Neurovascular Unit-Derived Extracellular Vesicles: From Their Physiopathological Roles to Their Clinical Applications in Acute Brain Injuries
Abstract
:1. Introduction
2. EVs in the Central Nervous System
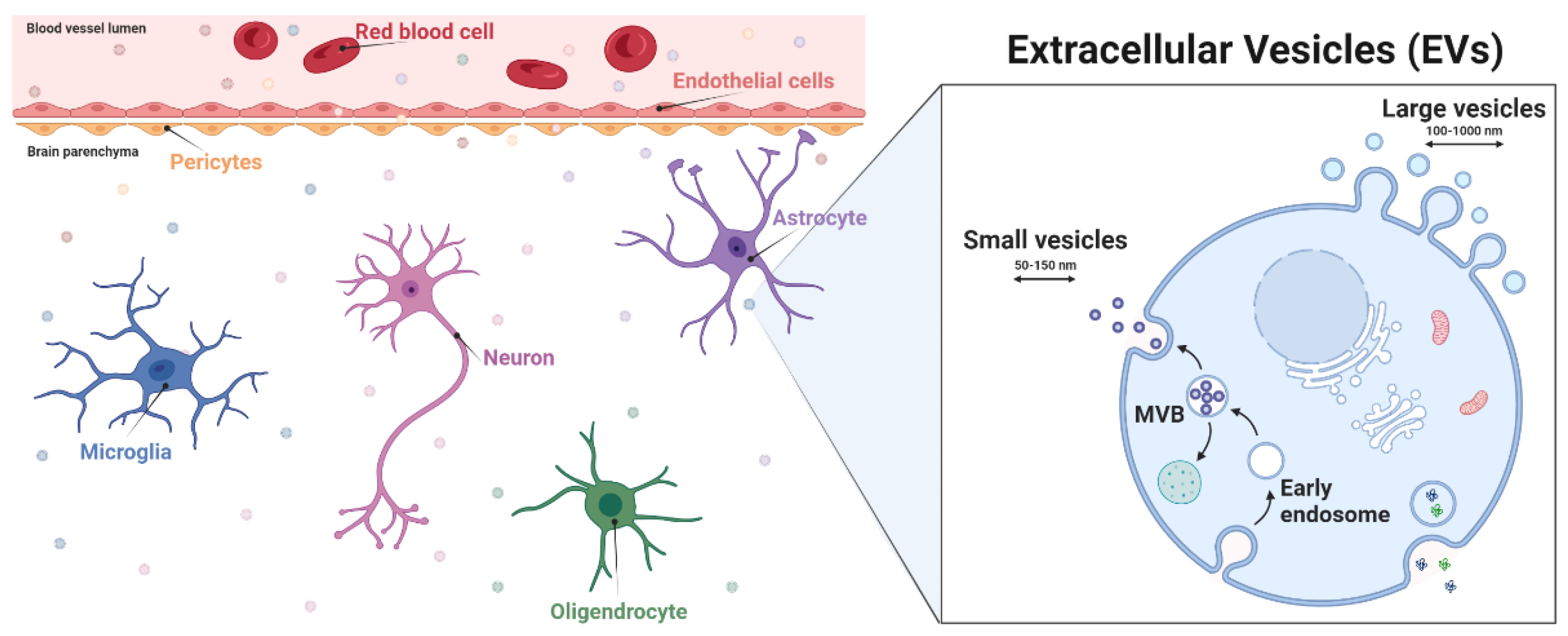
2.1. Neurovascular Unit Structure
2.2. Roles of EVs in the NVU
2.2.1. Roles of NVU Cell-Derived EVs in Physiological Conditions
2.2.2. Roles of NVU Cell-Derived EVs in Pathological Conditions
Neuroinflammation and EVs
Neurodegenerative Diseases and EVs
3. EVs in Acute Brain Injuries
3.1. Acute Brain Injuries
3.2. EVs’ Involvement in the Pathophysiology of Acute Brain Injuries
3.3. EVs as Biomarker Cargo in Acute Brain Injuries
3.3.1. EVs: An Interesting Reservoir of Molecular Biomarkers
3.3.2. EVs as Biomarker Cargo in Stroke
Current Biomarkers in Stroke
Potential EV-Associated Biomarkers in Stroke
3.3.3. EVs as Biomarker Cargo in TBI
Current Biomarkers in TBI
Potential EV-Associated Biomarkers in TBI
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gassama, Y.; Favereaux, A. Emerging Roles of Extracellular Vesicles in the Central Nervous System: Physiology, Pathology, and Therapeutic Perspectives. Front. Cell. Neurosci. 2021, 15, 7. [Google Scholar] [CrossRef] [PubMed]
- Tkach, M.; Théry, C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell 2016, 164, 1226–1232. [Google Scholar] [CrossRef]
- Brenna, S.; Krisp, C.; Altmeppen, H.C.; Magnus, T.; Puig, B. Brain-Derived Extracellular Vesicles in Health and Disease: A Methodological Perspective. Int. J. Mol. Sci. 2021, 22, 1365. [Google Scholar] [CrossRef]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding Light on the Cell Biology of Extracellular Vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Ciferri, M.C.; Quarto, R.; Tasso, R. Extracellular Vesicles as Biomarkers and Therapeutic Tools: From Pre-Clinical to Clinical Applications. Biology 2021, 10, 359. [Google Scholar] [CrossRef]
- Yue, B.; Yang, H.; Wang, J.; Ru, W.; Wu, J.; Huang, Y.; Lan, X.; Lei, C.; Chen, H. Exosome Biogenesis, Secretion and Function of Exosomal MiRNAs in Skeletal Muscle Myogenesis. Cell Prolif. 2020, 53, e12857. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Couch, Y.; Buzàs, E.I.; Di Vizio, D.; Gho, Y.S.; Harrison, P.; Hill, A.F.; Lötvall, J.; Raposo, G.; Stahl, P.D.; Théry, C.; et al. A Brief History of Nearly EV-erything–The Rise and Rise of Extracellular Vesicles. J. Extracell. Vesicles 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Hochberg, F.H.; Jones, P.S. Extracellular Vesicles: The Growth as Diagnostics and Therapeutics; a Survey. J. Extracell. Vesicles 2018, 7, 1438720. [Google Scholar] [CrossRef]
- Marostica, G.; Gelibter, S.; Gironi, M.; Nigro, A.; Furlan, R. Extracellular Vesicles in Neuroinflammation. Front. Cell Dev. Biol. 2021, 8, 1841. [Google Scholar] [CrossRef]
- Pouso, M.R.; Cairrao, E. Effect of Retinoic Acid on the Neurovascular Unit: A Review. Brain Res. Bull. 2022, 184, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Muoio, V.; Persson, P.B.; Sendeski, M.M. The Neurovascular Unit-Concept Review. Acta Physiol. 2014, 210, 790–798. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Ji, C.; Shao, A. Neurovascular Unit Dysfunction and Neurodegenerative Disorders. Front. Neurosci. 2020, 14, 334. [Google Scholar] [CrossRef] [PubMed]
- Hudson, N.; Campbell, M. Tight Junctions of the Neurovascular Unit. Front. Mol. Neurosci. 2021, 14. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Kanekiyo, T. Blood-Brain Barrier Dysfunction and the Pathogenesis of Alzheimer’s Disease. Int. J. Mol. Sci. 2017, 18, 1965. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Wong, L.-W.; Su, Y.; Huang, X.; Wang, N.; Chen, H.; Yi, C. Blood-Brain Barrier Integrity in the Pathogenesis of Alzheimer’s Disease. Front. Neuroendocrinol. 2020, 59, 100857. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood–Brain Barrier Breakdown in Alzheimer Disease and Other Neurodegenerative Disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef]
- Armulik, A.; Genové, G.; Mäe, M.; Nisancioglu, M.H.; Wallgard, E.; Niaudet, C.; He, L.; Norlin, J.; Lindblom, P.; Strittmatter, K.; et al. Pericytes Regulate the Blood–Brain Barrier. Nature 2010, 468, 557–561. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Ayyadurai, S.; Zlokovic, B.V. Pericytes of the Neurovascular Unit: Key Functions and Signaling Pathways. Nat. Neurosci. 2016, 19, 771–783. [Google Scholar] [CrossRef]
- Tietz, S.; Engelhardt, B. Brain Barriers: Crosstalk between Complex Tight Junctions and Adherens Junctions. J. Cell Biol. 2015, 209, 493–506. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, T.A.; El-Badri, N. Pericytes: The Role of Multipotent Stem Cells in Vascular Maintenance and Regenerative Medicine. Adv. Exp. Med. Biol. 2018, 1079, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Chen, Z.-L.; Norris, E.H.; Strickland, S. Astrocytic Laminin Regulates Pericyte Differentiation and Maintains Blood Brain Barrier Integrity. Nat. Commun. 2014, 5, 3413. [Google Scholar] [CrossRef]
- Skoff, R.P. Gliogenesis in Rat Optic Nerve: Astrocytes Are Generated in a Single Wave before Oligodendrocytes. Dev. Biol. 1990, 139, 149–168. [Google Scholar] [CrossRef]
- Thurgur, H.; Pinteaux, E. Microglia in the Neurovascular Unit: Blood–Brain Barrier–Microglia Interactions After Central Nervous System Disorders. Neuroscience 2019, 405, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, S.; Gritti, L.; Crooks, D.; Dombrowski, Y. Oligodendrocytes in Development, Myelin Generation and Beyond. Cells 2019, 8, 1424. [Google Scholar] [CrossRef]
- Barateiro, A.; Brites, D.; Fernandes, A. Oligodendrocyte Development and Myelination in Neurodevelopment: Molecular Mechanisms in Health and Disease. Curr. Pharm. Des. 2016, 22, 656–679. [Google Scholar] [CrossRef]
- Banerjee, S.; Bhat, M.A. Neuron-Glial Interactions in Blood-Brain Barrier Formation. Annu. Rev. Neurosci. 2007, 30, 235–258. [Google Scholar] [CrossRef]
- Segarra, M.; Aburto, M.R.; Hefendehl, J.; Acker-Palmer, A. Neurovascular Interactions in the Nervous System. Annu. Rev. Cell Dev. Biol. 2019, 35, 615–635. [Google Scholar] [CrossRef]
- Matsumoto, J.; Stewart, T.; Banks, W.A.; Zhang, J. The Transport Mechanism of Extracellular Vesicles at the Blood-Brain Barrier. Curr. Pharm. Des. 2017, 23, 6206–6214. [Google Scholar] [CrossRef]
- Busatto, S.; Morad, G.; Guo, P.; Moses, M.A. The Role of Extracellular Vesicles in the Physiological and Pathological Regulation of the Blood-Brain Barrier. FASEB Bioadv. 2021, 3, 665–675. [Google Scholar] [CrossRef]
- Saint-Pol, J.; Gosselet, F.; Duban-Deweer, S.; Pottiez, G.; Karamanos, Y. Targeting and Crossing the Blood-Brain Barrier with Extracellular Vesicles. Cells 2020, 9, 851. [Google Scholar] [CrossRef] [PubMed]
- Stremersch, S.; De Smedt, S.C.; Raemdonck, K. Therapeutic and Diagnostic Applications of Extracellular Vesicles. J. Control. Release 2016, 244, 167–183. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, A.; Silva, A.M.; Teixeira, J.H.; Gonçalves, R.M.; Almeida, M.I.; Barbosa, M.A.; Santos, S.G. Extracellular Vesicles: Intelligent Delivery Strategies for Therapeutic Applications. J. Control. Release 2018, 289, 56–69. [Google Scholar] [CrossRef]
- Oscar, P.; Wiklander, B.; Brennan, M.Á.; Lötvall, J.; Breakefield, X.O.; Andaloussi, S.E.L. Advances in Therapeutic Applications of Extracellular Vesicles. Sci. Transl. Med. 2019, 11, eaav8521. [Google Scholar] [CrossRef]
- Haqqani, A.S.; Delaney, C.E.; Tremblay, T.-L.; Sodja, C.; Sandhu, J.K.; Stanimirovic, D.B. Method for Isolation and Molecular Characterization of Extracellular Microvesicles Released from Brain Endothelial Cells. Fluids Barriers CNS 2013, 10, 4. [Google Scholar] [CrossRef]
- Dozio, V.; Sanchez, J.-C. Characterisation of Extracellular Vesicle-Subsets Derived from Brain Endothelial Cells and Analysis of Their Protein Cargo Modulation after TNF Exposure. J. Extracell. Vesicles 2017, 6, 1302705. [Google Scholar] [CrossRef]
- Schiera, G.; Proia, P.; Alberti, C.; Mineo, M.; Savettieri, G.; Di Liegro, I. Neurons Produce FGF2 and VEGF and Secrete Them at Least in Part by Shedding Extracellular Vesicles. J. Cell. Mol. Med. 2007, 11, 1384–1394. [Google Scholar] [CrossRef]
- Proia, P.; Schiera, G.; Mineo, M.; Ingrassia, A.M.R.; Santoro, G.; Savettieri, G.; Di Liegro, I. Astrocytes Shed Extracellular Vesicles That Contain Fibroblast Growth Factor-2 and Vascular Endothelial Growth Factor. Int. J. Mol. Med. 2008, 21, 63–67. [Google Scholar] [CrossRef]
- Attwell, D.; Mishra, A.; Hall, C.N.; O’Farrell, F.M.; Dalkara, T. What Is a Pericyte? J. Cereb. Blood Flow Metab. 2016, 36, 451–455. [Google Scholar] [CrossRef]
- Yuan, X.; Wu, Q.; Wang, P.; Jing, Y.; Yao, H.; Tang, Y.; Li, Z.; Zhang, H.; Xiu, R. Exosomes Derived From Pericytes Improve Microcirculation and Protect Blood–Spinal Cord Barrier After Spinal Cord Injury in Mice. Front. Neurosci. 2019, 13. [Google Scholar] [CrossRef]
- Willis, C.M.; Nicaise, A.M.; Bongarzone, E.R.; Givogri, M.; Reiter, C.R.; Heintz, O.; Jellison, E.R.; Sutter, P.A.; TeHennepe, G.; Ananda, G.; et al. Astrocyte Support for Oligodendrocyte Differentiation Can Be Conveyed via Extracellular Vesicles but Diminishes with Age. Sci. Rep. 2020, 10, 828. [Google Scholar] [CrossRef] [PubMed]
- Steiner, J.P.; Gardner, K.; Baines, A.; Bennett, V. Synapsin I: A Regulated Synaptic Vesicle Organizing Protein. Brain Res. Bull. 1987, 18, 777–785. [Google Scholar] [CrossRef]
- Wang, S.; Cesca, F.; Loers, G.; Schweizer, M.; Buck, F.; Benfenati, F.; Schachner, M.; Kleene, R. Synapsin I Is an Oligomannose-Carrying Glycoprotein, Acts as an Oligomannose-Binding Lectin, and Promotes Neurite Outgrowth and Neuronal Survival When Released via Glia-Derived Exosomes. J. Neurosci. 2011, 31, 7275–7290. [Google Scholar] [CrossRef] [PubMed]
- Bélanger, M.; Magistretti, P.J. The Role of Astroglia in Neuroprotection. Dialogues Clin. Neurosci. 2009, 11, 281–295. [Google Scholar] [CrossRef]
- Chen, Y.; Qin, C.; Huang, J.; Tang, X.; Liu, C.; Huang, K.; Xu, J.; Guo, G.; Tong, A.; Zhou, L. The Role of Astrocytes in Oxidative Stress of Central Nervous System: A Mixed Blessing. Cell Prolif. 2020, 53, e12781. [Google Scholar] [CrossRef]
- Ganfornina, M.D.; Do Carmo, S.; Lora, J.M.; Torres-Schumann, S.; Vogel, M.; Allhorn, M.; González, C.; Bastiani, M.J.; Rassart, E.; Sanchez, D. Apolipoprotein D Is Involved in the Mechanisms Regulating Protection from Oxidative Stress. Aging Cell 2008, 7, 506–515. [Google Scholar] [CrossRef]
- Ganfornina, M.D.; Do Carmo, S.; Martínez, E.; Tolivia, J.; Navarro, A.; Rassart, E.; Sanchez, D. ApoD, a Glia-Derived Apolipoprotein, Is Required for Peripheral Nerve Functional Integrity and a Timely Response to Injury. Glia 2010, 58, 1320–1334. [Google Scholar] [CrossRef]
- Pascua-Maestro, R.; González, E.; Lillo, C.; Ganfornina, M.D.; Falcón-Pérez, J.M.; Sanchez, D. Extracellular Vesicles Secreted by Astroglial Cells Transport Apolipoprotein D to Neurons and Mediate Neuronal Survival Upon Oxidative Stress. Front. Cell. Neurosci. 2018, 12, 526. [Google Scholar] [CrossRef]
- Taylor, A.R.; Robinson, M.B.; Gifondorwa, D.J.; Tytell, M.; Milligan, C.E. Regulation of Heat Shock Protein 70 Release in Astrocytes: Role of Signaling Kinases. Dev. Neurobiol. 2007, 67, 1815–1829. [Google Scholar] [CrossRef]
- Lemaire, Q.; Raffo-Romero, A.; Arab, T.; Van Camp, C.; Drago, F.; Forte, S.; Gimeno, J.-P.; Begard, S.; Colin, M.; Vizioli, J.; et al. Isolation of Microglia-Derived Extracellular Vesicles: Towards MiRNA Signatures and Neuroprotection. J. Nanobiotechnology 2019, 17, 119. [Google Scholar] [CrossRef]
- Potolicchio, I.; Carven, G.J.; Xu, X.; Stipp, C.; Riese, R.J.; Stern, L.J.; Santambrogio, L. Proteomic Analysis of Microglia-Derived Exosomes: Metabolic Role of the Aminopeptidase CD13 in Neuropeptide Catabolism. J. Immunol. 2005, 175, 2237–2243. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, Q.; Zhao, Z.-Z.; Xu, X.; Li, S.; Yin, H.; Li, L.; Zhang, J.; Wang, R. An Eco- and User-Friendly Herbicide. J. Agric. Food Chem. 2019, 67, 7783–7792. [Google Scholar] [CrossRef]
- Chivet, M.; Hemming, F.; Pernet-Gallay, K.; Fraboulet, S.; Sadoul, R. Emerging Role of Neuronal Exosomes in the Central Nervous System. Front. Physiol. 2012, 3, 145. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, Y.; Du, X.-F.; Li, J.; Zi, H.-X.; Bu, J.-W.; Yan, Y.; Han, H.; Du, J.-L. Neurons Secrete MiR-132-Containing Exosomes to Regulate Brain Vascular Integrity. Cell Res. 2017, 27, 882–897. [Google Scholar] [CrossRef] [PubMed]
- Blanchette, C.R.; Rodal, A.A. Mechanisms for Biogenesis and Release of Neuronal Extracellular Vesicles. Curr. Opin. Neurobiol. 2020, 63, 104–110. [Google Scholar] [CrossRef]
- DiSabato, D.J.; Quan, N.; Godbout, J.P. Neuroinflammation: The Devil Is in the Details. J. Neurochem. 2016, 139, 136–153. [Google Scholar] [CrossRef] [PubMed]
- Bazan, N.G.; Halabi, A.; Ertel, M.; Petasis, N.A. Chapter 34—Neuroinflammation. In Basic Neurochemistry, 8th ed.; Brady, S.T., Siegel, G.J., Albers, R.W., Price, D.L., Eds.; Academic Press: New York, NY, USA, 2012; pp. 610–620. ISBN 978-0-12-374947-5. [Google Scholar]
- Jayaraj, R.L.; Azimullah, S.; Beiram, R.; Jalal, F.Y.; Rosenberg, G.A. Neuroinflammation: Friend and Foe for Ischemic Stroke. J. Neuroinflammation 2019, 16, 142. [Google Scholar] [CrossRef]
- Shabab, T.; Khanabdali, R.; Moghadamtousi, S.Z.; Kadir, H.A.; Mohan, G. Neuroinflammation Pathways: A General Review. Int. J. Neurosci. 2017, 127, 624–633. [Google Scholar] [CrossRef]
- Naveed, M.; Zhou, Q.-G.; Han, F. Cerebrovascular Inflammation: A Critical Trigger for Neurovascular Injury? Neurochem. Int. 2019, 126, 165–177. [Google Scholar] [CrossRef]
- Sochocka, M.; Diniz, B.S.; Leszek, J. Inflammatory Response in the CNS: Friend or Foe? Mol. Neurobiol. 2017, 54, 8071–8089. [Google Scholar] [CrossRef] [Green Version]
- Anrather, J.; Iadecola, C. Inflammation and Stroke: An Overview. Neurotherapeutics 2016, 13, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Babu, R.; Bagley, J.H.; Di, C.; Friedman, A.H.; Adamson, C. Thrombin and Hemin as Central Factors in the Mechanisms of Intracerebral Hemorrhage–Induced Secondary Brain Injury and as Potential Targets for Intervention. Neurosurg. Focus 2012, 32, E8. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Tu, S.; Shao, A. Pathophysiological Mechanisms and Potential Therapeutic Targets in Intracerebral Hemorrhage. Front. Pharmacol. 2019, 10, 1079. [Google Scholar] [CrossRef]
- Yates, A.G.; Anthony, D.C.; Ruitenberg, M.J.; Couch, Y. Systemic Immune Response to Traumatic CNS Injuries—Are Extracellular Vesicles the Missing Link? Front. Immunol. 2019, 10, 2723. [Google Scholar] [CrossRef] [PubMed]
- Spiers, J.G.; Vassileff, N.; Hill, A.F. Neuroinflammatory Modulation of Extracellular Vesicle Biogenesis and Cargo Loading. NeuroMolecular Med. 2022. Online ahead of print. [Google Scholar] [CrossRef]
- Vaz, A.R.; Pinto, S.; Ezequiel, C.; Cunha, C.; Carvalho, L.A.; Moreira, R.; Brites, D. Phenotypic Effects of Wild-Type and Mutant SOD1 Expression in N9 Murine Microglia at Steady State, Inflammatory and Immunomodulatory Conditions. Front. Cell. Neurosci. 2019, 13, 109. [Google Scholar] [CrossRef]
- Kumar, A.; Stoica, B.A.; Loane, D.J.; Yang, M.; Abulwerdi, G.; Khan, N.; Kumar, A.; Thom, S.R.; Faden, A.I. Microglial-Derived Microparticles Mediate Neuroinflammation after Traumatic Brain Injury. J. Neuroinflammation 2017, 14, 47. [Google Scholar] [CrossRef]
- Datta Chaudhuri, A.; Dasgheyb, R.M.; DeVine, L.R.; Bi, H.; Cole, R.N.; Haughey, N.J. Stimulus-Dependent Modifications in Astrocyte-Derived Extracellular Vesicle Cargo Regulate Neuronal Excitability. Glia 2020, 68, 128–144. [Google Scholar] [CrossRef]
- Chaudhuri, A.D.; Dastgheyb, R.M.; Yoo, S.-W.; Trout, A.; Talbot, C.C.; Hao, H.; Witwer, K.W.; Haughey, N.J. TNFα and IL-1β Modify the MiRNA Cargo of Astrocyte Shed Extracellular Vesicles to Regulate Neurotrophic Signaling in Neurons. Cell Death Dis. 2018, 9, 363. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.-H. Neuroinflammation in Neurodegenerative Disorders: The Roles of Microglia and Astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef]
- Bhargava, P.; Nogueras-Ortiz, C.; Chawla, S.; Bæk, R.; Jørgensen, M.M.; Kapogiannis, D. Altered Levels of Toll-Like Receptors in Circulating Extracellular Vesicles in Multiple Sclerosis. Cells 2019, 8, 1058. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, F.; Montesinos, J.; Ureña-Peralta, J.R.; Guerri, C.; Pascual, M. TLR4 Participates in the Transmission of Ethanol-Induced Neuroinflammation via Astrocyte-Derived Extracellular Vesicles. J Neuroinflammation 2019, 16, 136. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Li, Z.; Gao, G.; Li, X.; Liao, Z.; Wang, Y.; Li, W.; Zhang, Y.; Liu, W. Combined Analysis of Surface Protein Profile and MicroRNA Expression Profile of Exosomes Derived from Brain Microvascular Endothelial Cells in Early Cerebral Ischemia. ACS Omega 2021, 6, 22410–22421. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Bai, X.; Zhang, A.; Huang, J.; Xu, S.; Zhang, J. Role of Exosomes in Central Nervous System Diseases. Front. Mol. Neurosci. 2019, 12, 240. [Google Scholar] [CrossRef]
- Peng, N.; Su, L. Progresses in Understanding Trauma-Induced Coagulopathy and the Underlying Mechanism. Chin. J. Traumatol. 2017, 20, 133–136. [Google Scholar] [CrossRef]
- Levi, M.; van der Poll, T. Two-Way Interactions between Inflammation and Coagulation. Trends Cardiovasc. Med. 2005, 15, 254–259. [Google Scholar] [CrossRef]
- Biró, É.; Sturk-Maquelin, K.N.; Vogel, G.M.T.; Meuleman, D.G.; Smit, M.J.; Hack, C.E.; Sturk, A.; Nieuwland, R. Human Cell-Derived Microparticles Promote Thrombus Formation in Vivo in a Tissue Factor-Dependent Manner. J. Thromb. Haemost. 2003, 1, 2561–2568. [Google Scholar] [CrossRef]
- Morel, N.; Morel, O.; Petit, L.; Hugel, B.; Cochard, J.-F.; Freyssinet, J.-M.; Sztark, F.; Dabadie, P. Generation of Procoagulant Microparticles in Cerebrospinal Fluid and Peripheral Blood After Traumatic Brain Injury. J. Trauma Acute Care Surg. 2008, 64, 698–704. [Google Scholar] [CrossRef]
- Nekludov, M.; Mobarrez, F.; Gryth, D.; Bellander, B.-M.; Wallen, H. Formation of Microparticles in the Injured Brain of Patients with Severe Isolated Traumatic Brain Injury. J. Neurotrauma 2014, 31, 1927–1933. [Google Scholar] [CrossRef]
- Nieuwland, R.; Berckmans, R.J.; Rotteveel-Eijkman, R.C.; Maquelin, K.N.; Roozendaal, K.J.; Jansen, P.G.; ten Have, K.; Eijsman, L.; Hack, C.E.; Sturk, A. Cell-Derived Microparticles Generated in Patients during Cardiopulmonary Bypass Are Highly Procoagulant. Circulation 1997, 96, 3534–3541. [Google Scholar] [CrossRef]
- Lalic-Cosic, S.; Dopsaj, V.; Kovac, M.; Mandic-Markovic, V.; Mikovic, Z.; Mobarrez, F.; Antovic, A. Phosphatidylserine Exposing Extracellular Vesicles in Pre-Eclamptic Patients. Front. Med. 2021, 8, 761453. [Google Scholar] [CrossRef] [PubMed]
- Owens, A.P.; Mackman, N. Microparticles in Hemostasis and Thrombosis. Circ. Res. 2011, 108, 1284–1297. [Google Scholar] [CrossRef] [PubMed]
- Berckmans, R.J.; Sturk, A.; van Tienen, L.M.; Schaap, M.C.L.; Nieuwland, R. Cell-Derived Vesicles Exposing Coagulant Tissue Factor in Saliva. Blood 2011, 117, 3172–3180. [Google Scholar] [CrossRef] [PubMed]
- Spronk, H.M.H.; ten Cate, H.; van der Meijden, P.E.J. Differential Roles of Tissue Factor and Phosphatidylserine in Activation of Coagulation. Thromb. Res. 2014, 133, S54–S56. [Google Scholar] [CrossRef]
- Midura, E.F.; Jernigan, P.L.; Kuethe, J.W.; Friend, L.A.; Veile, R.; Makley, A.T.; Caldwell, C.C.; Goodman, M.D. Microparticles Impact Coagulation after Traumatic Brain Injury. J. Surg. Res. 2015, 197, 25–31. [Google Scholar] [CrossRef]
- Tian, Y.; Salsbery, B.; Wang, M.; Yuan, H.; Yang, J.; Zhao, Z.; Wu, X.; Zhang, Y.; Konkle, B.A.; Thiagarajan, P.; et al. Brain-Derived Microparticles Induce Systemic Coagulation in a Murine Model of Traumatic Brain Injury. Blood 2015, 125, 2151–2159. [Google Scholar] [CrossRef]
- Wada, T.; Shiraishi, A.; Gando, S.; Yamakawa, K.; Fujishima, S.; Saitoh, D.; Kushimoto, S.; Ogura, H.; Abe, T.; Mayumi, T.; et al. Pathophysiology of Coagulopathy Induced by Traumatic Brain Injury Is Identical to That of Disseminated Intravascular Coagulation with Hyperfibrinolysis. Front. Med. 2021, 8, 767637. [Google Scholar] [CrossRef]
- Poplin, V.; Boulware, D.R.; Bahr, N.C. Methods for Rapid Diagnosis of Meningitis Etiology in Adults. Biomark. Med. 2020, 14, 459–479. [Google Scholar] [CrossRef]
- Scharfman, H.E. The Neurobiology of Epilepsy. Curr. Neurol. Neurosci. Rep. 2007, 7, 348–354. [Google Scholar] [CrossRef]
- Mckee, A.C.; Daneshvar, D.H. Chapter 4—The Neuropathology of Traumatic Brain Injury. In Handbook of Clinical Neurology; Grafman, J., Salazar, A.M., Eds.; Traumatic Brain Injury, Part I; Elsevier: Amsterdam, The Netherlands, 2015; Volume 127, pp. 45–66. [Google Scholar]
- Wolfe, C.D.A. The Impact of Stroke. Br. Med. Bull. 2000, 56, 275–286. [Google Scholar] [CrossRef] [Green Version]
- Kornek, B.; Lassmann, H. Neuropathology of Multiple Sclerosis—New Concepts. Brain Res. Bull. 2003, 61, 321–326. [Google Scholar] [CrossRef]
- Gammon, K. Neurodegenerative Disease: Brain Windfall. Nature 2014, 515, 299–300. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A Summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Balaji, V.; Kaniyappan, S.; Krüger, L.; Irsen, S.; Tepper, K.; Chandupatla, R.; Maetzler, W.; Schneider, A.; Mandelkow, E.; et al. The Release and Trans-Synaptic Transmission of Tau via Exosomes. Mol. Neurodegener. 2017, 12, 5. [Google Scholar] [CrossRef]
- Murphy, M.P.; LeVine, H. Alzheimer’s Disease and the Amyloid-Beta Peptide. J. Alzheimers Dis. 2010, 19, 311–323. [Google Scholar] [CrossRef]
- Achim, C.L.; Adame, A.; Dumaop, W.; Everall, I.P.; Masliah, E. Increased Accumulation of Intraneuronal Amyloid β in HIV-Infected Patients. J. Neuroimmune Pharmacol. 2009, 4, 190–199. [Google Scholar] [CrossRef]
- Xu, J.; Ikezu, T. The Comorbidity of HIV-Associated Neurocognitive Disorders and Alzheimer’s Disease: A Foreseeable Medical Challenge in Post-HAART Era. J. Neuroimmune Pharmacol. 2009, 4, 200–212. [Google Scholar] [CrossRef]
- Brew, B.J.; Crowe, S.M.; Landay, A.; Cysique, L.A.; Guillemin, G. Neurodegeneration and Ageing in the HAART Era. J. Neuroimmune Pharmacol. 2009, 4, 163–174. [Google Scholar] [CrossRef]
- András, I.E.; Leda, A.; Contreras, M.G.; Bertrand, L.; Park, M.; Skowronska, M.; Toborek, M. Extracellular Vesicles of the Blood-Brain Barrier: Role in the HIV-1 Associated Amyloid Beta Pathology. Mol. Cell. Neurosci. 2017, 79, 12–22. [Google Scholar] [CrossRef]
- Emmanouilidou, E.; Melachroinou, K.; Roumeliotis, T.; Garbis, S.D.; Ntzouni, M.; Margaritis, L.H.; Stefanis, L.; Vekrellis, K. Cell-Produced α-Synuclein Is Secreted in a Calcium-Dependent Manner by Exosomes and Impacts Neuronal Survival. J. Neurosci. 2010, 30, 6838–6851. [Google Scholar] [CrossRef] [Green Version]
- Silverman, J.M.; Christy, D.; Shyu, C.C.; Moon, K.-M.; Fernando, S.; Gidden, Z.; Cowan, C.M.; Ban, Y.; Stacey, R.G.; Grad, L.I.; et al. CNS-Derived Extracellular Vesicles from Superoxide Dismutase 1 (SOD1)G93A ALS Mice Originate from Astrocytes and Neurons and Carry Misfolded SOD1. J. Biol. Chem. 2019, 294, 3744–3759. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Sun, M.; Wang, F.; Geng, X.; Wang, F. Identification of Hub Genes Related to Alzheimer’s Disease and Major Depressive Disorder. Am. J. Alzheimer’s Dis. Other Dement. 2021, 36, 15333175211046123. [Google Scholar] [CrossRef]
- You, Y.; Muraoka, S.; Jedrychowski, M.P.; Hu, J.; McQuade, A.K.; Young-Pearse, T.; Aslebagh, R.; Shaffer, S.A.; Gygi, S.P.; Blurton-Jones, M.; et al. Human Neural Cell Type-Specific Extracellular Vesicle Proteome Defines Disease-Related Molecules Associated with Activated Astrocytes in Alzheimer’s Disease Brain. J. Extracell. Vesicles 2022, 11, e12183. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.-Y.; Wang, C.-Y.; Wang, T.; Li, Y.-C.; Wang, Z.-Y. Glial S100A6 Degrades β-Amyloid Aggregation through Targeting Competition with Zinc Ions. Aging Dis. 2019, 10, 756–769. [Google Scholar] [CrossRef] [PubMed]
- González-Molina, L.A.; Villar-Vesga, J.; Henao-Restrepo, J.; Villegas, A.; Lopera, F.; Cardona-Gómez, G.P.; Posada-Duque, R. Extracellular Vesicles From 3xTg-AD Mouse and Alzheimer’s Disease Patient Astrocytes Impair Neuroglial and Vascular Components. Front. Aging Neurosci. 2021, 13, 593927. [Google Scholar] [CrossRef]
- Lan, Y.L.; Zhao, J.; Ma, T.; Li, S. The Potential Roles of Aquaporin 4 in Alzheimer’s Disease. Mol. Neurobiol. 2016, 53, 5300–5309. [Google Scholar] [CrossRef]
- Zeppenfeld, D.M.; Simon, M.; Haswell, J.D.; D’Abreo, D.; Murchison, C.; Quinn, J.F.; Grafe, M.R.; Woltjer, R.L.; Kaye, J.; Iliff, J.J. Association of Perivascular Localization of Aquaporin-4 With Cognition and Alzheimer Disease in Aging Brains. JAMA Neurol. 2017, 74, 91–99. [Google Scholar] [CrossRef]
- Cohn, W.; Melnik, M.; Huang, C.; Teter, B.; Chandra, S.; Zhu, C.; McIntire, L.B.; John, V.; Gylys, K.H.; Bilousova, T. Multi-Omics Analysis of Microglial Extracellular Vesicles from Human Alzheimer’s Disease Brain Tissue Reveals Disease-Associated Signatures. Front. Pharmacol. 2021, 12, 766082. [Google Scholar] [CrossRef]
- Guo, M.; Wang, J.; Zhao, Y.; Feng, Y.; Han, S.; Dong, Q.; Cui, M.; Tieu, K. Microglial Exosomes Facilitate α-Synuclein Transmission in Parkinson’s Disease. Brain 2020, 143, 1476–1497. [Google Scholar] [CrossRef]
- Li, N.; Wu, Y.; Zhu, L.; Huang, Y.; Liu, Z.; Shi, M.; Soltys, D.; Zhang, J.; Chang, Q. Extracellular Microvesicles-Derived from Microglia Treated with Unaggregated α-Synuclein Attenuate Mitochondrial Fission and Toxicity-Induced by Parkinsonian Toxin MPP+. Biochem. Biophys. Res. Commun. 2019, 517, 642–647. [Google Scholar] [CrossRef]
- Yuyama, K.; Igarashi, Y. Exosomes as Carriers of Alzheimer’s Amyloid-ß. Front. Neurosci. 2017, 11, 229. [Google Scholar] [CrossRef] [PubMed]
- Yuyama, K.; Sun, H.; Sakai, S.; Mitsutake, S.; Okada, M.; Tahara, H.; Furukawa, J.; Fujitani, N.; Shinohara, Y.; Igarashi, Y. Decreased Amyloid-β Pathologies by Intracerebral Loading of Glycosphingolipid-Enriched Exosomes in Alzheimer Model Mice. J. Biol. Chem. 2014, 289, 24488–24498. [Google Scholar] [CrossRef]
- Go, A.S.; Mozaffarian, D.; Roger, V.L.; Benjamin, E.J.; Berry, J.D.; Borden, W.B.; Bravata, D.M.; Dai, S.; Ford, E.S.; Fox, C.S.; et al. Heart Disease and Stroke Statistics—2013 Update. Circulation 2013, 127, e6–e245. [Google Scholar] [CrossRef] [PubMed]
- Guedes, V.A.; Devoto, C.; Leete, J.; Sass, D.; Acott, J.D.; Mithani, S.; Gill, J.M. Extracellular Vesicle Proteins and MicroRNAs as Biomarkers for Traumatic Brain Injury. Front. Neurol. 2020, 11, 663. [Google Scholar] [CrossRef] [PubMed]
- The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 19 May 2021).
- Thelin, E.P.; Nelson, D.W.; Bellander, B.-M. A Review of the Clinical Utility of Serum S100B Protein Levels in the Assessment of Traumatic Brain Injury. Acta Neurochir. 2017, 159, 209–225. [Google Scholar] [CrossRef] [PubMed]
- Lagerstedt, L.; Egea-Guerrero, J.J.; Rodríguez-Rodríguez, A.; Bustamante, A.; Montaner, J.; El Rahal, A.; Andereggen, E.; Rinaldi, L.; Sarrafzadeh, A.; Schaller, K.; et al. Early Measurement of Interleukin-10 Predicts the Absence of CT Scan Lesions in Mild Traumatic Brain Injury. PLoS ONE 2018, 13, e0193278. [Google Scholar] [CrossRef]
- Meschia, J.F.; Brott, T. Ischaemic Stroke. Eur. J. Neurol. 2018, 25, 35–40. [Google Scholar] [CrossRef]
- Hong, S.-B.; Yang, H.; Manaenko, A.; Lu, J.; Mei, Q.; Hu, Q. Potential of Exosomes for the Treatment of Stroke. Cell Transpl. 2019, 28, 662–670. [Google Scholar] [CrossRef]
- Sun, M.K.; Passaro, A.P.; Latchoumane, C.-F.; Spellicy, S.E.; Bowler, M.; Goeden, M.; Martin, W.J.; Holmes, P.V.; Stice, S.L.; Karumbaiah, L. Extracellular Vesicles Mediate Neuroprotection and Functional Recovery after Traumatic Brain Injury. J. Neurotrauma 2020, 37, 1358–1369. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhou, Y.; Tian, Y.; Li, M.; Dong, J.; Zhang, J. Cellular Microparticles and Pathophysiology of Traumatic Brain Injury. Protein Cell 2017, 8, 801–810. [Google Scholar] [CrossRef] [Green Version]
- Dutta, D.; Khan, N.; Wu, J.; Jay, S.M. Extracellular Vesicles as an Emerging Frontier in Spinal Cord Injury Pathobiology and Therapy. Trends Neurosci. 2021, 44, 492–506. [Google Scholar] [CrossRef]
- Andrews, A.M.; Lutton, E.M.; Merkel, S.F.; Razmpour, R.; Ramirez, S.H. Mechanical Injury Induces Brain Endothelial-Derived Microvesicle Release: Implications for Cerebral Vascular Injury during Traumatic Brain Injury. Front. Cell. Neurosci. 2016, 10, 43. [Google Scholar] [CrossRef]
- Bohman, L.-E.; Riley, J.; Milovanova, T.N.; Sanborn, M.R.; Thom, S.R.; Armstead, W.M. Microparticles Impair Hypotensive Cerebrovasodilation and Cause Hippocampal Neuronal Cell Injury after Traumatic Brain Injury. J. Neurotrauma 2016, 33, 168–174. [Google Scholar] [CrossRef]
- Hazelton, I.; Yates, A.; Dale, A.; Roodselaar, J.; Akbar, N.; Ruitenberg, M.J.; Anthony, D.C.; Couch, Y. Exacerbation of Acute Traumatic Brain Injury by Circulating Extracellular Vesicles. J. Neurotrauma 2018, 35, 639–651. [Google Scholar] [CrossRef]
- Lackner, P.; Dietmann, A.; Beer, R.; Fischer, M.; Broessner, G.; Helbok, R.; Marxgut, J.; Pfausler, B.; Schmutzhard, E. Cellular Microparticles as a Marker for Cerebral Vasospasm in Spontaneous Subarachnoid Hemorrhage. Stroke 2010, 41, 2353–2357. [Google Scholar] [CrossRef]
- Sanborn, M.R.; Thom, S.R.; Bohman, L.-E.; Stein, S.C.; Levine, J.M.; Milovanova, T.; Maloney-Wilensky, E.; Frangos, S.; Kumar, M.A. Temporal Dynamics of Microparticle Elevation Following Subarachnoid Hemorrhage: Laboratory Investigation. J. Neurosurg. 2012, 117, 579–586. [Google Scholar] [CrossRef]
- Huang, M.; Hu, Y.-Y.; Dong, X.-Q. High Concentrations of Procoagulant Microparticles in the Cerebrospinal Fluid and Peripheral Blood of Patients with Acute Basal Ganglia Hemorrhage Are Associated with Poor Outcome. Surg. Neurol. 2009, 72, 481–489. [Google Scholar] [CrossRef]
- Kuriyama, N.; Nagakane, Y.; Hosomi, A.; Ohara, T.; Kasai, T.; Harada, S.; Takeda, K.; Yamada, K.; Ozasa, K.; Tokuda, T.; et al. Evaluation of Factors Associated With Elevated Levels of Platelet-Derived Microparticles in the Acute Phase of Cerebral Infarction. Clin. Appl. Thromb. Hemost. 2010, 16, 26–32. [Google Scholar] [CrossRef]
- Agouni, A.; Parray, A.S.; Akhtar, N.; Mir, F.A.; Bourke, P.J.; Joseph, S.; Morgan, D.M.; Santos, M.D.; Wadiwala, M.F.; Kamran, S.; et al. There Is Selective Increase in Pro-Thrombotic Circulating Extracellular Vesicles in Acute Ischemic Stroke and Transient Ischemic Attack: A Study of Patients From the Middle East and Southeast Asia. Front. Neurol. 2019, 10, 251. [Google Scholar] [CrossRef]
- Li, P.; Qin, C. Elevated Circulating VE-Cadherin+CD144+Endothelial Microparticles in Ischemic Cerebrovascular Disease. Thromb. Res. 2015, 135, 375–381. [Google Scholar] [CrossRef]
- Chiva-Blanch, G.; Suades, R.; Crespo, J.; Peña, E.; Padró, T.; Jiménez-Xarrié, E.; Martí-Fàbregas, J.; Badimon, L. Microparticle Shedding from Neural Progenitor Cells and Vascular Compartment Cells Is Increased in Ischemic Stroke. PLoS ONE 2016, 11, e0148176. [Google Scholar] [CrossRef] [PubMed]
- El-Gamal, H.; Parray, A.S.; Mir, F.A.; Shuaib, A.; Agouni, A. Circulating Microparticles as Biomarkers of Stroke: A Focus on the Value of Endothelial- and Platelet-Derived Microparticles. J. Cell. Physiol. 2019, 234, 16739–16754. [Google Scholar] [CrossRef] [PubMed]
- Simak, J.; Gelderman, M.P.; Yu, H.; Wright, V.; Baird, A.E. Circulating Endothelial Microparticles in Acute Ischemic Stroke: A Link to Severity, Lesion Volume and Outcome. J. Thromb. Haemost. 2006, 4, 1296–1302. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Chopp, M. Exosomes in Stroke Pathogenesis and Therapy. J. Clin. Investig. 2016, 126, 1190–1197. [Google Scholar] [CrossRef]
- Biomarkers and Surrogate Endpoints: Preferred Definitions and Conceptual Framework. Clin. Pharmacol. Ther. 2001, 69, 89–95. [CrossRef]
- Whiteley, W.; Tian, Y.; Jickling, G.C. Blood Biomarkers in Stroke: Research and Clinical Practice. Int. J. Stroke 2012, 7, 435–439. [Google Scholar] [CrossRef]
- Stenz, K.T.; Just, J.; Blauenfeldt, R.A.; Drasbek, K.R. Extracellular Vesicles in Acute Stroke Diagnostics. Biomedicines 2020, 8, 248. [Google Scholar] [CrossRef]
- Monteiro-Reis, S.; Carvalho-Maia, C.; Bart, G.; Vainio, S.J.; Pedro, J.; Silva, E.R.; Sales, G.; Henrique, R.; Jerónimo, C. Secreted Extracellular Vesicle Molecular Cargo as a Novel Liquid Biopsy Diagnostics of Central Nervous System Diseases. Int. J. Mol. Sci. 2021, 22, 3267. [Google Scholar] [CrossRef]
- Lee, B.; Newberg, A. Neuroimaging in Traumatic Brain Imaging. NeuroRx 2005, 2, 372–383. [Google Scholar] [CrossRef]
- Fassbender, K.; Walter, S.; Grunwald, I.Q.; Merzou, F.; Mathur, S.; Lesmeister, M.; Liu, Y.; Bertsch, T.; Grotta, J.C. Prehospital Stroke Management in the Thrombectomy Era. Lancet Neurol. 2020, 19, 601–610. [Google Scholar] [CrossRef]
- Ma, H.; Campbell, B.C.V.; Parsons, M.W.; Churilov, L.; Levi, C.R.; Hsu, C.; Kleinig, T.J.; Wijeratne, T.; Curtze, S.; Dewey, H.M.; et al. Thrombolysis Guided by Perfusion Imaging up to 9 Hours after Onset of Stroke. N. Engl. J. Med. 2019, 380, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.; Silva, I.; Pinto, I.M.; Maia, L.F. Timely and Blood-Based Multiplex Molecular Profiling of Acute Stroke. Life 2021, 11, 816. [Google Scholar] [CrossRef] [PubMed]
- Chalela, J.A.; Kidwell, C.S.; Nentwich, L.M.; Luby, M.; Butman, J.A.; Demchuk, A.M.; Hill, M.D.; Patronas, N.; Latour, L.; Warach, S. Magnetic Resonance Imaging and Computed Tomography in Emergency Assessment of Patients with Suspected Acute Stroke: A Prospective Comparison. Lancet 2007, 369, 293–298. [Google Scholar] [CrossRef]
- Aguiar de Sousa, D.; von Martial, R.; Abilleira, S.; Gattringer, T.; Kobayashi, A.; Gallofré, M.; Fazekas, F.; Szikora, I.; Feigin, V.; Caso, V.; et al. Access to and Delivery of Acute Ischaemic Stroke Treatments: A Survey of National Scientific Societies and Stroke Experts in 44 European Countries. Eur. Stroke J. 2019, 4, 13–28. [Google Scholar] [CrossRef]
- Dagonnier, M.; Donnan, G.A.; Davis, S.M.; Dewey, H.M.; Howells, D.W. Acute Stroke Biomarkers: Are We There Yet? Front. Neurol. 2021, 12, 619721. [Google Scholar] [CrossRef]
- Glushakova, O.; Glushakov, A.; Miller, E.; Valadka, A.; Hayes, R. Biomarkers for Acute Diagnosis and Management of Stroke in Neurointensive Care Units. Brain Circ. 2016, 2, 28. [Google Scholar] [CrossRef]
- Montaner, J.; Ramiro, L.; Simats, A.; Tiedt, S.; Makris, K.; Jickling, G.C.; Debette, S.; Sanchez, J.-C.; Bustamante, A. Multilevel Omics for the Discovery of Biomarkers and Therapeutic Targets for Stroke. Nat. Rev. Neurol. 2020, 16, 247–264. [Google Scholar] [CrossRef]
- Allard, L.; Burkhard, P.R.; Lescuyer, P.; Burgess, J.A.; Walter, N.; Hochstrasser, D.F.; Sanchez, J.-C. PARK7 and Nucleoside Diphosphate Kinase A as Plasma Markers for the Early Diagnosis of Stroke. Clin. Chem. 2005, 51, 2043–2051. [Google Scholar] [CrossRef]
- Bustamante, A.; Penalba, A.; Orset, C.; Azurmendi, L.; Llombart, V.; Simats, A.; Pecharroman, E.; Ventura, O.; Ribó, M.; Vivien, D.; et al. Blood Biomarkers to Differentiate Ischemic and Hemorrhagic Strokes. Neurology 2021, 96, e1928–e1939. [Google Scholar] [CrossRef]
- Bustamante, A.; López-Cancio, E.; Pich, S.; Penalba, A.; Giralt, D.; García-Berrocoso, T.; Ferrer-Costa, C.; Gasull, T.; Hernández-Pérez, M.; Millan, M.; et al. Blood Biomarkers for the Early Diagnosis of Stroke. Stroke 2017, 48, 2419–2425. [Google Scholar] [CrossRef]
- Bustamante, A.; Díaz-Fernández, B.; Giralt, D.; Boned, S.; Pagola, J.; Molina, C.A.; García-Berrocoso, T.; Kanse, S.M.; Montaner, J. Factor Seven Activating Protease (FSAP) Predicts Response to Intravenous Thrombolysis in Acute Ischemic Stroke. Int. J. Stroke 2016, 11, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Foerch, C.; Niessner, M.; Back, T.; Bauerle, M.; De Marchis, G.M.; Ferbert, A.; Grehl, H.; Hamann, G.F.; Jacobs, A.; Kastrup, A.; et al. Diagnostic Accuracy of Plasma Glial Fibrillary Acidic Protein for Differentiating Intracerebral Hemorrhage and Cerebral Ischemia in Patients with Symptoms of Acute Stroke. Clin. Chem. 2012, 58, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.M.; Stillings, S.A.; Leclerc, J.L.; Phillips, H.; Edwards, N.J.; Robicsek, S.A.; Hoh, B.L.; Blackburn, S.; Doré, S. Role of Interleukin-10 in Acute Brain Injuries. Front. Neurol. 2017, 8, 244. [Google Scholar] [CrossRef]
- Isenegger, J.; Meier, N.; Lämmle, B.; Alberio, L.; Fischer, U.; Nedeltchev, K.; Gralla, J.; Kohler, H.-P.; Mattle, H.P.; Arnold, M. D-Dimers Predict Stroke Subtype When Assessed Early. CED 2010, 29, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Katsanos, A.H.; Makris, K.; Stefani, D.; Koniari, K.; Gialouri, E.; Lelekis, M.; Chondrogianni, M.; Zompola, C.; Dardiotis, E.; Rizos, I.; et al. Plasma Glial Fibrillary Acidic Protein in the Differential Diagnosis of Intracerebral Hemorrhage. Stroke 2017, 48, 2586–2588. [Google Scholar] [CrossRef]
- Laskowitz, D.T.; Kasner, S.E.; Saver, J.; Remmel, K.S.; Jauch, E.C. BRAIN Study Group Clinical Usefulness of a Biomarker-Based Diagnostic Test for Acute Stroke: The Biomarker Rapid Assessment in Ischemic Injury (BRAIN) Study. Stroke 2009, 40, 77–85. [Google Scholar] [CrossRef]
- Llombart, V.; García-Berrocoso, T.; Bustamante, A.; Giralt, D.; Rodriguez-Luna, D.; Muchada, M.; Penalba, A.; Boada, C.; Hernández-Guillamon, M.; Montaner, J. Plasmatic Retinol-Binding Protein 4 and Glial Fibrillary Acidic Protein as Biomarkers to Differentiate Ischemic Stroke and Intracerebral Hemorrhage. J. Neurochem. 2016, 136, 416–424. [Google Scholar] [CrossRef]
- Llombart, V.; Antolin-Fontes, A.; Bustamante, A.; Giralt, D.; Rost, N.S.; Furie, K.; Shibazaki, K.; Biteker, M.; Castillo, J.; Rodríguez-Yáñez, M.; et al. B-Type Natriuretic Peptides Help in Cardioembolic Stroke Diagnosis: Pooled Data Meta-Analysis. Stroke 2015, 46, 1187–1195. [Google Scholar] [CrossRef]
- Luger, S.; Jæger, H.S.; Dixon, J.; Bohmann, F.O.; Schaefer, J.; Richieri, S.P.; Larsen, K.; Hov, M.R.; Bache, K.G.; Foerch, C.; et al. Diagnostic Accuracy of Glial Fibrillary Acidic Protein and Ubiquitin Carboxy-Terminal Hydrolase-L1 Serum Concentrations for Differentiating Acute Intracerebral Hemorrhage from Ischemic Stroke. Neurocrit. Care 2020, 33, 39–48. [Google Scholar] [CrossRef]
- Luger, S.; Witsch, J.; Dietz, A.; Hamann, G.F.; Minnerup, J.; Schneider, H.; Sitzer, M.; Wartenberg, K.E.; Niessner, M.; Foerch, C.; et al. Glial Fibrillary Acidic Protein Serum Levels Distinguish between Intracerebral Hemorrhage and Cerebral Ischemia in the Early Phase of Stroke. Clin. Chem. 2017, 63, 377–385. [Google Scholar] [CrossRef] [Green Version]
- Misra, S.; Montaner, J.; Ramiro, L.; Arora, R.; Talwar, P.; Nath, M.; Kumar, A.; Kumar, P.; Pandit, A.K.; Mohania, D.; et al. Blood Biomarkers for the Diagnosis and Differentiation of Stroke: A Systematic Review and Meta-Analysis. Int. J. Stroke 2020, 15, 704–721. [Google Scholar] [CrossRef] [PubMed]
- Montaner, J.; Mendioroz, M.; Delgado, P.; García-Berrocoso, T.; Giralt, D.; Merino, C.; Ribó, M.; Rosell, A.; Penalba, A.; Fernández-Cadenas, I.; et al. Differentiating Ischemic from Hemorrhagic Stroke Using Plasma Biomarkers: The S100B/RAGE Pathway. J. Proteom. 2012, 75, 4758–4765. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-Y.; Kim, M.-H.; Kim, O.-J.; Ahn, H.-J.; Song, J.-Y.; Jeong, J.-Y.; Oh, S.-H. Plasma Heart-Type Fatty Acid Binding Protein Level in Acute Ischemic Stroke: Comparative Analysis with Plasma S100B Level for Diagnosis of Stroke and Prediction of Long-Term Clinical Outcome. Clin. Neurol. Neurosurg. 2013, 115, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Pachón, A.; López-Cancio, E.; Bustamante, A.; Pérez de la Ossa, N.; Millán, M.; Hernández-Pérez, M.; Garcia-Berrocoso, T.; Cardona, P.; Rubiera, M.; Serena, J.; et al. D-Dimer as Predictor of Large Vessel Occlusion in Acute Ischemic Stroke. Stroke 2021, 52, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Kobeissy, F.; Alawieh, A.; Li, N.; Li, N.; Zibara, K.; Zoltewicz, S.; Guingab-Cagmat, J.; Larner, S.F.; Ding, Y.; et al. Assessment of Serum UCH-L1 and GFAP in Acute Stroke Patients. Sci. Rep. 2016, 6, 24588. [Google Scholar] [CrossRef]
- Reynolds, M.A.; Kirchick, H.J.; Dahlen, J.R.; Anderberg, J.M.; McPherson, P.H.; Nakamura, K.K.; Laskowitz, D.T.; Valkirs, G.E.; Buechler, K.F. Early Biomarkers of Stroke. Clin. Chem. 2003, 49, 1733–1739. [Google Scholar] [CrossRef]
- Turck, N.; Robin, X.; Walter, N.; Fouda, C.; Hainard, A.; Sztajzel, R.; Wagner, G.; Hochstrasser, D.F.; Montaner, J.; Burkhard, P.R.; et al. Blood Glutathione S-Transferase-π as a Time Indicator of Stroke Onset. PLoS ONE 2012, 7, e43830. [Google Scholar] [CrossRef]
- Barr, T.L.; Latour, L.L.; Lee, K.-Y.; Schaewe, T.J.; Luby, M.; Chang, G.S.; El-Zammar, Z.; Alam, S.; Hallenbeck, J.M.; Kidwell, C.S.; et al. Blood–Brain Barrier Disruption in Humans Is Independently Associated With Increased Matrix Metalloproteinase-9. Stroke 2010, 41, e123–e128. [Google Scholar] [CrossRef]
- Castellanos, M.; Sobrino, T.; Millán, M.; García, M.; Arenillas, J.; Nombela, F.; Brea, D.; Perez de la Ossa, N.; Serena, J.; Vivancos, J.; et al. Serum Cellular Fibronectin and Matrix Metalloproteinase-9 as Screening Biomarkers for the Prediction of Parenchymal Hematoma after Thrombolytic Therapy in Acute Ischemic Stroke: A Multicenter Confirmatory Study. Stroke 2007, 38, 1855–1859. [Google Scholar] [CrossRef]
- Castellanos, M.; Leira, R.; Serena, J.; Blanco, M.; Pedraza, S.; Castillo, J.; Dávalos, A. Plasma Cellular-Fibronectin Concentration Predicts Hemorrhagic Transformation after Thrombolytic Therapy in Acute Ischemic Stroke. Stroke 2004, 35, 1671–1676. [Google Scholar] [CrossRef] [Green Version]
- Castellanos, M.; Leira, R.; Serena, J.; Pumar, J.M.; Lizasoain, I.; Castillo, J.; Dávalos, A. Plasma Metalloproteinase-9 Concentration Predicts Hemorrhagic Transformation in Acute Ischemic Stroke. Stroke 2003, 34, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Foerch, C.; Wunderlich, M.T.; Dvorak, F.; Humpich, M.; Kahles, T.; Goertler, M.; Alvarez-Sabín, J.; Wallesch, C.W.; Molina, C.A.; Steinmetz, H.; et al. Elevated Serum S100B Levels Indicate a Higher Risk of Hemorrhagic Transformation after Thrombolytic Therapy in Acute Stroke. Stroke 2007, 38, 2491–2495. [Google Scholar] [CrossRef]
- Hsu, P.-J.; Chen, C.-H.; Yeh, S.-J.; Tsai, L.-K.; Tang, S.-C.; Jeng, J.-S. High Plasma D-Dimer Indicates Unfavorable Outcome of Acute Ischemic Stroke Patients Receiving Intravenous Thrombolysis. Cereb. Dis. 2016, 42, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Montaner, J.; Ribó, M.; Monasterio, J.; Molina, C.A.; Alvarez-Sabín, J. Thrombin-Activable Fibrinolysis Inhibitor Levels in the Acute Phase of Ischemic Stroke. Stroke 2003, 34, 1038–1040. [Google Scholar] [CrossRef] [PubMed]
- Ribo, M.; Montaner, J.; Molina, C.A.; Arenillas, J.F.; Santamarina, E.; Quintana, M.; Alvarez-Sabín, J. Admission Fibrinolytic Profile Is Associated With Symptomatic Hemorrhagic Transformation in Stroke Patients Treated With Tissue Plasminogen Activator. Stroke 2004, 35, 2123–2127. [Google Scholar] [CrossRef]
- Serena, J.; Blanco, M.; Castellanos, M.; Silva, Y.; Vivancos, J.; Moro, M.A.; Leira, R.; Lizasoain, I.; Castillo, J.; Dávalos, A. The Prediction of Malignant Cerebral Infarction by Molecular Brain Barrier Disruption Markers. Stroke 2005, 36, 1921–1926. [Google Scholar] [CrossRef] [PubMed]
- Brea, D.; Sobrino, T.; Blanco, M.; Cristobo, I.; Rodríguez-González, R.; Rodríguez-Yañez, M.; Moldes, O.; Agulla, J.; Leira, R.; Castillo, J. Temporal Profile and Clinical Significance of Serum Neuron-Specific Enolase and S100 in Ischemic and Hemorrhagic Stroke. Clin. Chem. Lab. Med. 2009, 47, 1513–1518. [Google Scholar] [CrossRef]
- González-García, S.; González-Quevedo, A.; Fernández-Concepción, O.; Peña-Sánchez, M.; Menéndez-Saínz, C.; Hernández-Díaz, Z.; Arteche-Prior, M.; Pando-Cabrera, A.; Fernández-Novales, C. Short-Term Prognostic Value of Serum Neuron Specific Enolase and S100B in Acute Stroke Patients. Clin. Biochem. 2012, 45, 1302–1307. [Google Scholar] [CrossRef]
- Hou, H.; Xiang, X.; Pan, Y.; Li, H.; Meng, X.; Wang, Y. Association of Level and Increase in D-Dimer With All-Cause Death and Poor Functional Outcome After Ischemic Stroke or Transient Ischemic Attack. J. Am. Heart Assoc. 2021, 10, e018600. [Google Scholar] [CrossRef]
- Vila, N.; Castillo, J.; Dávalos, A.; Esteve, A.; Planas, A.M.; Chamorro, Á. Levels of Anti-Inflammatory Cytokines and Neurological Worsening in Acute Ischemic Stroke. Stroke 2003, 34, 671–675. [Google Scholar] [CrossRef] [Green Version]
- Wunderlich, M.T.; Hanhoff, T.; Goertler, M.; Spener, F.; Glatz, J.F.C.; Wallesch, C.-W.; Pelsers, M.M.A.L. Release of Brain–Type and Heart–Type Fatty Acid–Binding Proteins in Serum after Acute Ischaemic Stroke. J. Neurol. 2005, 252, 718–724. [Google Scholar] [CrossRef]
- Yang, X.; Gao, S.; Ding, J.; Chen, Y.; Zhou, X.; Wang, J.-E. Plasma D-Dimer Predicts Short-Term Poor Outcome after Acute Ischemic Stroke. PLoS ONE 2014, 9, e89756. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Bao, J.; Wang, Y.; Pan, S. S100β as a Biomarker for Differential Diagnosis of Intracerebral Hemorrhage and Ischemic Stroke. Neurol. Res. 2016, 38, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Foerch, C.; Singer, O.C.; Neumann-Haefelin, T.; du Mesnil de Rochemont, R.; Steinmetz, H.; Sitzer, M. Evaluation of Serum S100B as a Surrogate Marker for Long-Term Outcome and Infarct Volume in Acute Middle Cerebral Artery Infarction. Arch. Neurol. 2005, 62, 1130–1134. [Google Scholar] [CrossRef] [PubMed]
- Lorenzano, S.; Rost, N.S.; Khan, M.; Li, H.; Batista, L.M.; Chutinet, A.; Green, R.E.; Thankachan, T.K.; Thornell, B.; Muzikansky, A.; et al. Early Molecular Oxidative Stress Biomarkers of Ischemic Penumbra in Acute Stroke. Neurology 2019, 93, e1288–e1298. [Google Scholar] [CrossRef]
- Rodríguez-Yáñez, M.; Sobrino, T.; Arias, S.; Vázquez-Herrero, F.; Brea, D.; Blanco, M.; Leira, R.; Castellanos, M.; Serena, J.; Vivancos, J.; et al. Early Biomarkers of Clinical–Diffusion Mismatch in Acute Ischemic Stroke. Stroke 2011, 42, 2813–2818. [Google Scholar] [CrossRef]
- Chen, Y.; Xiao, Y.; Lin, Z.; Xiao, X.; He, C.; Bihl, J.C.; Zhao, B.; Ma, X.; Chen, Y. The Role of Circulating Platelets Microparticles and Platelet Parameters in Acute Ischemic Stroke Patients. J. Stroke Cereb. Dis. 2015, 24, 2313–2320. [Google Scholar] [CrossRef]
- Dong, X.-Q.; Huang, M.; Hu, Y.-Y.; Yu, W.-H.; Zhang, Z.-Y. Time Course of Plasma Microparticle Concentrations after Acute Spontaneous Basal Ganglia Hemorrhage. Acta Neurol. Scand. 2011, 123, 280–288. [Google Scholar] [CrossRef]
- Ji, Q.; Ji, Y.; Peng, J.; Zhou, X.; Chen, X.; Zhao, H.; Xu, T.; Chen, L.; Xu, Y. Increased Brain-Specific MiR-9 and MiR-124 in the Serum Exosomes of Acute Ischemic Stroke Patients. PLoS ONE 2016, 11, e0163645. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, L.; Chen, B.; Huang, S.; Zeng, C.; Wu, H.; Chen, C.; Long, F. Increased Serum Exosomal MiR-134 Expression in the Acute Ischemic Stroke Patients. BMC Neurol. 2018, 18, 198. [Google Scholar] [CrossRef]
- Chen, Y.; Song, Y.; Huang, J.; Qu, M.; Zhang, Y.; Geng, J.; Zhang, Z.; Liu, J.; Yang, G.-Y. Increased Circulating Exosomal MiRNA-223 Is Associated with Acute Ischemic Stroke. Front. Neurol. 2017, 8, 57. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, D.-B.; Li, R.-Y.; Zhou, X.; Yu, D.-J.; Lan, X.-Y.; Li, J.-P.; Liu, J.-L. Diagnosis of Hyperacute and Acute Ischaemic Stroke: The Potential Utility of Exosomal MicroRNA-21-5p and MicroRNA-30a-5p. Cereb. Dis. 2018, 45, 204–212. [Google Scholar] [CrossRef]
- Kalani, M.Y.S.; Alsop, E.; Meechoovet, B.; Beecroft, T.; Agrawal, K.; Whitsett, T.G.; Huentelman, M.J.; Spetzler, R.F.; Nakaji, P.; Kim, S.; et al. Extracellular MicroRNAs in Blood Differentiate between Ischaemic and Haemorrhagic Stroke Subtypes. J. Extracell. Vesicles 2020, 9, 1713540. [Google Scholar] [CrossRef] [PubMed]
- Couch, Y.; Akbar, N.; Davis, S.; Fischer, R.; Dickens, A.M.; Neuhaus, A.A.; Burgess, A.I.; Rothwell, P.M.; Buchan, A.M. Inflammatory Stroke Extracellular Vesicles Induce Macrophage Activation. Stroke 2017, 48, 2292–2296. [Google Scholar] [CrossRef] [PubMed]
- Mitaki, S.; Wada, Y.; Sheikh, A.M.; Yamaguchi, S.; Nagai, A. Proteomic Analysis of Extracellular Vesicles Enriched Serum Associated with Future Ischemic Stroke. Sci. Rep. 2021, 11, 24024. [Google Scholar] [CrossRef]
- Datta, A.; Chen, C.P.; Sze, S.K. Discovery of Prognostic Biomarker Candidates of Lacunar Infarction by Quantitative Proteomics of Microvesicles Enriched Plasma. PLoS ONE 2014, 9, e94663. [Google Scholar] [CrossRef]
- Bazarian, J.J.; Biberthaler, P.; Welch, R.D.; Lewis, L.M.; Barzo, P.; Bogner-Flatz, V.; Gunnar Brolinson, P.; Büki, A.; Chen, J.Y.; Christenson, R.H.; et al. Serum GFAP and UCH-L1 for Prediction of Absence of Intracranial Injuries on Head CT (ALERT-TBI): A Multicentre Observational Study. Lancet Neurol. 2018, 17, 782–789. [Google Scholar] [CrossRef]
- Papa, L.; Zonfrillo, M.R.; Welch, R.D.; Lewis, L.M.; Braga, C.F.; Tan, C.N.; Ameli, N.J.; Lopez, M.A.; Haeussler, C.A.; Mendez Giordano, D.; et al. Evaluating Glial and Neuronal Blood Biomarkers GFAP and UCH-L1 as Gradients of Brain Injury in Concussive, Subconcussive and Non-Concussive Trauma: A Prospective Cohort Study. BMJ Paediatr. Open 2019, 3, e000473. [Google Scholar] [CrossRef]
- Pattinson, C.L.; Shahim, P.; Taylor, P.; Dunbar, K.; Guedes, V.A.; Motamedi, V.; Lai, C.; Devoto, C.; Peyer, J.; Roy, M.J.; et al. Elevated Tau in Military Personnel Relates to Chronic Symptoms Following Traumatic Brain Injury. J. Head Trauma Rehabil. 2020, 35, 66–73. [Google Scholar] [CrossRef]
- Shahim, P.; Tegner, Y.; Marklund, N.; Blennow, K.; Zetterberg, H. Neurofilament Light and Tau as Blood Biomarkers for Sports-Related Concussion. Neurology 2018, 90, e1780–e1788. [Google Scholar] [CrossRef] [Green Version]
- Shahim, P.; Zetterberg, H.; Tegner, Y.; Blennow, K. Serum Neurofilament Light as a Biomarker for Mild Traumatic Brain Injury in Contact Sports. Neurology 2017, 88, 1788–1794. [Google Scholar] [CrossRef] [PubMed]
- Lagerstedt, L.; Egea-Guerrero, J.J.; Bustamante, A.; Rodríguez-Rodríguez, A.; Rahal, A.E.; Quintana-Diaz, M.; García-Armengol, R.; Prica, C.M.; Andereggen, E.; Rinaldi, L.; et al. Combining H-FABP and GFAP Increases the Capacity to Differentiate between CT-Positive and CT-Negative Patients with Mild Traumatic Brain Injury. PLoS ONE 2018, 13, e0200394. [Google Scholar] [CrossRef] [PubMed]
- ABDCx TBICheck Has Obtained the CE Mark. Available online: https://tbicheck.com/news/tbicheck-has-obtained-the-ce-mark (accessed on 7 July 2022).
- Abbott Abbott Receives FDA 510(k) Clearance for the First Rapid Handheld Blood Test for Concussions. Available online: https://abbott.mediaroom.com/2021-01-11-Abbott-Receives-FDA-510-k-Clearance-for-the-First-Rapid-Handheld-Blood-Test-for-Concussions (accessed on 7 March 2022).
- Undén, J.; Ingebrigtsen, T.; Romner, B. The Scandinavian Neurotrauma Committee (SNC) Scandinavian Guidelines for Initial Management of Minimal, Mild and Moderate Head Injuries in Adults: An Evidence and Consensus-Based Update. BMC Med. 2013, 11, 50. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Hemphill, M.; Yang, Z.; Beard, K.; Sewell, E.; Shallcross, J.; Schweizer, M.; Sandsmark, D.K.; Diaz-Arrastia, R.; Kim, J.; et al. Multi-Dimensional Mapping of Brain-Derived Extracellular Vesicle MicroRNA Biomarker for Traumatic Brain Injury Diagnostics. J. Neurotrauma 2020, 37, 2424–2434. [Google Scholar] [CrossRef] [PubMed]
- Beard, K.; Yang, Z.; Haber, M.; Flamholz, M.; Diaz-Arrastia, R.; Sandsmark, D.; Meaney, D.F.; Issadore, D. Extracellular Vesicles as Distinct Biomarker Reservoirs for Mild Traumatic Brain Injury Diagnosis. Brain Commun. 2021, 3, fcab151. [Google Scholar] [CrossRef] [PubMed]
- Flynn, S.; Leete, J.; Shahim, P.; Pattinson, C.; Guedes, V.A.; Lai, C.; Devoto, C.; Qu, B.-X.; Greer, K.; Moore, B.; et al. Extracellular Vesicle Concentrations of Glial Fibrillary Acidic Protein and Neurofilament Light Measured 1 Year after Traumatic Brain Injury. Sci. Rep. 2021, 11, 3896. [Google Scholar] [CrossRef]
- Gill, J.; Mustapic, M.; Diaz-Arrastia, R.; Lange, R.; Gulyani, S.; Diehl, T.; Motamedi, V.; Osier, N.; Stern, R.A.; Kapogiannis, D. Higher Exosomal Tau, Amyloid-Beta 42 and IL-10 Are Associated with Mild TBIs and Chronic Symptoms in Military Personnel. Brain Inj. 2018, 32, 1359–1366. [Google Scholar] [CrossRef]
- Goetzl, E.J.; Elahi, F.M.; Mustapic, M.; Kapogiannis, D.; Pryhoda, M.; Gilmore, A.; Gorgens, K.A.; Davidson, B.; Granholm, A.-C.; Ledreux, A. Altered Levels of Plasma Neuron-Derived Exosomes and Their Cargo Proteins Characterize Acute and Chronic Mild Traumatic Brain Injury. FASEB J. 2019, 33, 5082–5088. [Google Scholar] [CrossRef]
- Kenney, K.; Qu, B.-X.; Lai, C.; Devoto, C.; Motamedi, V.; Walker, W.C.; Levin, H.S.; Nolen, T.; Wilde, E.A.; Diaz-Arrastia, R.; et al. Higher Exosomal Phosphorylated Tau and Total Tau among Veterans with Combat-Related Repetitive Chronic Mild Traumatic Brain Injury. Brain Inj. 2018, 32, 1276–1284. [Google Scholar] [CrossRef]
- Mondello, S.; Guedes, V.A.; Lai, C.; Czeiter, E.; Amrein, K.; Kobeissy, F.; Mechref, Y.; Jeromin, A.; Mithani, S.; Martin, C.; et al. Circulating Brain Injury Exosomal Proteins Following Moderate-to-Severe Traumatic Brain Injury: Temporal Profile, Outcome Prediction and Therapy Implications. Cells 2020, 9, 977. [Google Scholar] [CrossRef] [Green Version]
- Stern, R.A.; Tripodis, Y.; Baugh, C.M.; Fritts, N.G.; Martin, B.M.; Chaisson, C.; Cantu, R.C.; Joyce, J.A.; Shah, S.; Ikezu, T.; et al. Preliminary Study of Plasma Exosomal Tau as a Potential Biomarker for Chronic Traumatic Encephalopathy. J. Alzheimer’s Dis. 2016, 51, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Winston, C.N.; Romero, H.K.; Ellisman, M.; Nauss, S.; Julovich, D.A.; Conger, T.; Hall, J.R.; Campana, W.; O’Bryant, S.E.; Nievergelt, C.M.; et al. Assessing Neuronal and Astrocyte Derived Exosomes From Individuals with Mild Traumatic Brain Injury for Markers of Neurodegeneration and Cytotoxic Activity. Front. Neurosci. 2019, 13, 1005. [Google Scholar] [CrossRef] [PubMed]
- Ghai, V.; Fallen, S.; Baxter, D.; Scherler, K.; Kim, T.-K.; Zhou, Y.; Meabon, J.S.; Logsdon, A.F.; Banks, W.A.; Schindler, A.G.; et al. Alterations in Plasma MicroRNA and Protein Levels in War Veterans with Chronic Mild Traumatic Brain Injury. J. Neurotrauma 2020, 37, 1418–1430. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; He, C.; Hao, Y.; Wang, L.; Li, L.; Zhu, G. Prospects and Challenges of Extracellular Vesicle-Based Drug Delivery System: Considering Cell Source. Drug Deliv. 2020, 27, 585–598. [Google Scholar] [CrossRef] [PubMed]
- Kocheril, P.A.; Moore, S.C.; Lenz, K.D.; Mukundan, H.; Lilley, L.M. Progress Toward a Multiomic Understanding of Traumatic Brain Injury: A Review. Biomark. Insights 2022, 17, 11772719221105145. [Google Scholar] [CrossRef]
- Ramos-Cejudo, J.; Wisniewski, T.; Marmar, C.; Zetterberg, H.; Blennow, K.; de Leon, M.J.; Fossati, S. Traumatic Brain Injury and Alzheimer’s Disease: The Cerebrovascular Link. EBioMedicine 2018, 28, 21–30. [Google Scholar] [CrossRef]
- Clayton, A.; Buschmann, D.; Byrd, J.B.; Carter, D.R.F.; Cheng, L.; Compton, C.; Daaboul, G.; Devitt, A.; Falcon-Perez, J.M.; Gardiner, C.; et al. Summary of the ISEV Workshop on Extracellular Vesicles as Disease Biomarkers, Held in Birmingham, UK, during December 2017. J. Extracell. Vesicles 2018, 7, 1473707. [Google Scholar] [CrossRef]
- Coumans, F.A.W.; Brisson, A.R.; Buzas, E.I.; Dignat-George, F.; Drees, E.E.E.; El-Andaloussi, S.; Emanueli, C.; Gasecka, A.; Hendrix, A.; Hill, A.F.; et al. Methodological Guidelines to Study Extracellular Vesicles. Circ. Res. 2017, 120, 1632–1648. [Google Scholar] [CrossRef]
- Liang, Y.; Lehrich, B.M.; Zheng, S.; Lu, M. Emerging Methods in Biomarker Identification for Extracellular Vesicle-Based Liquid Biopsy. J. Extracell. Vesicles 2021, 10, e12090. [Google Scholar] [CrossRef]

| EV populations as Biomarkers | Application | Cohort | EV Detection Method | Reference |
|---|---|---|---|---|
| Plasma endothelial-, activated platelet-, erythrocytes-, granulocytes, and leukocytes-derived EVs | Detection of AIS and TIA | AIS (n = 66) and TIA (n = 21) patients and healthy participants (n = 24) | Flow Cytometry | [132] |
| Plasma platelet-derived EVs | Detection of AIS | AIS patients with LAA (n = 53) or SAO (n = 59) and healthy participants (n = 35) | Flow Cytometry | [190] |
| Plasma endothelial-, platelet-, neuronal precursor cell-, circulating immune cells-derived EVs | Detection of IS | AIS patients (n = 44) and high-risk cardiovascular participants (n = 44) | Flow Cytometry | [134] |
| Plasma, and CSF PS+ EVs | Detection of ICH | ICH patients (n = 36) and controls (n = 10) | Biotinylation of Annexin V | [130] |
| Plasma platelet-derived EVs | Detection of SAO and LAA | SAO (n = 34), LAA (n = 41), cardioembolism (n = 20), and undetermined etiology (n = 15) patients and healthy participants (n = 61) | Immunoassay | [131] |
| Plasma endothelial-, leukocyte- and erythrocyte-derived EVs | Detection of SAH | SAH patients (n = 20) and healthy participants (n = 20) | Flow Cytometry | [128] |
| Plasma endothelial-derived EVs | Detection of AIS | AIS patients (n = 68) and healthy participants (n = 61) | Flow Cytometry | [133] |
| Plasma endothelial-, erythrocyte-, neutrophil- and platelet-derived EVs | Detection of SAH | SAH patients (n = 22) and healthy participants (n = 13) | Flow Cytometry | [129] |
| Plasma PS+ endothelial-derived EVs | Detection of AIS | AIS patients (n = 41) and healthy participants (n = 23) | Flow Cytometry | [136] |
| Biomarker | Sample Type | Application | Cohort | EV Isolation Method | Reference |
|---|---|---|---|---|---|
| miR-9, miR-124 | Serum | Detection of AIS | AIS patients (n = 65) and healthy participants (n = 66) | ExoQuick (System Biosciences) | [192] |
| miR-134 | Serum | Detection of AIS | AIS patients (n = 50) and healthy participants (n = 50) | ExoQuick (System Biosciences) | [193] |
| miR-223 | Serum | Detection of AIS | AIS patients (n = 50) | ExoQuick (System Biosciences) | [194] |
| miR-21-5p, miR-30a-5p | Plasma | Detection of IS | hyperacute (n = 15), acute (n = 55), subacute (n = 31) and recovery phase (n = 32) IS patients and healthy participants (n = 24) | ExoRNeasy (QIAGEN) | [195] |
| miR-27b-3p, miR-146b-5p | Plasma | Detection of AIS | IS (n = 21), IPH (n = 19) and SAH (n = 17) patients | ExoRNeasy (QIAGEN) | [196] |
| A2MG, C1Q, C1R, HRG | Serum | Detection of AIS | AIS patients (n = 38) and healthy participants | Ultracentrifugation | [197] |
| Biomarker Candidates | Application | EV Population | Cohort | EV Isolation Methods | Reference |
|---|---|---|---|---|---|
| GFAP, IL-16 | Detection of acute mTBI | Plasma GluR2+ brain-derived EVs | mTBI patients (n = 47), healthy (n = 39) and orthopedically injured (n = 7) participants | TENPO nanofluidic platform [209] | [210] |
| GFAP, NFL | Detection of 1-year TBI | Serum EVs | TBI patients (n = 72) and healthy participants (n = 20) | ExoQuick (System Biosciences) | [211] |
| Aβ42, IL-10, tau | Detection of mTBI | Plasma L1CAM+ neuron-derived EVs | mTBI military personnel (n = 42) vs. healthy participants (n = 22) | ExoQuick (System Biosciences) + L1CAM immunoprecipitation | [212] |
| Aβ42, AQ4, IL-16, NKCC1, P-T181, P-S396-tau, PRPc, UCHL-1, Synaptogyrin-3 | Detection of acute and chronic mTBI | Plasma L1CAM+ neuron-derived EVs | Acute (n = 18) and chronic (n = 14) mTBI and healthy participants (n = 21) | ExoQuick (System Biosciences) + L1CAM immunoprecipitation | [213] |
| Tau, p-tau | Detection of repetitive mTBI | Plasma EVs | Repetitive mTBI (n = 56), with 1–2 mTBI (n = 94) and without TBI (n = 45) participants | ExoQuick (System Biosciences) | [214] |
| GFAP, tau, UCHL-1 | Detection of moderate–severe TBI | Serum EVs | Moderate-severe TBI patients (n = 21) | ExoQuick (System Biosciences) | [215] |
| Tau | Detection of chronic traumatic encephalopathy (CTE) | Plasma EVs | Former NFL players with CTE and repetitive TBI (n = 78) and participants with a reported history of TBI (n = 15) | Size Exclusion Chromatography (Agarose Bead Technologies) | [216] |
| Aβ42, neurogranin | Detection of mTBI | Plasma L1CAM+ neuron- and GLAST+ astrocyte-derived EVs | With mTBI (n = 19) and without mTBI (n = 20) military personnel | ExoQuick (System Biosciences) + L1CAM or GLAST immunocapture + FACS selection | [217] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reymond, S.; Vujić, T.; Sanchez, J.-C. Neurovascular Unit-Derived Extracellular Vesicles: From Their Physiopathological Roles to Their Clinical Applications in Acute Brain Injuries. Biomedicines 2022, 10, 2147. https://doi.org/10.3390/biomedicines10092147
Reymond S, Vujić T, Sanchez J-C. Neurovascular Unit-Derived Extracellular Vesicles: From Their Physiopathological Roles to Their Clinical Applications in Acute Brain Injuries. Biomedicines. 2022; 10(9):2147. https://doi.org/10.3390/biomedicines10092147
Chicago/Turabian StyleReymond, Sandrine, Tatjana Vujić, and Jean-Charles Sanchez. 2022. "Neurovascular Unit-Derived Extracellular Vesicles: From Their Physiopathological Roles to Their Clinical Applications in Acute Brain Injuries" Biomedicines 10, no. 9: 2147. https://doi.org/10.3390/biomedicines10092147
APA StyleReymond, S., Vujić, T., & Sanchez, J.-C. (2022). Neurovascular Unit-Derived Extracellular Vesicles: From Their Physiopathological Roles to Their Clinical Applications in Acute Brain Injuries. Biomedicines, 10(9), 2147. https://doi.org/10.3390/biomedicines10092147








