but not on normal cells. Int. J. Cancer 1995, 61, 272–279. [Google Scholar] [CrossRef]
- Breuninger, S.; Stangl, S.; Werner, C.; Sievert, W.; Lobinger, D.; Foulds, G.A.; Wagner, S.; Pickhard, A.; Piontek, G.; Kokowski, K.; et al. Membrane HSP70-a novel target for the isolation of circulating tumor cells after epithelial-to-mesenchymal transition. Front. Oncol. 2018, 8, 497. [Google Scholar] [CrossRef]
- Freund, E.; Liedtke, K.R.; van der Linde, J.; Metelmann, H.R.; Heidecke, C.D.; Partecke, L.I.; Bekeschus, S. Physical plasma-treated saline promotes an immunogenic phenotype in CT26 colon cancer cells in vitro and in vivo. Sci. Rep. 2019, 9, 634. [Google Scholar] [CrossRef]
- Arneth, B. Tumor microenvironment. Medicina 2020, 56, 15. [Google Scholar] [CrossRef]
- Al Hrout, A.; Cervantes-Gracia, K.; Chahwan, R.; Amin, A. Modelling liver cancer microenvironment using a novel 3D culture system. Sci. Rep. 2022, 12, 8003. [Google Scholar] [CrossRef]
- Benne, N.; van Duijn, J.; Kuiper, J.; Jiskoot, W.; Slutter, B. Orchestrating immune responses: How size, shape and rigidity affect the immunogenicity of particulate vaccines. J. Control Release 2016, 234, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Binder, C.; Torzewski, M.; Witztum, J. C-reactive protein binds to both oxidized LDL and apoptotic cells through recognition of a common ligand: Phosphorylcholine of oxidized phospholipids. Proc. Natl. Acad. Sci. USA 2002, 99, 13043–13048. [Google Scholar] [CrossRef] [PubMed]
- Podrez, E.; Poliakov, E.; Shen, Z.; Zhang, R.; Deng, Y.; Sun, M.; Finton, P.; Shan, L.; Gugiu, B.; Fox, P.; et al. Identification of a novel family of oxidized phospholipids that serve as ligands for the macrophage scavenger receptor CD36. J. Biol. Chem. 2002, 277, 38503–38516. [Google Scholar] [CrossRef]
- Miller, Y.I.; Choi, S.H.; Wiesner, P.; Fang, L.; Harkewicz, R.; Hartvigsen, K.; Boullier, A.; Gonen, A.; Diehl, C.J.; Que, X.; et al. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ. Res. 2011, 108, 235–248. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, S.-H.; Tsai, G.-Y.; Wang, M.-J.; Chen, S.-Y. Effects of Cancer Cell-Derived Nanovesicle Vaccines Produced by the Oxidative Stress-Induced Expression of DAMP and Spontaneous Release/Filter Extrusion in the Interplay of Cancer Cells and Macrophages. Biomedicines 2022, 10, 1977. https://doi.org/10.3390/biomedicines10081977
Lin S-H, Tsai G-Y, Wang M-J, Chen S-Y. Effects of Cancer Cell-Derived Nanovesicle Vaccines Produced by the Oxidative Stress-Induced Expression of DAMP and Spontaneous Release/Filter Extrusion in the Interplay of Cancer Cells and Macrophages. Biomedicines. 2022; 10(8):1977. https://doi.org/10.3390/biomedicines10081977
Chicago/Turabian StyleLin, Song-Hsien, Guan-Ying Tsai, Meng-Jiy Wang, and Szu-Yuan Chen. 2022. "Effects of Cancer Cell-Derived Nanovesicle Vaccines Produced by the Oxidative Stress-Induced Expression of DAMP and Spontaneous Release/Filter Extrusion in the Interplay of Cancer Cells and Macrophages" Biomedicines 10, no. 8: 1977. https://doi.org/10.3390/biomedicines10081977
APA StyleLin, S. -H., Tsai, G. -Y., Wang, M. -J., & Chen, S. -Y. (2022). Effects of Cancer Cell-Derived Nanovesicle Vaccines Produced by the Oxidative Stress-Induced Expression of DAMP and Spontaneous Release/Filter Extrusion in the Interplay of Cancer Cells and Macrophages. Biomedicines, 10(8), 1977. https://doi.org/10.3390/biomedicines10081977





