Effects of Achieving Sustained Virologic Response after Direct-Acting Antiviral Agents on Long-Term Liver Fibrosis in Diabetics vs. in Non-Diabetic Patients with Chronic Hepatitis C Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Molecular Determination of HCV RNA and Genotype
2.3. Diagnosis of T2DM Patients
2.4. FibroMax Test
2.5. Statistical Analysis
3. Results
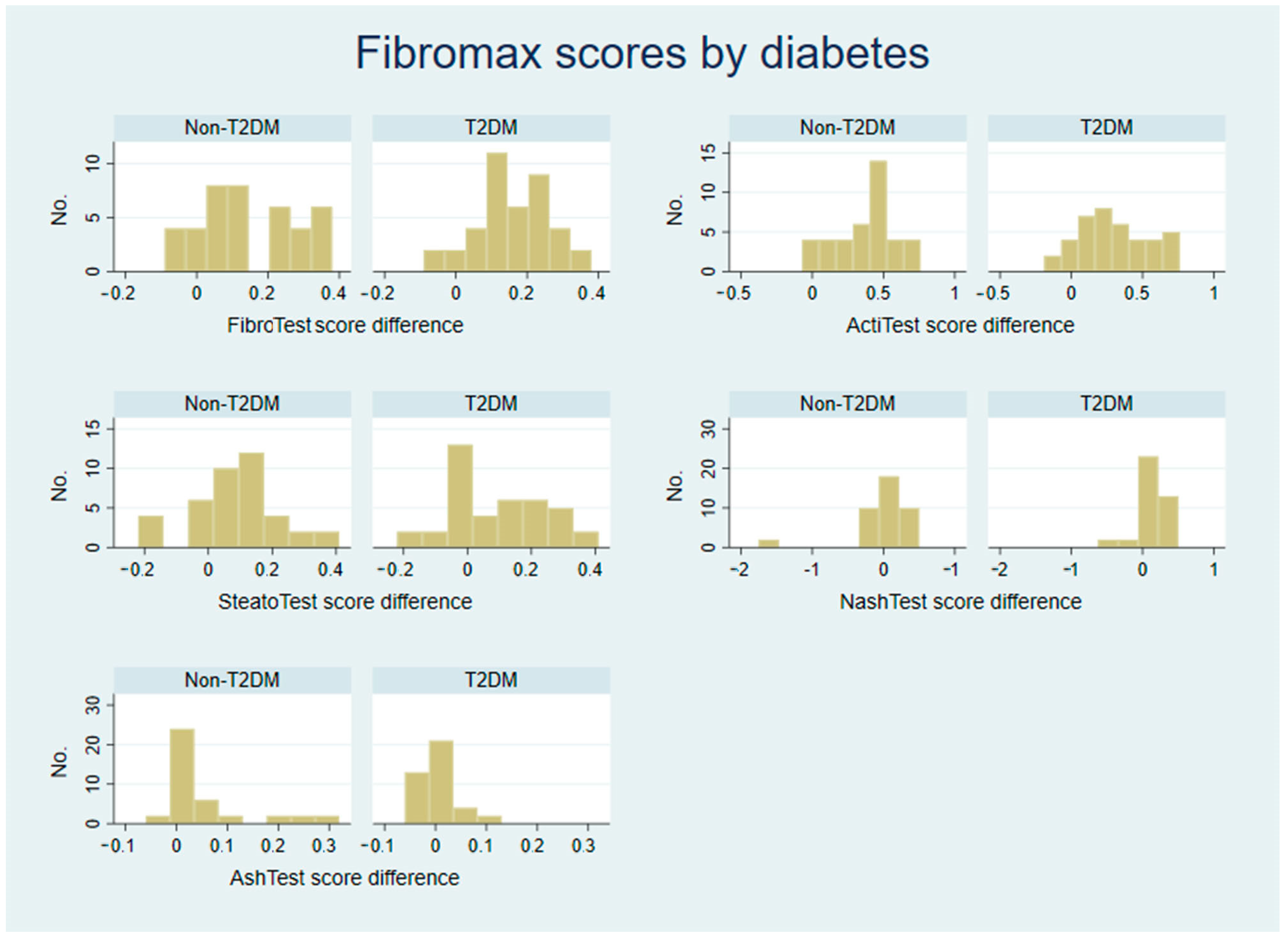
3.1. Analysis of Raw FibroMax Scores
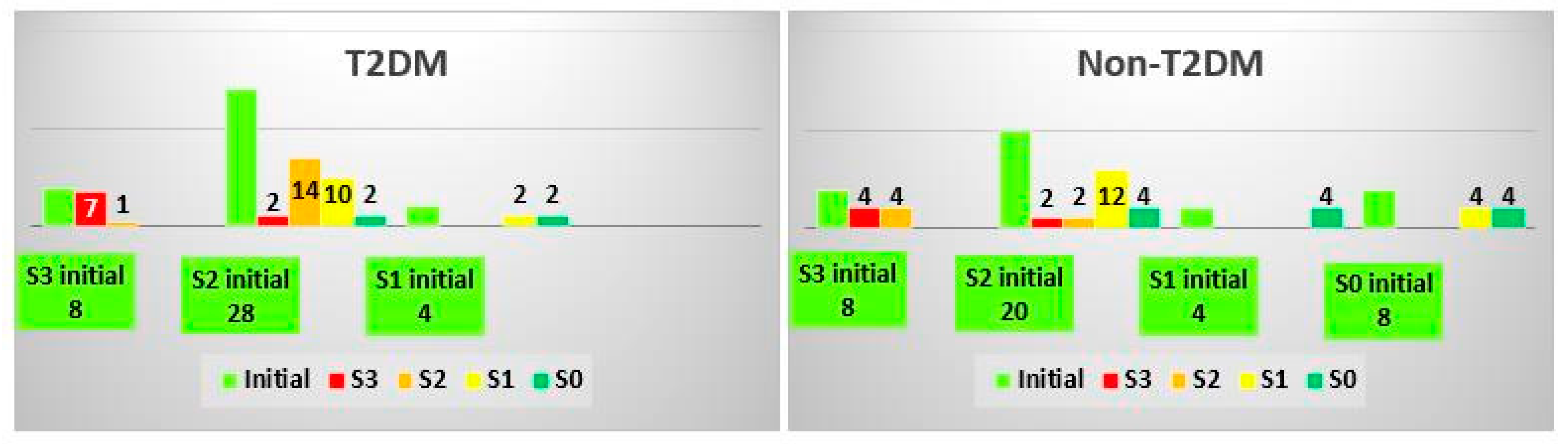
3.2. Analysis of FibroTest Classes
3.3. Analysis of ActiTest Classes
3.4. Analysis of SteatoTest Classes
3.5. Analysis of NashTest Classes
3.6. Analysis of AshTest Classes
3.7. Analysis of the Impact of Previous Hepatitis Treatment on the Effect of Current Treatment with DAAs on Liver Fibrosis
3.8. Regression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Global Health Sector Strategy on Viral Hepatitis 2016–2021; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Cooke, G.S.; Andrieux-Meyer, I.; Applegate, T.L.; Atun, R.; Burry, J.R.; Cheinquer, H.; Dusheiko, G.; Feld, J.J.; Gore, C.; Griswold, M.G.; et al. Accelerating the elimination of viral hepatitis: A Lancet Gastroenterology & Hepatology Commission. Lancet Gastroenterol. Hepatol. 2019, 4, 135–184. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Filipe, A.; Sreenu, V.; Hughes, J.; Aranday-Cortes, E.; Irving, W.L.; Foster, G.R.; Agarwal, K.; Rosenberg, W.; Macdonald, D.; Richardson, P.; et al. Response to DAA therapy in the NHS England Early Access Programme for rare HCV subtypes from low and middle income countries. J. Hepatol. 2017, 67, 1348–1350. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.Y.K.; Ou, J.J. Autophagy in HCV Replication and Protein Trafficking. Int. J. Mol. Sci. 2021, 22, 1089. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, L.; Nevola, R.; Franci, G.; Perrella, A.; Corvino, G.; Marrone, A.; Berretta, M.; Morone, M.V.; Galdiero, M.; Giordano, M.; et al. Risk of Hepatocellular Carcinoma after HCV Clearance by Direct-Acting Antivirals Treatment Predictive Factors and Role of Epigenetics. Cancers 2020, 12, 1351. [Google Scholar] [CrossRef]
- Kamal, A.M.; Mitrut, P.; Docea, A.O.; Sosoi, S.S.; Kamal, C.K.; Mitrut, R.; Margaritescu, D.; Calina, D.; Banciu, C.; Tica, O.S.; et al. Double therapy with pegylated interferon and ribavirin for chronic hepatitis c. A pharmacogenetic guide for predicting adverse events. Farmacia 2017, 65, 877–884. [Google Scholar]
- Drazilova, S.; Gazda, J.; Janicko, M.; Jarcuska, P. Chronic Hepatitis C Association with Diabetes Mellitus and Cardiovascular Risk in the Era of DAA Therapy. Can. J. Gastroenterol. Hepatol. 2018, 2018, 6150861. [Google Scholar] [CrossRef]
- Docea, A.O.; Gofita, E.; Calina, D.; Ioan, Z.S.; Valcea, D.I.; Mitrut, P. Autoimmune disorders due to double antiviral therapy with peginterferon and ribavirin in patients with hepatitis c virus infection. Farmacia 2016, 64, 605–611. [Google Scholar]
- Rehman, G.U.; Ali, M.; Shah, F.; Iqbal, A.; Ahmad, A.; Hayat, Z.; Islam, B.; Ali, F.; Ikramullah; Jamal, Y.; et al. Prevalence of Diabetes Type 2 in Hepatitis C Infected Patients in Kpk, Pakistan. BioMed Res. Int. 2017, 2017, 2416281. [Google Scholar] [CrossRef]
- Zignego, A.L.; Ferri, C.; Pileri, S.A.; Caini, P.; Bianchi, F.B.; Italian Association of the Study of Liver Commission on Extrahepatic Manifestations of HCV Infection. Extrahepatic manifestations of Hepatitis C Virus infection: A general overview and guidelines for a clinical approach. Dig. Liver Dis. 2007, 39, 2–17. [Google Scholar] [CrossRef]
- Romero-Gomez, M.; Del Mar Viloria, M.; Andrade, R.J.; Salmeron, J.; Diago, M.; Fernandez-Rodriguez, C.M.; Corpas, R.; Cruz, M.; Grande, L.; Vazquez, L.; et al. Insulin resistance impairs sustained response rate to peginterferon plus ribavirin in chronic hepatitis C patients. Gastroenterology 2005, 128, 636–641. [Google Scholar] [CrossRef]
- Yair-Sabag, S.; Nussinson, E.; Ben-Assuli, O.; Shibli, F.; Shahbari, A.; Zelber-Sagi, S. Retrospective study of the associations between hepatitis C virus infection and metabolic factors. World J. Hepatol. 2016, 8, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, L.; De Michina, A.; Misciagna, G.; Guerra, V.; Di Leo, A. Virus C hepatitis and type 2 diabetes: A cohort study in southern Italy. Am. J. Gastroenterol. 2013, 108, 1108–1111. [Google Scholar] [CrossRef] [PubMed]
- Soma, J.; Saito, T.; Taguma, Y.; Chiba, S.; Sato, H.; Sugimura, K.; Ogawa, S.; Ito, S. High prevalence and adverse effect of hepatitis C virus infection in type II diabetic-related nephropathy. J. Am. Soc. Nephrol. 2000, 11, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Jin, M.; Yang, M.; Liu, K.; Li, J.W. Type 2 diabetes mellitus and the risk of hepatitis C virus infection: A systematic review. Sci. Rep. 2013, 3, 2981. [Google Scholar] [CrossRef]
- Weinman, S.A.; Belalcazar, L.M. Hepatitis C: A metabolic liver disease. Gastroenterology 2004, 126, 917–919. [Google Scholar] [CrossRef]
- Laskus, T.; Radkowski, M.; Wang, L.F.; Vargas, H.; Rakela, J. Search for hepatitis C virus extrahepatic replication sites in patients with acquired immunodeficiency syndrome: Specific detection of negative-strand viral RNA in various tissues. Hepatology 1998, 28, 1398–1401. [Google Scholar] [CrossRef]
- Sheikh, M.Y.; Choi, J.; Qadri, I.; Friedman, J.E.; Sanyal, A.J. Hepatitis C virus infection: Molecular pathways to metabolic syndrome. Hepatology 2008, 47, 2127–2133. [Google Scholar] [CrossRef]
- Banerjee, S.; Saito, K.; Ait-Goughoulte, M.; Meyer, K.; Ray, R.B.; Ray, R. Hepatitis C virus core protein upregulates serine phosphorylation of insulin receptor substrate-1 and impairs the downstream akt/protein kinase B signaling pathway for insulin resistance. J. Virol. 2008, 82, 2606–2612. [Google Scholar] [CrossRef]
- Matsumoto, M.; Ogawa, W.; Teshigawara, K.; Inoue, H.; Miyake, K.; Sakaue, H.; Kasuga, M. Role of the insulin receptor substrate 1 and phosphatidylinositol 3-kinase signaling pathway in insulin-induced expression of sterol regulatory element binding protein 1c and glucokinase genes in rat hepatocytes. Diabetes 2002, 51, 1672–1680. [Google Scholar] [CrossRef]
- Lonardo, A.; Adinolfi, L.E.; Petta, S.; Craxi, A.; Loria, P. Hepatitis C and diabetes: The inevitable coincidence? Expert Rev. Anti Infect 2009, 7, 293–308. [Google Scholar] [CrossRef]
- Allison, M.E.; Wreghitt, T.; Palmer, C.R.; Alexander, G.J. Evidence for a link between hepatitis C virus infection and diabetes mellitus in a cirrhotic population. J. Hepatol. 1994, 21, 1135–1139. [Google Scholar] [CrossRef]
- Mehta, S.H.; Brancati, F.L.; Strathdee, S.A.; Pankow, J.S.; Netski, D.; Coresh, J.; Szklo, M.; Thomas, D.L. Hepatitis C virus infection and incident type 2 diabetes. Hepatology 2003, 38, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Knobler, H.; Schihmanter, R.; Zifroni, A.; Fenakel, G.; Schattner, A. Increased risk of type 2 diabetes in noncirrhotic patients with chronic hepatitis C virus infection. Mayo Clin. Proc. 2000, 75, 355–359. [Google Scholar] [CrossRef] [PubMed]
- White, D.L.; Ratziu, V.; El-Serag, H.B. Hepatitis C infection and risk of diabetes: A systematic review and meta-analysis. J. Hepatol. 2008, 49, 831–844. [Google Scholar] [CrossRef] [PubMed]
- Naing, C.; Mak, J.W.; Ahmed, S.I.; Maung, M. Relationship between hepatitis C virus infection and type 2 diabetes mellitus: Meta-analysis. World J. Gastroenterol. 2012, 18, 1642–1651. [Google Scholar] [CrossRef]
- Missiha, S.B.; Ostrowski, M.; Heathcote, E.J. Disease progression in chronic hepatitis C: Modifiable and nonmodifiable factors. Gastroenterology 2008, 134, 1699–1714. [Google Scholar] [CrossRef]
- Ruhl, C.E.; Menke, A.; Cowie, C.C.; Everhart, J.E. Relationship of hepatitis C virus infection with diabetes in the U.S. population. Hepatology 2014, 60, 1139–1149. [Google Scholar] [CrossRef]
- Bose, S.K.; Shrivastava, S.; Meyer, K.; Ray, R.B.; Ray, R. Hepatitis C virus activates the mTOR/S6K1 signaling pathway in inhibiting IRS-1 function for insulin resistance. J. Virol. 2012, 86, 6315–6322. [Google Scholar] [CrossRef]
- Zein, N.N.; Abdulkarim, A.S.; Wiesner, R.H.; Egan, K.S.; Persing, D.H. Prevalence of diabetes mellitus in patients with end-stage liver cirrhosis due to hepatitis C, alcohol, or cholestatic disease. J. Hepatol. 2000, 32, 209–217. [Google Scholar] [CrossRef]
- Ramachandran, T.M.; Rajneesh, A.H.R.; Zacharia, G.S.; Adarsh, R.P. Cirrhosis of Liver and Diabetes Mellitus: The Diabolic Duo? J. Clin. Diagn. Res. 2017, 11, OC01–OC05. [Google Scholar] [CrossRef]
- Memon, M.S.; Arain, Z.I.; Naz, F.; Zaki, M.; Kumar, S.; Burney, A.A. Prevalence of type 2 diabetes mellitus in hepatitis C virus infected population: A Southeast Asian study. J. Diabetes Res. 2013, 2013, 539361. [Google Scholar] [CrossRef] [PubMed]
- Custro, N.; Carroccio, A.; Ganci, A.; Scafidi, V.; Campagna, P.; Di Prima, L.; Montalto, G. Glycemic homeostasis in chronic viral hepatitis and liver cirrhosis. Diabetes Metab 2001, 27, 476–481. [Google Scholar] [PubMed]
- Nwankiti, O.O.; Ndako, J.A.; Echeonwu, G.O.; Olabode, A.O.; Nwosuh, C.I.; Onovoh, E.M.; Okeke, L.A.; Akinola, J.O.; Duru, B.N.; Nwagbo, I.O.; et al. Hepatitis C Virus infection in apparentenly healthy individuals with family history of diabetes in Vom, Plateau State Nigeria. Virol. J. 2009, 6, 110. [Google Scholar] [CrossRef][Green Version]
- Huang, Y.W.; Yang, S.S.; Fu, S.C.; Wang, T.C.; Hsu, C.K.; Chen, D.S.; Hu, J.T.; Kao, J.H. Increased risk of cirrhosis and its decompensation in chronic hepatitis C patients with new-onset diabetes: A nationwide cohort study. Hepatology 2014, 60, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Nkontchou, G.; Bastard, J.P.; Ziol, M.; Aout, M.; Cosson, E.; Ganne-Carrie, N.; Grando-Lemaire, V.; Roulot, D.; Capeau, J.; Trinchet, J.C.; et al. Insulin resistance, serum leptin, and adiponectin levels and outcomes of viral hepatitis C cirrhosis. J. Hepatol. 2010, 53, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, L.; Perrella, A.; Guarino, M.; De Luca, M.; Piai, G.; Coppola, N.; Pafundi, P.C.; Ciardiello, F.; Fasano, M.; Martinelli, E.; et al. Incidence and risk factors of early HCC occurrence in HCV patients treated with direct acting antivirals: A prospective multicentre study. J. Transl. Med. 2019, 17, 292. [Google Scholar] [CrossRef]
- Qing, S.; Ji, D.; Li, B.; Li, F.; Wang, Y.; Niu, X.; Ling, B.; Meng, Y.; Lau, G.; Chen, G. Improvement of glucose and lipid metabolism with pegylated interferon-a plus ribavirin therapy in Chinese patients chronically infected with genotype 1b hepatitis C virus. Ann. Saudi Med. 2015, 35, 293–297. [Google Scholar] [CrossRef]
- Pais, R.; Rusu, E.; Zilisteanu, D.; Circiumaru, A.; Micu, L.; Voiculescu, M.; Poynard, T.; Ratziu, V. Prevalence of steatosis and insulin resistance in patients with chronic hepatitis B compared with chronic hepatitis C and non-alcoholic fatty liver disease. Eur. J. Intern. Med. 2015, 26, 30–36. [Google Scholar] [CrossRef]
- Friedman, S.L. Mechanisms of hepatic fibrogenesis. Gastroenterology 2008, 134, 1655–1669. [Google Scholar] [CrossRef]
- Hui, J.M.; Kench, J.; Farrell, G.C.; Lin, R.; Samarasinghe, D.; Liddle, C.; Byth, K.; George, J. Genotype-specific mechanisms for hepatic steatosis in chronic hepatitis C infection. J. Gastroenterol. Hepatol. 2002, 17, 873–881. [Google Scholar] [CrossRef]
- Hsu, C.S.; Liu, C.H.; Liu, C.J.; Hsu, S.J.; Chen, C.L.; Hwang, J.J.; Lai, M.Y.; Chen, P.J.; Chen, D.S.; Kao, J.H. Association of metabolic profiles with hepatic fibrosis in chronic hepatitis C patients with genotype 1 or 2 infection. J. Gastroenterol. Hepatol. 2010, 25, 970–977. [Google Scholar] [CrossRef]
- Svegliati-Baroni, G.; Bugianesi, E.; Bouserhal, T.; Marini, F.; Ridolfi, F.; Tarsetti, F.; Ancarani, F.; Petrelli, E.; Peruzzi, E.; Lo Cascio, M.; et al. Post-load insulin resistance is an independent predictor of hepatic fibrosis in virus C chronic hepatitis and in non-alcoholic fatty liver disease. Gut 2007, 56, 1296–1301. [Google Scholar] [CrossRef] [PubMed]
- Cioboată, R.; Găman, A.; Traşcă, D.; Ungureanu, A.; Docea, A.O.; Tomescu, P.; Gherghina, F.; Arsene, A.L.; Badiu, C.; Tsatsakis, A.M.; et al. Pharmacological management of non-alcoholic fatty liver disease: Atorvastatin versus pentoxifylline. Exp. Ther. Med. 2017, 13, 2375–2381. [Google Scholar] [CrossRef] [PubMed]
- Fartoux, L.; Chazouilleres, O.; Wendum, D.; Poupon, R.; Serfaty, L. Impact of steatosis on progression of fibrosis in patients with mild hepatitis C. Hepatology 2005, 41, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Tag-Adeen, M.; Sabra, A.M.; Akazawa, Y.; Ohnita, K.; Nakao, K. Impact of hepatitis C virus genotype-4 eradication following direct acting antivirals on liver stiffness measurement. Hepat. Med. 2017, 9, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, H.L.; Utay, N.S. Hepatic steatosis in HCV-infected persons in the direct-acting antiviral era. Trop. Dis. Travel Med. Vaccines 2016, 2, 21. [Google Scholar] [CrossRef] [PubMed]
- Poynard, T.; Ratziu, V.; McHutchison, J.; Manns, M.; Goodman, Z.; Zeuzem, S.; Younossi, Z.; Albrecht, J. Effect of treatment with peginterferon or interferon alfa-2b and ribavirin on steatosis in patients infected with hepatitis C. Hepatology 2003, 38, 75–85. [Google Scholar] [CrossRef]
- Hum, J.; Jou, J.H.; Green, P.K.; Berry, K.; Lundblad, J.; Hettinger, B.D.; Chang, M.; Ioannou, G.N. Improvement in Glycemic Control of Type 2 Diabetes After Successful Treatment of Hepatitis C Virus. Diabetes Care 2017, 40, 1173–1180. [Google Scholar] [CrossRef]
- Sasso, F.C.; Pafundi, P.C.; Caturano, A.; Galiero, R.; Vetrano, E.; Nevola, R.; Petta, S.; Fracanzani, A.L.; Coppola, C.; Di Marco, V.; et al. Impact of direct acting antivirals (DAAs) on cardiovascular events in HCV cohort with pre-diabetes. Nutr. Metab Cardiovasc Dis. 2021, 31, 2345–2353. [Google Scholar] [CrossRef]
- Adinolfi, L.E.; Petta, S.; Fracanzani, A.L.; Coppola, C.; Narciso, V.; Nevola, R.; Rinaldi, L.; Calvaruso, V.; Staiano, L.; Di Marco, V.; et al. Impact of hepatitis C virus clearance by direct-acting antiviral treatment on the incidence of major cardiovascular events: A prospective multicentre study. Atherosclerosis 2020, 296, 40–47. [Google Scholar] [CrossRef]
- Adinolfi, L.E.; Petta, S.; Fracanzani, A.L.; Nevola, R.; Coppola, C.; Narciso, V.; Rinaldi, L.; Calvaruso, V.; Pafundi, P.C.; Lombardi, R.; et al. Reduced incidence of type 2 diabetes in patients with chronic hepatitis C virus infection cleared by direct-acting antiviral therapy: A prospective study. Diabetes Obes. Metab. 2020, 22, 2408–2416. [Google Scholar] [CrossRef] [PubMed]
- Iacob, S.; Cerban, R.; Pietrareanu, C.; Ester, C.; Iacob, R.; Gheorghe, C.; Popescu, I.; Gheorghe, L. 100% sustained virological response and fibrosis improvement in real-life use of direct acting antivirals in genotype-1b recurrent hepatitis C following liver transplantation. J. Gastrointestin. Liver Dis. 2018, 27, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Borrego, A.; Healey, D.; Negre, B.; Christofi, M.; Sabharwal, S.; Ludwig, D.A.; Chung, R.T.; Jonas, M.M. Influence of body mass index on outcome of pediatric chronic hepatitis C virus infection. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 191–197. [Google Scholar] [CrossRef] [PubMed]




| Non-T2DM Group | T2DM Group | ||
|---|---|---|---|
| Variable | Category | ||
| Sex (no., %) | Female | 26 (65.00%) | 23 (57.50%) |
| Male | 14 (35.00%) | 17 (42.50%) | |
| Habitat (no., %) | Rural | 22 (55.00%) | 22 (55.00%) |
| Urban | 18 (45.00%) | 18 (45.00%) | |
| Prior treatment (no., %) | Yes | 16 (40.00%) | 5 (12.50%) * |
| No | 24 (60.00%) | 35 (87.50%) * | |
| HCV genotype | 1b | 40 (100%) | 40 (100%) |
| Age (years) (median, IQ range) | 65 (58–71) | 65 (59–70) | |
| ALT (IU/L) (median, IQ range) | 85 (61.5–131) | 82 (59–108) | |
| AST (IU/L) (median, IQ range) | 68 (49–92) | 72 (54–88) | |
| TB (mg/dL) (median, IQ range) | 0.78 (0.62–1.03) | 0.67 (0.54–0.88) | |
| BMI | 24.70 (22.83–28.73) | 25.85 (24.65–29.00) | |
| TC (mg/dL) (median, IQ range) | 144 (128–174) | 172 (141–264) * | |
| Triglycerides (mg/dL) (median, IQ range) | 104 (64–138) | 104 (73–126) | |
| APOA1 (mg/dL) (median, IQ range) | 1.39 (1.23–1.56) | 1.47 (1.35–1.63) |
| Parameter | Statistic | FibroTest | ActiTest | SteatoTest | NashTest | AshTest | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Initial | Final | Initial | Final | Initial | Final | Initial | Final | Initial | Final | ||
| Non-T2DM | Median | 0.74 | 0.54 | 0.62 | 0.16 | 0.57 | 0.42 | 0.50 | 0.50 | 0.04 | 0.02 |
| IQ range | 0.67–0.84 | 0.42–0.78 | 0.50–0.78 | 0.11–0.39 | 0.36–0.63 | 0.25–0.75 | 0.25–0.75 | 0.25–0.70 | 0.01–0.11 | 0.01–0.04 | |
| T2DM | Median | 0.71 | 0.54 | 0.70 | 0.24 | 0.56 | 0.52 | 0.50 | 0.25 | 0.02 | 0.03 |
| IQ range | 0.64–0.85 | 0.46–0.68 | 0.53–0.74 | 0.12–0.62 | 0.49–0.65 | 0.40–0.66 | 0.25–0.75 | 0.25–0.59 | 0.01–0.04 | 0.01–0.04 | |
| p value | 0.326 | 0.672 | 0.500 | 0.189 | 0.699 | 0.086 | 0.503 | 0.150 | 0.024 * | 0.270 | |
| Parameter | Statistic | FibroTest Score Decrease | ActiTest Score Decrease | SteatoTest Score Decrease | NashTest Score Decrease | AshTest Score Decrease |
|---|---|---|---|---|---|---|
| Non-T2DM | Median | 0.13 (17.57%) | 0.41 (66.13%) | 0.09 (15.71%) | 0.00 (0%) | 0.01 (50.00%) |
| IQ range | 0.04–0.27 | 0.20–0.50 | 0.02–0.17 | −0.17–0.14 | −0.01–0.04 | |
| T2DM | Median | 0.16 (22.54%) | 0.22 (31.43%) | 0.04 (7.14%) | 0.00 (0%) | 0.00 (0%) |
| IQ range | 0.10–0.25 | 0.08–0.51 | −0.04–0.20 | 0.00–0.25 | −0.03–0.01 | |
| p value | 0.413 | 0.272 | 0.441 | 0.082 | 0.009 * |
| Parameter | Statistic | Glucose Level (mg/dL) | ||
|---|---|---|---|---|
| Initial | Final | Decrease Level | ||
| Non-T2DM | Median | 105.5 | 101.0 | 2.0 |
| IQ range | 96.5–113.0 | 93.0–109.0 | −9–11.0 | |
| T2DM | Median | 176.0 * | 137.0 * | 27.0 * |
| IQ range | 141.0–264.0 | 110.0–239.0 | 106.0–7.0 | |
| Variable | FibroTest | ActiTest | SteatoTest | NashTest | AshTest |
|---|---|---|---|---|---|
| Baseline value | 0.893 ± 0.082 p < 0.001 * | 0.682 ± 0.120 p < 0.001 * | 0.675 ± 0.112 p < 0.001 * | 0.250 ± 0.189 p = 0.190 | 0.134 ± 0.048 p = 0.007 * |
| Diabetes, type 2 | −0.013 ± 0.027 p = 0.641 | 0.054 ± 0.047 p = 0.261 | 0.011 ± 0.034 p = 0.747 | −0.146 ± 0.079 p = 0.068 | 0.014 ± 0.008 p = 0.088 |
| Treatment-naive patient | −0.024 ± 0.034 p = 0.475 | −0.086 ± 0.060 p = 0.156 | −0.009 ± 0.041 p = 0.025 * | 0.007 ± 0.100 p = 0.946 | 0.001 ± 0.010 p = 0.980 |
| BMI | 0.015 ± 0.004 p < 0.001 * | 0.028 ± 0.006 p < 0.001 * | 0.010 ± 0.005 p = 0.027 * | 0.015 ± 0.001 p = 0.142 | 0.001 ± 0.001 p = 0.129 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popescu, M.-S.; Firu, D.-M.; Pădureanu, V.; Mărginean, C.M.; Mitruț, R.; Arsene, A.L.; Mărgăritescu, D.N.; Calina, D.; Docea, A.O.; Mitruț, P. Effects of Achieving Sustained Virologic Response after Direct-Acting Antiviral Agents on Long-Term Liver Fibrosis in Diabetics vs. in Non-Diabetic Patients with Chronic Hepatitis C Infection. Biomedicines 2022, 10, 2093. https://doi.org/10.3390/biomedicines10092093
Popescu M-S, Firu D-M, Pădureanu V, Mărginean CM, Mitruț R, Arsene AL, Mărgăritescu DN, Calina D, Docea AO, Mitruț P. Effects of Achieving Sustained Virologic Response after Direct-Acting Antiviral Agents on Long-Term Liver Fibrosis in Diabetics vs. in Non-Diabetic Patients with Chronic Hepatitis C Infection. Biomedicines. 2022; 10(9):2093. https://doi.org/10.3390/biomedicines10092093
Chicago/Turabian StylePopescu, Marian-Sorin, Dan-Mihai Firu, Vlad Pădureanu, Cristina Maria Mărginean, Radu Mitruț, Andreea Letitia Arsene, Dragoș Nicolae Mărgăritescu, Daniela Calina, Anca Oana Docea, and Paul Mitruț. 2022. "Effects of Achieving Sustained Virologic Response after Direct-Acting Antiviral Agents on Long-Term Liver Fibrosis in Diabetics vs. in Non-Diabetic Patients with Chronic Hepatitis C Infection" Biomedicines 10, no. 9: 2093. https://doi.org/10.3390/biomedicines10092093
APA StylePopescu, M.-S., Firu, D.-M., Pădureanu, V., Mărginean, C. M., Mitruț, R., Arsene, A. L., Mărgăritescu, D. N., Calina, D., Docea, A. O., & Mitruț, P. (2022). Effects of Achieving Sustained Virologic Response after Direct-Acting Antiviral Agents on Long-Term Liver Fibrosis in Diabetics vs. in Non-Diabetic Patients with Chronic Hepatitis C Infection. Biomedicines, 10(9), 2093. https://doi.org/10.3390/biomedicines10092093










