Matrin3 (MATR3) Expression Is Associated with Hemophagocytosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Recruitment
2.2. Real-Time qRT-PCR to Quantify MATR3 and NF-κB Expression
2.3. Clinical Endpoint
2.4. RNA Interference-Mediated MATR3-Knockdown in THP1 Cells and Cell Culture
2.5. Phagocytosis of Necrotic and Apoptotic Cells
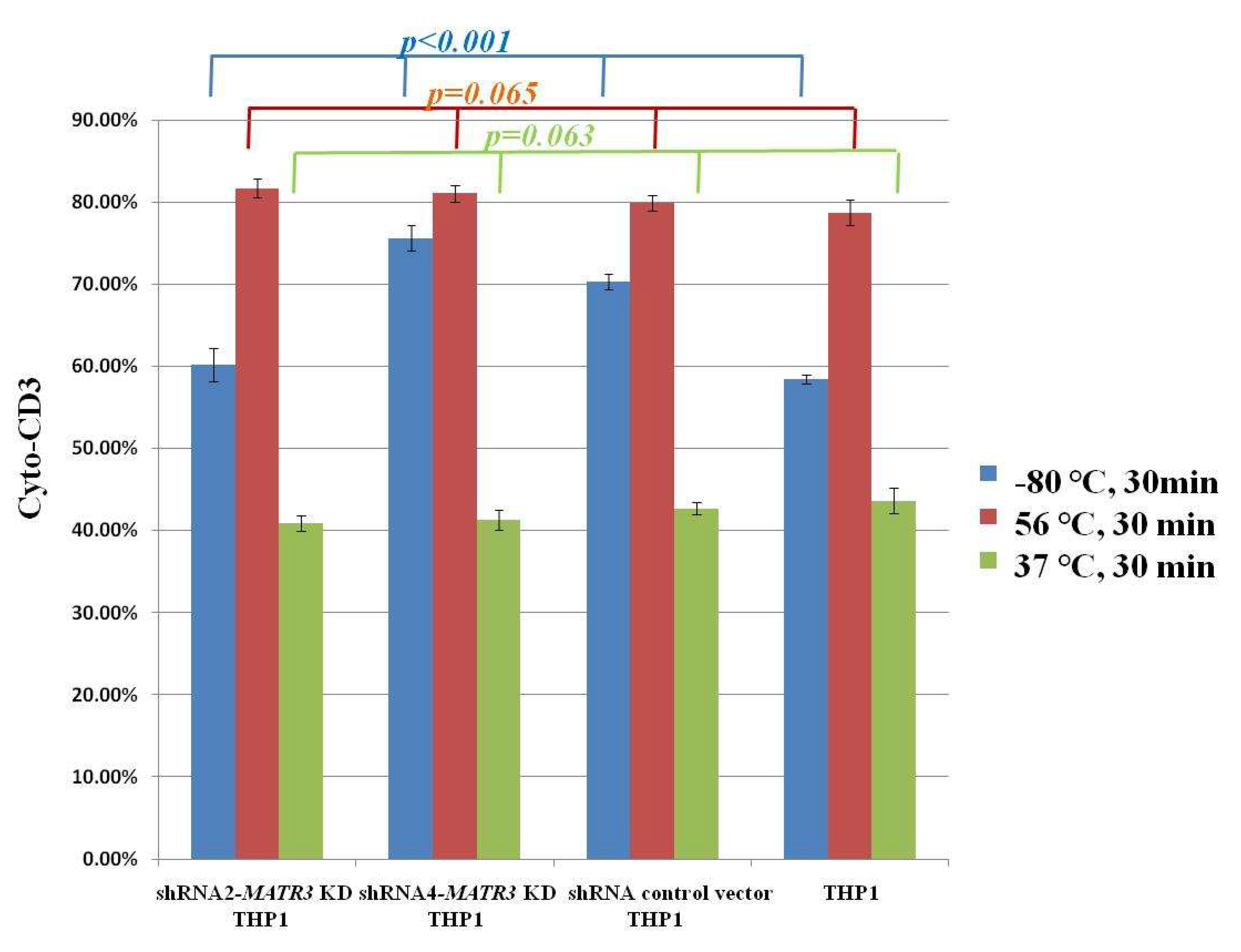
2.6. Hemophagocytosis
2.7. Flow Cytometry for Determining Phagocytosis
2.8. Fluorescent Microscope
2.9. Statistics
3. Results
3.1. Patients’ Characteristics
3.2. Downregulation of MATR3 in Patients with Hemophagocytosis
3.3. Similar NFκB and MATR3mRNA Expression in Different Disease Etiologies
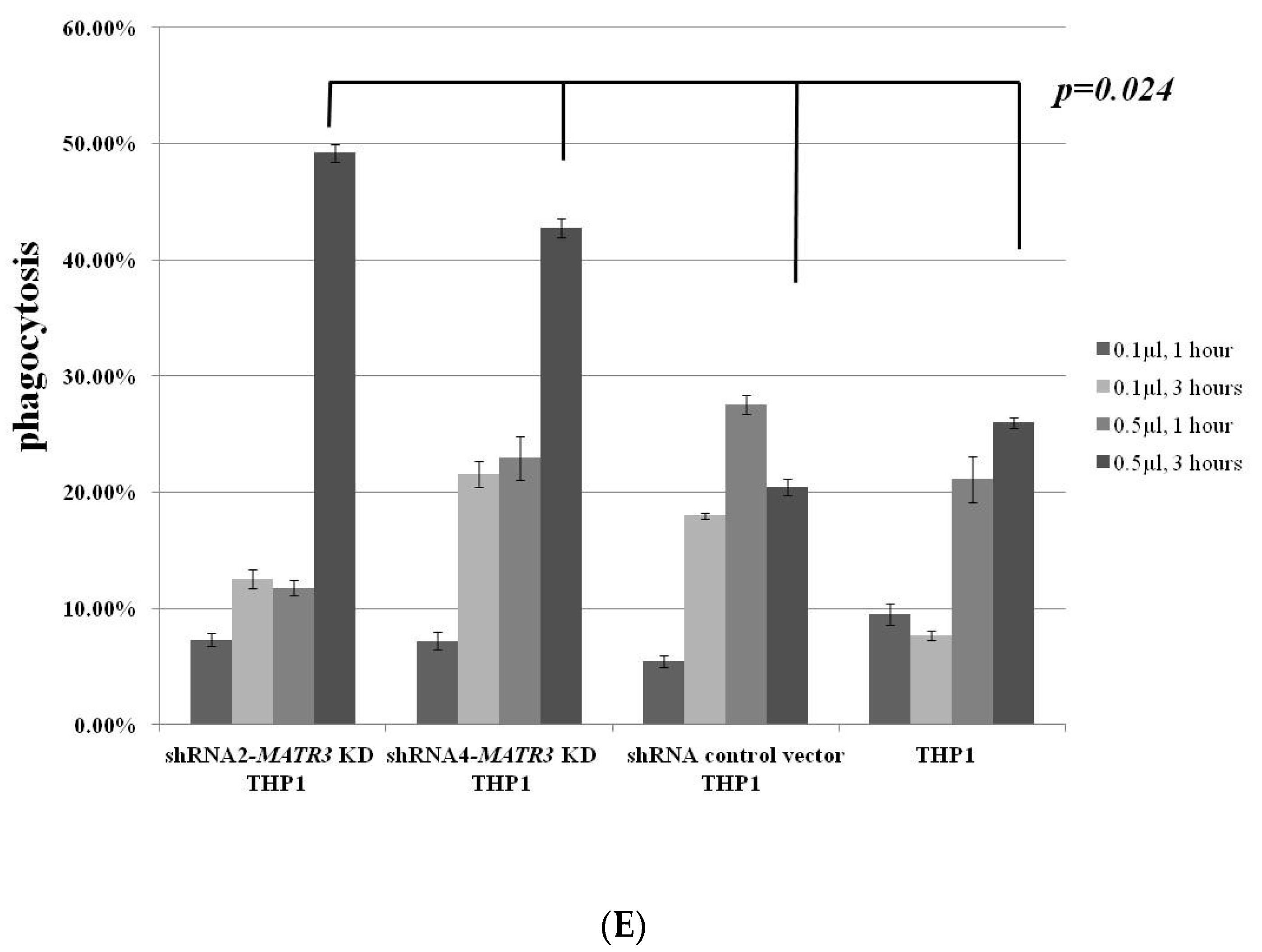
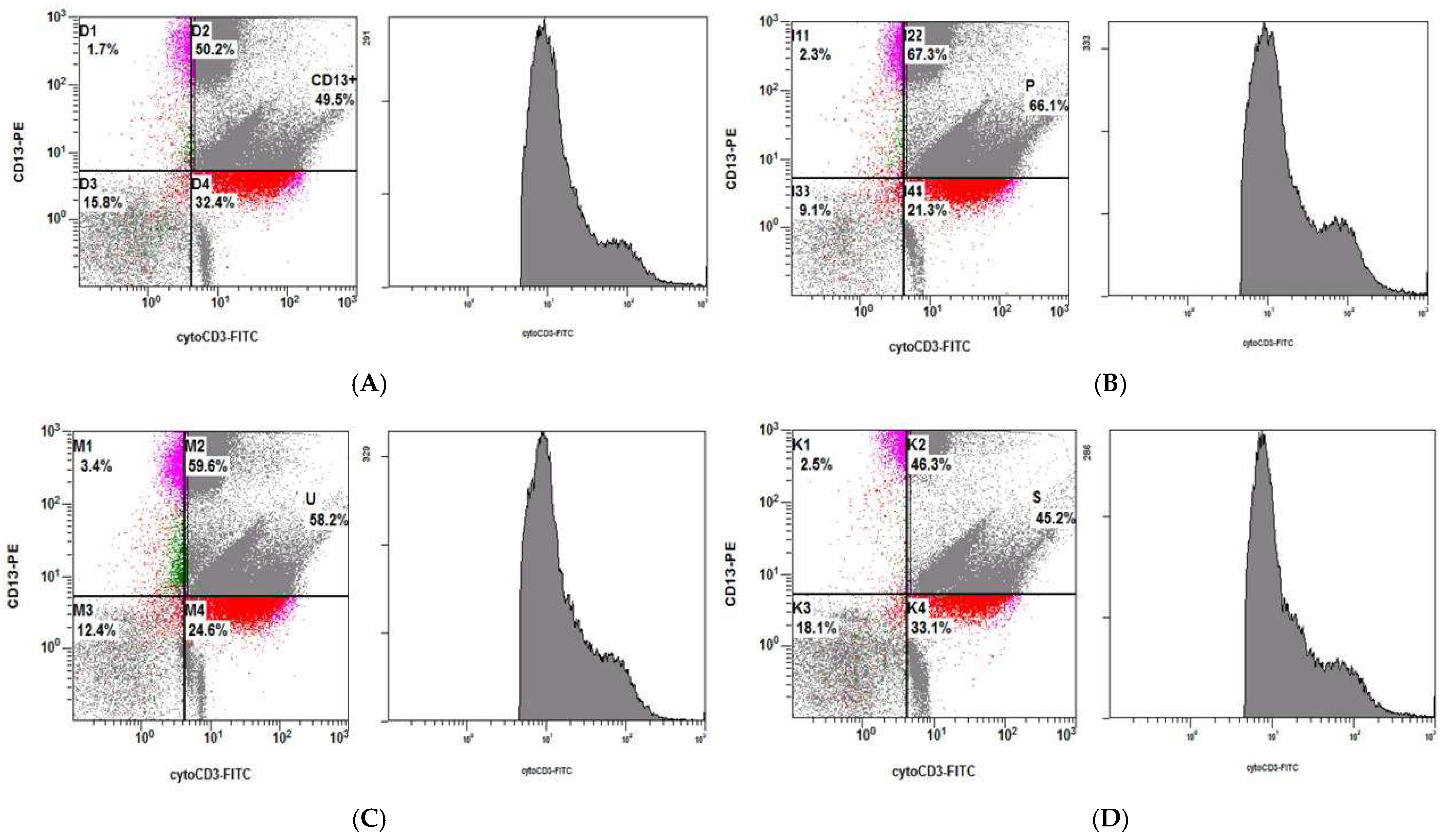
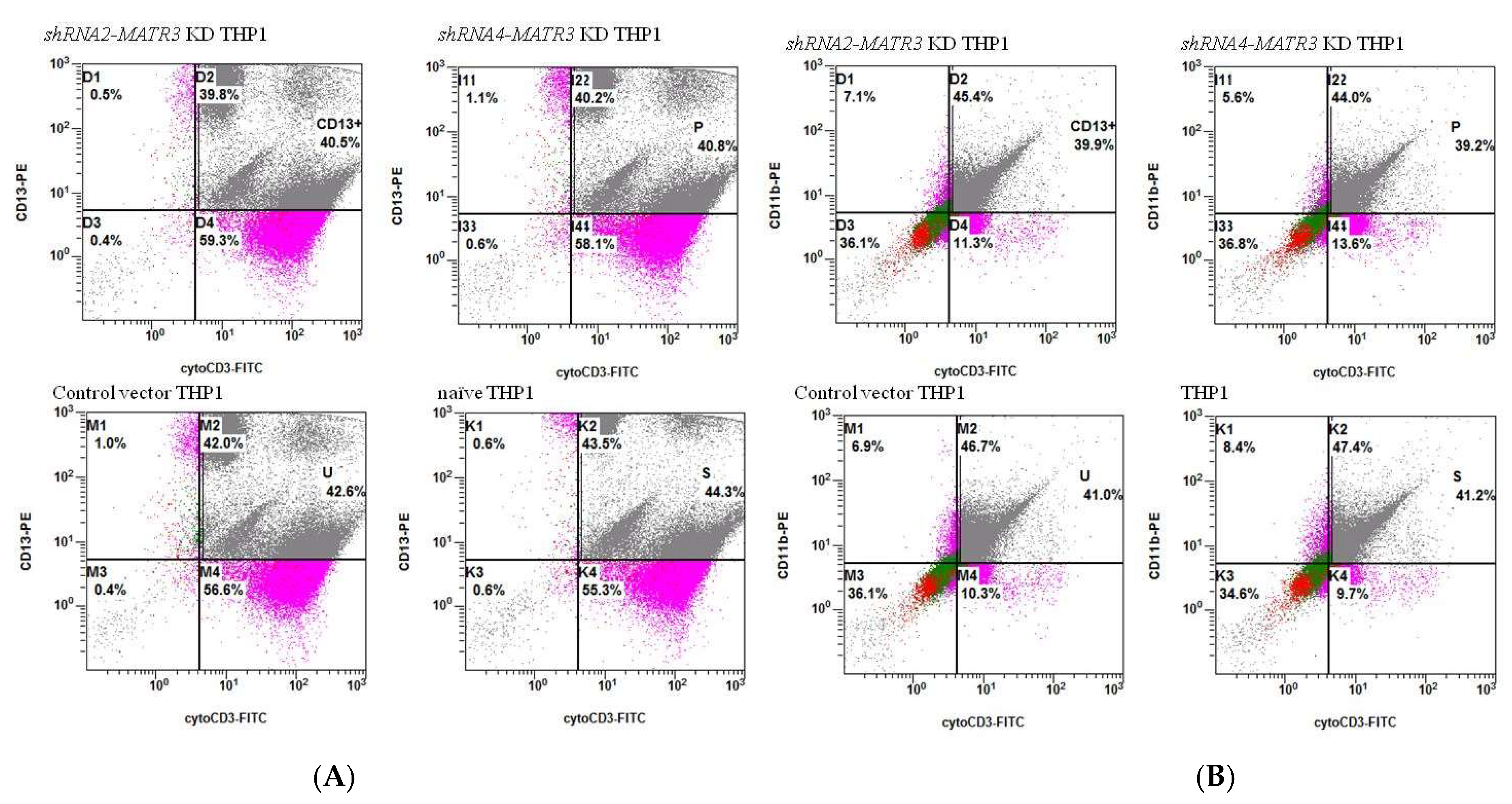
3.4. Enhanced Phagocytosis in shRNA-MATR3-KD THP1 Cells
3.4.1. Increased Phagocytosis of Latex Beads in shRNA-MATR3-KD THP1 Cells
3.4.2. Phagocytosis of Necrotic Jurkat E6 Cells Increase in shRNA-MATR3-KD THP1 Cells
3.4.3. Phagocytosis of Apoptotic Jurkat E6 Cells by shRNA-MATR3-KD THP1 and Control THP1 Cells
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Janka, G.E. Hemophagocytic syndrome. Blood Rev. 2007, 21, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Henter, J.I.; Arico, M.; Elinder, G.; Imashuku, S.; Janka, G. Familial hemophagocytic lymphohistiocytosis. Primary hemophagocytic lymphohistiocytosis. Hematol. Oncol. Clin. N. Am. 1998, 12, 417–433. [Google Scholar] [CrossRef]
- Arico, M.; Nespoli, L.; Maccario, R.; Montagna, D.; Bonetti, F.; Caselli, D.; Burgio, G.R. Natural cytotoxicity impairment in familial haemophagocytic lymphohistiocytosis. Arch. Dis. Child. 1988, 63, 292–296. [Google Scholar] [CrossRef]
- Janka, G.E.; Lehmberg, K. Hemophagocytic syndromes: An update. Blood Rev. 2014, 28, 135–142. [Google Scholar] [CrossRef]
- Filipovich, A.H. The expanding spectrum of hemophagocytic lymphohistiocytosis. Curr. Opin. Allergy Clin. Immunol. 2011, 11, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Pagel, J.; Beutel, K.; Lehmberg, K.; Koch, F.; Maul-Pavicic, A.; Rohlfs, A.K.; Al-Jefri, A.; Beier, R.; Bomme Ousager, L.; Ehlert, K.; et al. Distinct mutations in STXBP2 are associated with variable clinical presentations in patients with familial hemophagocytic lymphohistiocytosis type 5 (FHL5). Blood 2012, 119, 60166024. [Google Scholar] [CrossRef]
- Ramos-Casals, M.; Brito-Zerón, P.; López-Guillermo, A.; Khamashta, M.A.; Bosch, X. Adult haemophagocytic syndrome. Lancet 2014, 383, 1503–1516. [Google Scholar] [CrossRef]
- Imashuku, S. Treatment of Epstein-Barr virus-related hemophagocytic lymphohistiocytosis (EBV-HLH); update 2010. J. Pediatr. Hematol. Oncol. 2011, 33, 35–39. [Google Scholar] [CrossRef]
- Nikiforow, S.; Berliner, N. The unique aspects of presentation and diagnosis of hemophagocytic lymphohistiocytosis in adults. Hematol. Am. Soc. Hematol. Educ. Program 2015, 2015, 183–189. [Google Scholar] [CrossRef]
- Oeckinghaus, A.; Ghosh, S. The NF-κB Family of Transcription Factors and Its Regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef]
- Chuang, H.C.; Lay, J.D.; Hsieh, W.C.; Su, I.J. Pathogenesis and mechanism of disease progression from hemophagocytic lymphohistiocytosis to Epstein–Barr virus-associated T-cell lymphoma: Nuclear factor-κB pathway as a potential therapeutic target. Cancer Sci. 2007, 98, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Chuang, H.C.; Lay, J.D.; Hsieh, W.C.; Wang, H.C.; Chang, Y.; Chuang, S.E.; Su, I.J. Epstein-Barr virus LMP1 inhibits the expression of SAP gene and upregulates Th1 cytokines in the pathogenesis of hemophagocytic syndrome. Blood 2005, 106, 3090–3096. [Google Scholar] [CrossRef] [PubMed]
- Morchikh, M.; Cribier, A.; Raffel, R.; Amraoui, S.; Cau, J.; Severac, D.; Dubois, E.; Schwartz, O.; Bennasser, Y.; Benkirane, M. HEXIM1 and NEAT1 Long Non-coding RNA Form a Multi-subunit Complex that Regulates DNA-Mediated Innate Immune Response. Mol. Cell 2017, 67, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Gewurz, B.E.; Mar, J.C.; Padi, M.; Zhao, B.; Shinners, N.P.; Takasaki, K.; Bedoya, E.; Zou, J.Y.; Cahir-McFarland, E.; Quackenbush, J.; et al. Canonical NF-kappaB activation is essential for Epstein-Barr virus latent membrane protein 1 TES2/CTAR2 gene regulation. J. Virol. 2011, 85, 6764–6773. [Google Scholar]
- Franz, S.; Herrmann, K.; Fürnrohr, B.G.; Sheriff, A.; Frey, B.; Gaipl, U.S.; Voll, R.E.; Kalden, J.R.; Jäck, H.M.; Herrmann, M. After shrinkage apoptotic cells expose internal membrane-derived epitopes on their plasma membranes. Cell Death Differ. 2007, 14, 733–742. [Google Scholar] [CrossRef]
- Freire-de-Lima, C.G.; Nascimento, D.O.; Soares, M.B.; Bozza, P.T.; Castro-Faria-Neto, H.C.; de Mello, F.G.; DosReis, G.A.; Lopes, M.F. Uptake of apoptotic cells drives the growth of a pathogenic trypanosome in macrophages. Nature 2000, 403, 199–203. [Google Scholar] [CrossRef]
- Lemaire, S.; Mingeot-Leclercq, M.P.; Tulkens, P.M.; Van Bambeke, F. Study of macrophage functions in murine J774 cells and Human activated THP1 cells exposed to oritavancin, a lipoglycopeptide with high cellular accumulation. Antimicrob. Agents Chemother. 2014, 58, 2059–2066. [Google Scholar] [CrossRef]
- Hsu, M.J.; Lee, S.S.; Lee, S.T.; Lin, W.W. Signaling mechanisms of enhanced neutrophil phagocytosis and chemotaxis by the polysaccharide purified from Ganoderma lucidum. Br. J. Pharm. 2003, 139, 289–298. [Google Scholar] [CrossRef]
- Yang, W.C.; Tsai, W.C.; Lin, P.M.; Yang, M.Y.; Liu, Y.C.; Chang, C.S.; Yu, W.H.; Lin, S.F. Human BDH2, an anti-apoptosis factor, is a novel poor prognostic factor for de novo cytogenetically normal acute myeloid leukemia. J. Biomed. Sci. 2013, 20, 58. [Google Scholar] [CrossRef]
- Brisse, E.; Wouters, C.H.; Matthys, P. Advances in the pathogenesis of primary and secondary haemophagocytic lymphohistiocytosis: Differences and similarities. Br. J. Haematol. 2016, 174, 203–217. [Google Scholar] [CrossRef]
- Zoref-Lorenz, A.; Murakami, J.; Hofstetter, L.; Iyer, S.; Alotaibi, A.S.; Mohamed, S.F.; Miller, P.G.; Guber, E.; Weinstein, S.; Yacobovich, J.; et al. An improved index for diagnosis and mortality prediction in malignancy-associated hemophagocytic lymphohistiocytosis. Blood 2022, 139, 1098–1110. [Google Scholar] [CrossRef] [PubMed]
- Henter, J.I.; Horne, A.; Aricó, M.; Egeler, R.M.; Filipovich, A.H.; Imashuku, S.; Ladisch, S.; McClain, K.; Webb, D.; Winiarski, J.; et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr. Blood Cancer 2007, 48, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Fardet, L.; Galicier, L.; Lambotte, O.; Marzac, C.; Aumont, C.; Chahwan, D.; Coppo, P.; Hejblum, G. Development and validation of the H Score, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol. 2014, 66, 2613–2620. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Weitzman, S.; Abdelhaleem, M. The role of hemophagocytosis in bone marrow aspirates in the diagnosis of hemophagocytic lymphohistiocytosis. Pediatr. Blood Cancer 2008, 235, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Carvelli, J.; Piperoglou, C.; Farnarier, C.; Vely, F.; Mazodier, K.; Audonnet, S.; Nitschke, P.; Bole-Feysot, C.; Boucekine, M.; Cambon, A.; et al. Functional and genetic testing in adults with HLH reveals an inflammatory profile rather than a cytotoxicity defect. Blood 2020, 136, 542–552. [Google Scholar] [CrossRef]
- Ahn, J.S.; Rew, S.Y.; Shin, M.G.; Kim, H.R.; Yang, D.H.; Cho, D.; Kim, S.H.; Bae, S.Y.; Lee, S.R.; Kim, Y.K.; et al. Clinical significance of clonality and Epstein-Barr virus infection in adult patients with hemophagocytic lymphohistiocytosis. Am. J. Hematol. 2010, 85, 719–722. [Google Scholar] [CrossRef]
- Chen, C.C.; Manning, A.M. Transcriptional regulation of endothelial cell adhesion molecules: A dominant role for NF-kappa B. Agents Actions Suppl. 1995, 47, 135–141. [Google Scholar]
- Kopp, E.B.; Ghosh, S. NF-kappa B and rel proteins in innate immunity. Adv. Immunol. 1995, 58, 1–27. [Google Scholar]
- Wissink, S.; van de Stolpe, A.; Caldenhoven, E.; Koenderman, L.; van der Saag, P.T. NF-kappa B/Rel family members regulating the ICAM-1 promoter in monocytic THP-1 cells. Immunobiology 1997, 198, 50–64. [Google Scholar] [CrossRef]
- Salton, M.; Elkon, R.; Borodina, T.; Davydov, A.; Yaspo, M.L.; Halperin, E.; Shiloh, Y. Matrin 3 Binds and Stabilizes mRNA. PLoS ONE 2011, 6, e23882. [Google Scholar] [CrossRef]
- Przygodzka, P.; Boncela, J.; Cierniewski, C.S. Matrin 3 as a key regulator of endothelial cell survival. Exp. Cell Res. 2011, 317, 802–811. [Google Scholar] [CrossRef] [PubMed]
- Shih, V.F.S.; Tsui, R.; Caldwell, A.; Hoffmann, A. A single NFκB system for both canonical and non-canonical signaling. Cell Res. 2011, 21, 86–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Death (n) | Survival (n) | p-Value | |
|---|---|---|---|
| Age (y/o) (95%CI) | 51.55 (43.82–59.27) (33) | 52.52 (44.09–60.96) (21) | 0.866 |
| Sex (male/female) | 19/14 | 13/8 | 0.489 |
| Ferritin (mg/dL) (95%CI) | 11,847.1714 (2417.56–21,276.79) (21) | 12,073.13 (−7646.19–31,792.45) (10) | 0.98 |
| Ferritin > 3000 ng/mL (%) | 11/21 (52.38%) | 3/10 (30%) | 0.218 |
| Triglyceride mg/dL (95%CI) | 218.6 (126.59–310.61) (17) | 179.7 (111.55–247.85) (10) | 0.532 |
| Fibrinogen (95%CI) | 308.43 (225.42–391.45) (23) | 354.11 (245.83–462.40) (9) | 0.523 |
| WBC (/μL) (95%CI) | 5754.23 (2357.76–9150.70) (26) | 5884.17 ± 7279.51 (1258.99–10,509.34) (12) | 0.964 |
| Hgb (g/dL) (95%CI) | 8.996 (8.05–9.94) (26) | 8.142 (7.34–8.94) (12) | 0.246 |
| Platelet (×1000/μL) (95%CI) | 70.538 (48.25–92.83) (26) | 66 (36.20–95.80) (12) | 0.807 |
| LDH IU/L (95%CI) | 774.39 ± 996.96 (26) | 406.67 ± 307.27 (12) | 0.222 |
| Β2-microglobulin μg/L (95%CI) | 750.62 (371.71–1177.07) (13) | 284.57 (211.43–601.90) (7) | 0.194 |
| EBV status | 33 | 21 | 0.390 |
| EBNA Ab (+) (%) | 15 (45.45%) | 13 (61.90%) | |
| EBV DNA (+) (%) | 2 (6.06%) | 4 (19.05%) | |
| Both negative (%) | 4 (12.12%) | 1 (4.76%) | |
| Unknown (%) | 14 (42.42%) | 7 (33.33%) | |
| CMV status | 33 | 21 | 0.661 |
| CMV (+) (%) | 1 (3.03%) | 0 | |
| CMV (−) (%) | 6 (18.18%) | 3 (14.29%) | |
| Unknown (%) | 26 (78.79%) | 18 (85.71%) | |
| VZV status VZV (+) (%) VZV (−) (%) Unknown (%) | 33 | 21 | 0.446 |
| 3 (9.1%) | 2 (9.52%) | ||
| 0 | 1 (4.76%) | ||
| 30 (90.91%) | 18 (85.71%) | ||
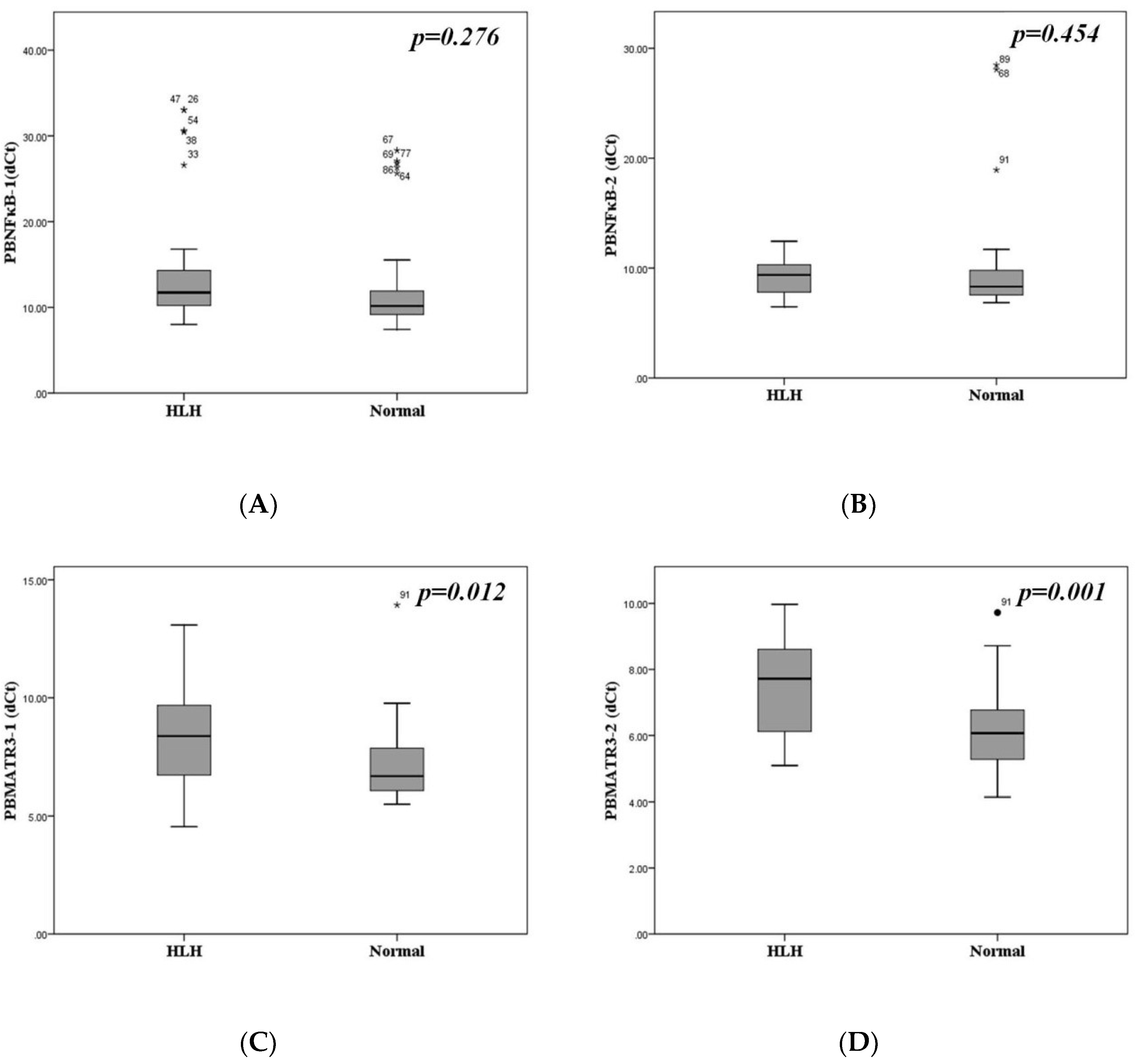
| PBNFκB-1 (dCt) (95%CI) | 13.913 (10.09–17.74) (16) | 16.731 (10.34–25.12) (9) | 0.092 |
| PBNFκB-2 (dCt) (95%CI) | 9.711 (8.84–10.58) (15) | 8.738 (7.46–10.01) (9) | 0.474 |
| PBMATR3-1 (dCt) (95%CI) | 8.511 (7.71–9.31) (16) | 8.491 (6.58–10.40) (9) | 0.162 |
| PBMATR3-2 (dCt) (95%CI) | 7.751 (6.97–8.51) (16) | 7.262 (6.19–8.33) (9) | 0.914 |
| BMNFκB-1 (dCt) (95%CI) | 20.194 (16.50–23.88) (28) | 15.976 (11.73–20.22) (16) | 0.011 * |
| BMNFκB-2 (dCt) (95%CI) | 13.534 (10.78–16.29) (28) | 11.946 (9.16–14.74) (17) | 0.144 |
| BMMATR3-1 (dCt) (95%CI) | 9.382 (8.34–10.43) (30) | 8.404 (7.02–9.79) (17) | 0.827 |
| BMMATR3-2 (dCt) (95%CI) | 11.272 (8.15–14.39) (28) | 8.855 (6.26–11.45) (17) | 0.017 * |
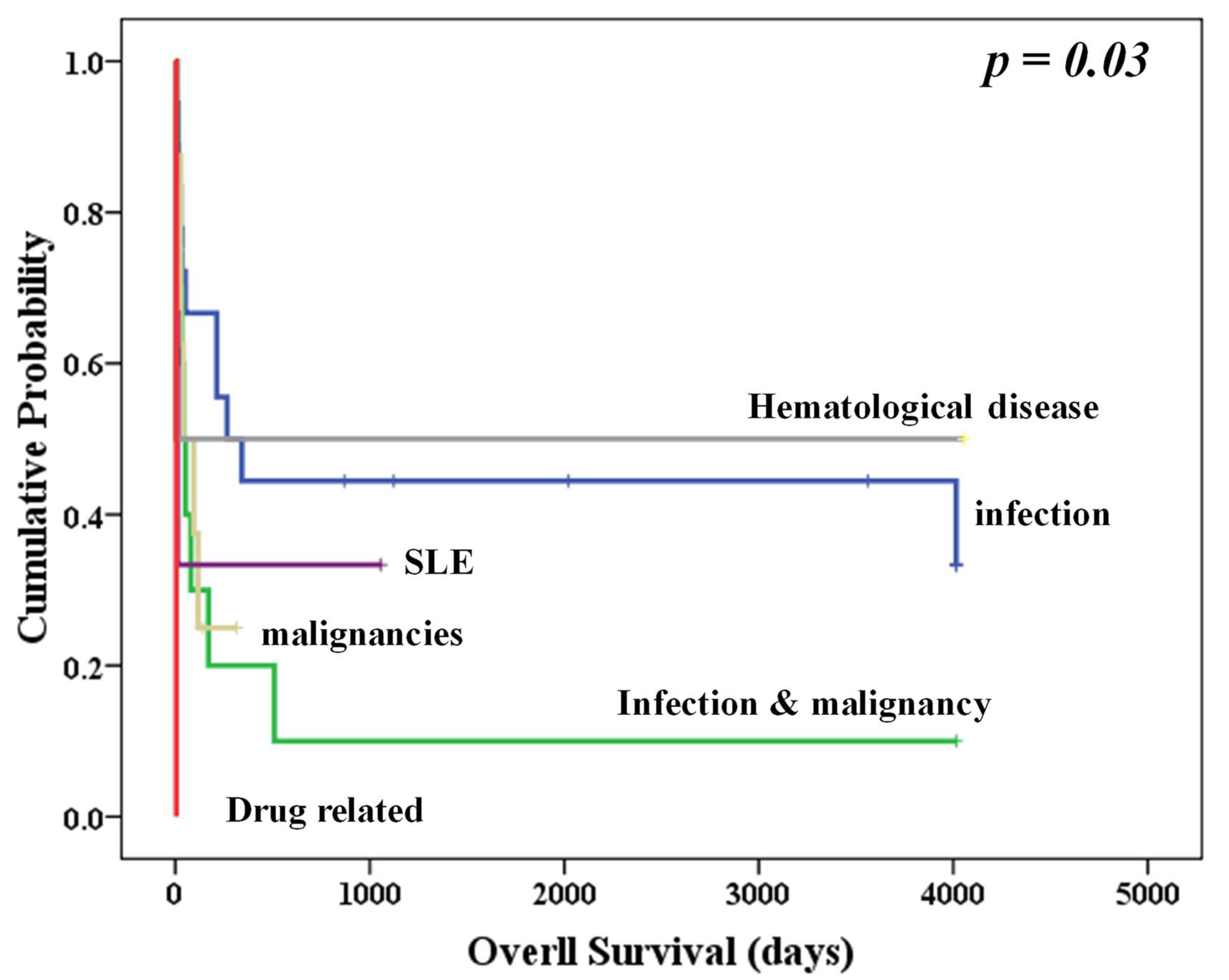
| Etiology | No. of Patients | Death (n = 32) | Survival (n = 18) | p |
|---|---|---|---|---|
| Infection (%) | 20 | 12 (60%) | 8 (40%) | 0.966 |
| Infection and malignancy (%) # | 9 | 6 (67%) | 3 (33%) | |
| Malignancy (%) # | 15 | 10 (67%) | 5 (33%) | |
| SLE (%) | 2 | 1 (50%) | 1 (50%) | |
| Vit B12 deficiency (%) | 1 | 1 (100%) | 0 (0) | |
| Hematological disease (%) * | 2 | 1 (50%) | 1 (50%) | |
| Drug-related | 1 | 1 (100%) | 0 |
| Cause (n) | Infection | Infection and Malignancy | Malignancies * | Hematological Diseases | SLE | Drug-Related | p-Value |
|---|---|---|---|---|---|---|---|
| PBNFκB-1 (dCt) (n, 95%CI) | 14.916 (8.94–20.90) (10) | 18.922 (6.15–31.69) (5) | 16.290 (7.33–25.25) (6) | 9.470 (1) | 0 | 0 | 0.746 |
| PBNFκB-2 (dCt) (n, 95%CI) | 9.556 (8.35–10.76) (10) | 9.208 (6.95–11.47) (5) | 9.410 (7.56–11.266) (6) | 6.720 (1) | 0 | 0 | 0.492 |
| PBMATR3-1 (dCt) (n, 95%CI) | 8.254 (6.93–9.58) (10) | 9.190 (5.81–12.57) (5) | 8.162 (6.59–9.73) (6) | 6.620 (1) | 0 | 0 | 0.642 |
| PBMATR3-2 (dCt) (n, 95%CI) | 7.593 (6.39–8.79) (10) | 7.632 (5.99–9.27) (5) | 7.460 (6.02–8.90) (6) | 5.570 (1) | 0 | 0 | 0.649 |
| BMNFκB-1 (dCt) (n, 95%CI) | 17.584 (12.60–22.57) (15) | 19.678 (5.99–9.27) (12) | 21.644 (14.75–28.54) (9) | 19.480 (−77.34–116.30) (2) | 29.820 (1) | 11.090 (1) | 0.698 |
| BMNFκB-2 (dCt) (n, 95%CI) | 13.745 (9.91–17.58) (16) | 11.304 (7.10–15.51) (12) | 12.817 (8.35–17.29) (9) | 18.520 (−90.4–127.54) (2) | 18.40 (1) | 10.680 (1) | 0.715 |
| BMMATR3-1 (dCt) (n, 95%CI) | 9.572 (8.08–11.07) (16) | 7.804 (6.54–9.06) (12) | 8.850 (7.32–10.38) (9) | 8.987 (5.35–12.63) (3) | 8.765 (−39.07–56.60) (2) | 13.640 (1) | 0.261 |
| BMMATR3-2 (dCt) (n, 95%CI) | 11.571 (7.20–15.94) (16) | 8.366 (4.42–12.31) (12) | 9.622 (4.41–14.83) (9) | 17.340 (−106.6–141.35) (2) | 12.260 (1) | 9.60 (1) | 0.683 |
| Ferritin (n, 95%CI) | 15,807.364 (–2231.861) (14) | 17,677.380 (−3803.19–39,157.95) (5) | 4368.457 (−2060.83–10,797.74) (7) | 247.850 (−1088.20–1583.91) (2) | 20,000 (1) | 7298 (1) | 0.489 |
| Age (n, 95%CI) | 50.24 (39.87–60.60) (21) | 51.44 (42.39–60.48) (16) | 50.22 (32.86–67.58) (9) | 73.33 (49.85–96.81) (3) | 64 (2) | 66 (1) | 0.855 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.-C.; Lin, S.-F.; Wu, S.-C.; Shu, C.-W. Matrin3 (MATR3) Expression Is Associated with Hemophagocytosis. Biomedicines 2022, 10, 2161. https://doi.org/10.3390/biomedicines10092161
Yang W-C, Lin S-F, Wu S-C, Shu C-W. Matrin3 (MATR3) Expression Is Associated with Hemophagocytosis. Biomedicines. 2022; 10(9):2161. https://doi.org/10.3390/biomedicines10092161
Chicago/Turabian StyleYang, Wen-Chi, Sheng-Fung Lin, Shih-Chi Wu, and Chih-Wen Shu. 2022. "Matrin3 (MATR3) Expression Is Associated with Hemophagocytosis" Biomedicines 10, no. 9: 2161. https://doi.org/10.3390/biomedicines10092161





