Silencing of LLGL2 Suppresses the Estradiol-Induced BPH-1 Cell Proliferation through the Regulation of Autophagy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Experiments and Immunohistochemical Staining
2.2. Cell Culture and Reagents
2.3. siRNA and Plasmid Transfection
2.4. Cell Viability Assay
2.5. Observation of Cell Confluency
2.6. Flow Cytometry
2.7. Western Blot Analysis
2.8. Immunofluorescence
2.9. Detection of Autophagy Flux
2.10. Statistical Analysis
3. Results
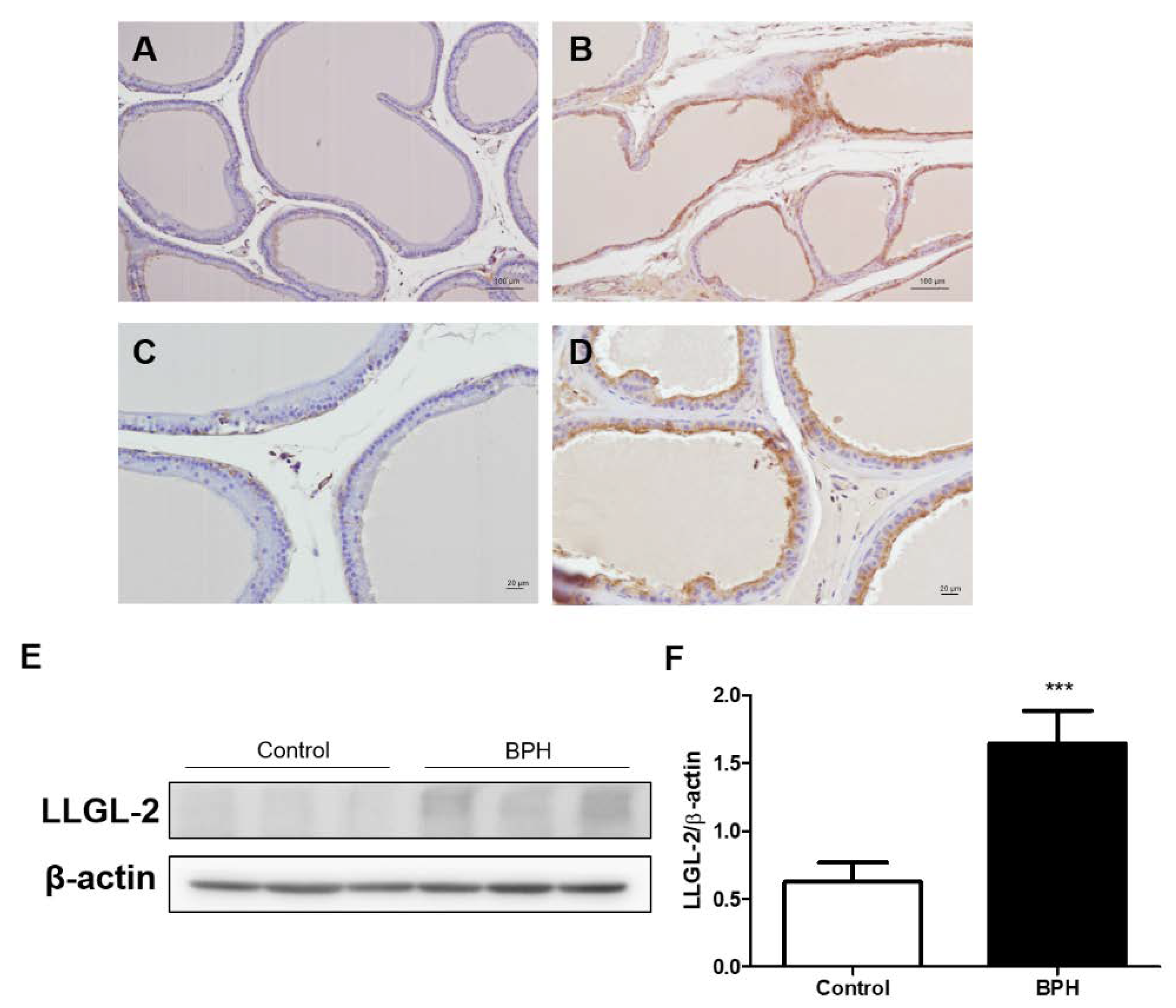
3.1. LLGL-2 Expression in Testosterone-Induced Rat BPH Animal Model
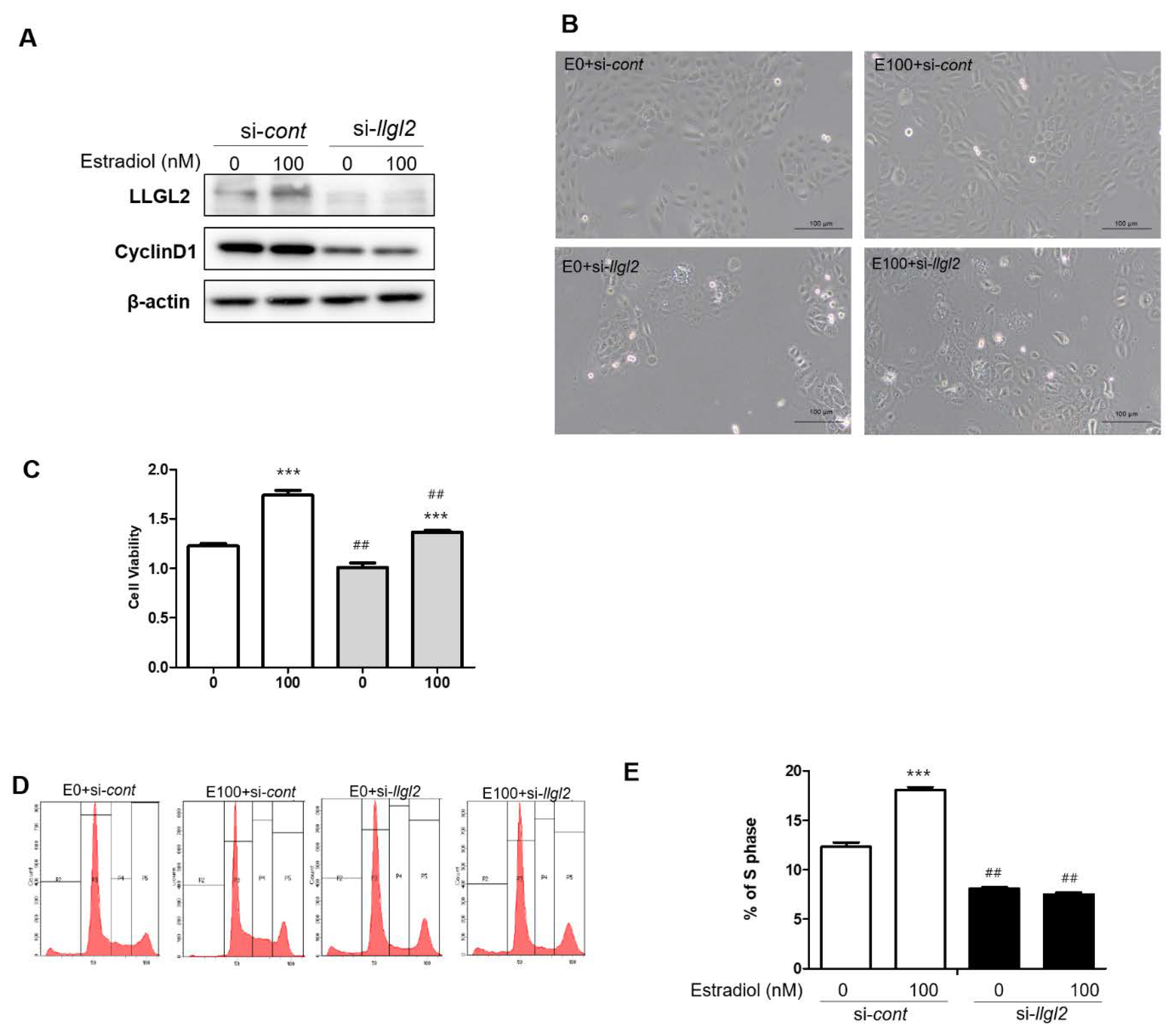
3.2. LLGL2 Expression in Estradiol-Induced BPH-1 Cell Proliferation
3.3. Inhibition of LLGL2 Expression Induces Autophagy
3.4. Overexpression of LLGL2 Promotes the Proliferation of BPH-1 Cells
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thorpe, A.; Neal, D. Benign prostatic hyperplasia. Lancet 2003, 361, 1359–1367. [Google Scholar] [CrossRef]
- Carson, C., 3rd; Rittmaster, R. The role of dihydrotestosterone in benign prostatic hyperplasia. Urology 2003, 61, 2–7. [Google Scholar] [CrossRef]
- Nicholson, T.M.; Ricke, W.A. Androgens and estrogens in benign prostatic hyperplasia: Past, present and future. Differentiation 2011, 82, 184–199. [Google Scholar] [CrossRef] [PubMed]
- Ellem, S.J.; Risbridger, G.P. The dual, opposing roles of estrogen in the prostate. Ann. N. Y. Acad. Sci. 2009, 1155, 174–186. [Google Scholar] [CrossRef]
- Mechler, B.M.; McGinnis, W.; Gehring, W.J. Molecular cloning of lethal(2)giant larvae, a recessive oncogene of Drosophila melanogaster. EMBO J. 1985, 4, 1551–1557. [Google Scholar] [CrossRef]
- Bilder, D. Epithelial polarity and proliferation control: Links from the Drosophila neoplastic tumor suppressors. Genes. Dev. 2004, 18, 1909–1925. [Google Scholar] [CrossRef]
- Martin-Belmonte, F.; Perez-Moreno, M. Epithelial cell polarity, stem cells and cancer. Nat. Rev. Cancer 2011, 12, 23–38. [Google Scholar] [CrossRef]
- Albertson, R.; Doe, C.Q. Dlg, Scrib and Lgl regulate neuroblast cell size and mitotic spindle asymmetry. Nat. Cell Biol. 2003, 5, 166–170. [Google Scholar] [CrossRef]
- Betschinger, J.; Mechtler, K.; Knoblich, J.A. The Par complex directs asymmetric cell division by phosphorylating the cytoskeletal protein Lgl. Nature 2003, 422, 326–330. [Google Scholar] [CrossRef]
- Schimanski, C.C.; Schmitz, G.; Kashyap, A.; Bosserhoff, A.K.; Bataille, F.; Schafer, S.C.; Lehr, H.A.; Berger, M.R.; Galle, P.R.; Strand, S.; et al. Reduced expression of Hugl-1, the human homologue of Drosophila tumour suppressor gene lgl, contributes to progression of colorectal cancer. Oncogene 2005, 24, 3100–3109. [Google Scholar] [CrossRef]
- Grifoni, D.; Garoia, F.; Bellosta, P.; Parisi, F.; De Biase, D.; Collina, G.; Strand, D.; Cavicchi, S.; Pession, A. aPKCzeta cortical loading is associated with Lgl cytoplasmic release and tumor growth in Drosophila and human epithelia. Oncogene 2007, 26, 5960–5965. [Google Scholar] [CrossRef] [PubMed]
- Biesterfeld, S.; Kauhausen, A.; Kost, C.; Gockel, I.; Schimanski, C.C.; Galle, P.R. Preservation of HUGL-1 expression as a favourable prognostic factor in pancreatic carcinoma. Anticancer Res. 2012, 32, 3153–3159. [Google Scholar] [PubMed]
- Ohshiro, T.; Yagami, T.; Zhang, C.; Matsuzaki, F. Role of cortical tumour-suppressor proteins in asymmetric division of Drosophila neuroblast. Nature 2000, 408, 593–596. [Google Scholar] [CrossRef]
- Russ, A.; Louderbough, J.M.; Zarnescu, D.; Schroeder, J.A. Hugl1 and Hugl2 in mammary epithelial cells: Polarity, proliferation, and differentiation. PLoS ONE 2012, 7, e47734. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, A.; Zimmerman, T.; Ergul, N.; Bosserhoff, A.; Hartman, U.; Alla, V.; Bataille, F.; Galle, P.R.; Strand, S.; Strand, D. The human Lgl polarity gene, Hugl-2, induces MET and suppresses Snail tumorigenesis. Oncogene 2013, 32, 1396–1407. [Google Scholar] [CrossRef]
- Saito, Y.; Li, L.; Coyaud, E.; Luna, A.; Sander, C.; Raught, B.; Asara, J.M.; Brown, M.; Muthuswamy, S.K. LLGL2 rescues nutrient stress by promoting leucine uptake in ER(+) breast cancer. Nature 2019, 569, 275–279. [Google Scholar] [CrossRef]
- Leng, S.; Xie, F.; Liu, J.; Shen, J.; Quan, G.; Wen, T. LLGL2 Increases Ca(2+) Influx and Exerts Oncogenic Activities via PI3K/AKT Signaling Pathway in Hepatocellular Carcinoma. Front. Oncol. 2021, 11, 683629. [Google Scholar] [CrossRef]
- La Vignera, S.; Condorelli, R.A.; Russo, G.I.; Morgia, G.; Calogero, A.E. Endocrine control of benign prostatic hyperplasia. Andrology 2016, 4, 404–411. [Google Scholar] [CrossRef]
- Mathiassen, S.G.; De Zio, D.; Cecconi, F. Autophagy and the Cell Cycle: A Complex Landscape. Front. Oncol. 2017, 7, 51. [Google Scholar] [CrossRef]
- Santana-Codina, N.; Mancias, J.D.; Kimmelman, A.C. The Role of Autophagy in Cancer. Annu. Rev. Cancer Biol. 2017, 1, 19–39. [Google Scholar] [CrossRef]
- McNeal, J. Pathology of benign prostatic hyperplasia. Insight into etiology. Urol. Clin. N. Am. 1990, 17, 477–486. [Google Scholar] [CrossRef]
- Morales, A. Androgen replacement therapy and prostate safety. Eur. Urol. 2002, 41, 113–120. [Google Scholar] [CrossRef]
- Vermeulen, A.; Kaufman, J.M.; Goemaere, S.; van Pottelberg, I. Estradiol in elderly men. Aging. Male. 2002, 5, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Klionsky, D.J.; Emr, S.D. Autophagy as a regulated pathway of cellular degradation. Science 2000, 290, 1717–1721. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xu, P.; Chen, D.; Fan, X.; Xu, Y.; Li, M.; Yang, X.; Wang, C. Roles of autophagy-related genes Beclin-1 and LC3 in the development and progression of prostate cancer and benign prostatic hyperplasia. Biomed. Rep. 2013, 1, 855–860. [Google Scholar] [CrossRef]
- Zhang, N.; Ji, N.; Jiang, W.M.; Li, Z.Y.; Wang, M.; Wen, J.M.; Li, Y.; Chen, X.; Chen, J.M. Hypoxia-induced autophagy promotes human prostate stromal cells survival and ER-stress. Biochem. Biophys. Res. Commun. 2015, 464, 1107–1112. [Google Scholar] [CrossRef]
- Li, M.; Yang, X.; Wang, H.; Xu, E.; Xi, Z. Inhibition of androgen induces autophagy in benign prostate epithelial cells. Int. J. Urol. 2014, 21, 195–199. [Google Scholar] [CrossRef]
- Jiang, C.Y.; Yang, B.Y.; Zhao, S.; Shao, S.H.; Bei, X.Y.; Shi, F.; Sun, Q.; Deng, Z.; Wang, X.H.; Han, B.M.; et al. Deregulation of ATG9A by impaired AR signaling induces autophagy in prostate stromal fibroblasts and promotes BPH progression. Cell Death Dis. 2018, 9, 431. [Google Scholar] [CrossRef]
- Levine, B.; Kroemer, G. Autophagy in the pathogenesis of disease. Cell 2008, 132, 27–42. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.-H.; Hong, G.-L.; Kim, Y.-J.; Lee, H.-J.; Jung, J.-Y. Silencing of LLGL2 Suppresses the Estradiol-Induced BPH-1 Cell Proliferation through the Regulation of Autophagy. Biomedicines 2022, 10, 1981. https://doi.org/10.3390/biomedicines10081981
Kim K-H, Hong G-L, Kim Y-J, Lee H-J, Jung J-Y. Silencing of LLGL2 Suppresses the Estradiol-Induced BPH-1 Cell Proliferation through the Regulation of Autophagy. Biomedicines. 2022; 10(8):1981. https://doi.org/10.3390/biomedicines10081981
Chicago/Turabian StyleKim, Kyung-Hyun, Geum-Lan Hong, Yae-Ji Kim, Hui-Ju Lee, and Ju-Young Jung. 2022. "Silencing of LLGL2 Suppresses the Estradiol-Induced BPH-1 Cell Proliferation through the Regulation of Autophagy" Biomedicines 10, no. 8: 1981. https://doi.org/10.3390/biomedicines10081981





