Contribution of the HIV-1 Envelope Glycoprotein to AIDS Pathogenesis and Clinical Progression
Abstract
:1. Introduction
2. HIV-1 Envs from LTNP-EC Individuals Present Inefficient Viral Functions, Associated with the Natural Control of the Infection and the Non-Progressor Clinical Phenotype
3. Fully Functional HIV-1 Envs Are Linked to Viremia and Progressor Clinical Phenotypes
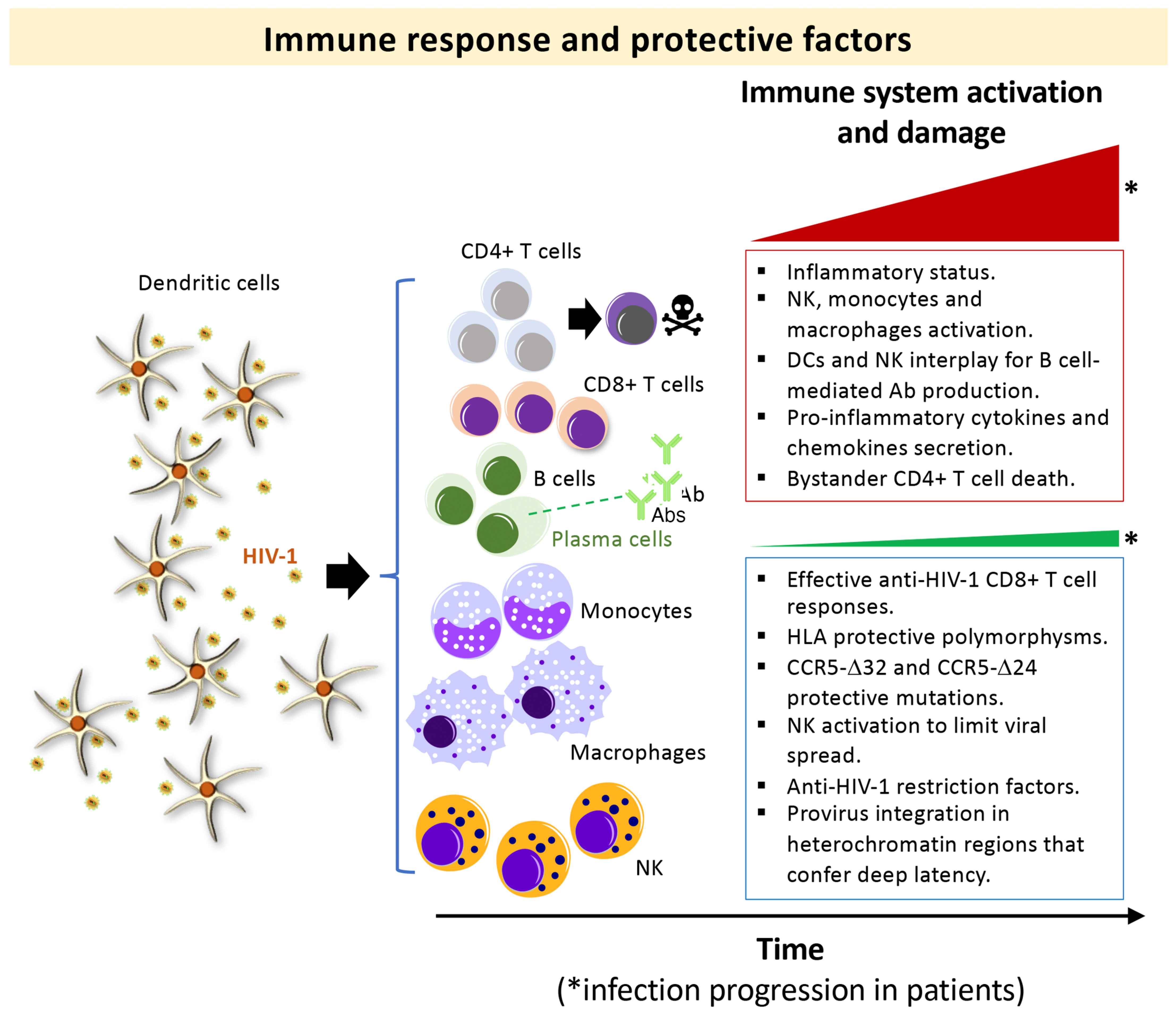
4. Role of the Viral Env Complex in Signal Transmission in Other Cellular Process and Cell Death
5. Discussion

6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Simon, V.; Ho, D.D.; Abdool Karim, Q. HIV/AIDS epidemiology, pathogenesis, prevention, and treatment. Lancet 2006, 368, 489–504. [Google Scholar] [CrossRef]
- Douek, D.C.; Picker, L.J.; Koup, R.A. T cell dynamics in HIV-1 infection. Annu. Rev. Immunol. 2003, 21, 265–304. [Google Scholar] [CrossRef] [PubMed]
- Moir, S.; Chun, T.W.; Fauci, A.S. Pathogenic mechanisms of HIV disease. Annu. Rev. Pathol. 2011, 6, 223–248. [Google Scholar] [CrossRef]
- McCune, J.M. The dynamics of CD4+ T-cell depletion in HIV disease. Nature 2001, 410, 974–979. [Google Scholar] [CrossRef]
- Deeks, S.G.; Overbaugh, J.; Phillips, A.; Buchbinder, S. HIV infection. Nat. Rev. Dis. Primers 2015, 1, 15035. [Google Scholar] [CrossRef] [PubMed]
- Ramratnam, B.; Bonhoeffer, S.; Binley, J.; Hurley, A.; Zhang, L.; Mittler, J.E.; Markowitz, M.; Moore, J.P.; Perelson, A.S.; Ho, D.D. Rapid production and clearance of HIV-1 and hepatitis C virus assessed by large volume plasma apheresis. Lancet 1999, 354, 1782–1785. [Google Scholar] [CrossRef]
- Simon, V.; Ho, D.D. HIV-1 dynamics in vivo: Implications for therapy. Nat. Rev. Microbiol. 2003, 1, 181–190. [Google Scholar] [CrossRef]
- Sepkowitz, K.A. AIDS—The first 20 years. N. Engl. J. Med. 2001, 344, 1764–1772. [Google Scholar] [CrossRef]
- Hazenberg, M.D.; Hamann, D.; Schuitemaker, H.; Miedema, F. T cell depletion in HIV-1 infection: How CD4+ T cells go out of stock. Nat. Immunol. 2000, 1, 285–289. [Google Scholar] [CrossRef]
- Borkowsky, W.; Rigaud, M.; Krasinski, K.; Moore, T.; Lawrence, R.; Pollack, H. Cell-mediated and humoral immune responses in children infected with human immunodeficiency virus during the first four years of life. J. Pediatr. 1992, 120, 371–375. [Google Scholar] [CrossRef]
- Musey, L.K.; Krieger, J.N.; Hughes, J.P.; Schacker, T.W.; Corey, L.; McElrath, M.J. Early and persistent human immunodeficiency virus type 1 (HIV-1)-specific T helper dysfunction in blood and lymph nodes following acute HIV-1 infection. J. Infect. Dis. 1999, 180, 278–284. [Google Scholar] [CrossRef]
- Kelley, C.F.; Barbour, J.D.; Hecht, F.M. The relation between symptoms, viral load, and viral load set point in primary HIV infection. J. Acquir. Immune Defic. Syndr. 2007, 45, 445–448. [Google Scholar] [CrossRef]
- Katzenstein, T.L.; Pedersen, C.; Nielsen, C.; Lundgren, J.D.; Jakobsen, P.H.; Gerstoft, J. Longitudinal serum HIV RNA quantification: Correlation to viral phenotype at seroconversion and clinical outcome. Aids 1996, 10, 167–173. [Google Scholar] [CrossRef]
- Sterling, T.R.; Vlahov, D.; Astemborski, J.; Hoover, D.R.; Margolick, J.B.; Quinn, T.C. Initial plasma HIV-1 RNA levels and progression to AIDS in women and men. N. Engl. J. Med. 2001, 344, 720–725. [Google Scholar] [CrossRef]
- Prins, H.A.B.; Verbon, A.; Boucher, C.A.B.; Rokx, C. Ending the epidemic: Critical role of primary HIV infection. Neth J. Med. 2017, 75, 321–327. [Google Scholar]
- Andersson, S.; Norrgren, H.; da Silva, Z.; Biague, A.; Bamba, S.; Kwok, S.; Christopherson, C.; Biberfeld, G.; Albert, J. Plasma viral load in HIV-1 and HIV-2 singly and dually infected individuals in Guinea-Bissau, West Africa: Significantly lower plasma virus set point in HIV-2 infection than in HIV-1 infection. Arch. Intern. Med. 2000, 160, 3286–3293. [Google Scholar] [CrossRef]
- O’Brien, W.A.; Hartigan, P.M.; Martin, D.; Esinhart, J.; Hill, A.; Benoit, S.; Rubin, M.; Simberkoff, M.S.; Hamilton, J.D. Changes in plasma HIV-1 RNA and CD4+ lymphocyte counts and the risk of progression to AIDS. Veterans Affairs Cooperative Study Group on AIDS. N. Engl. J. Med. 1996, 334, 426–431. [Google Scholar] [CrossRef]
- McPhee, E.; Grabowski, M.K.; Gray, R.H.; Ndyanabo, A.; Ssekasanvu, J.; Kigozi, G.; Makumbi, F.; Serwadda, D.; Quinn, T.C.; Laeyendecker, O. Short Communication: The Interaction of HIV Set Point Viral Load and Subtype on Disease Progression. AIDS Res. Hum. Retroviruses 2019, 35, 49–51. [Google Scholar] [CrossRef]
- Ananworanich, J.; Chomont, N.; Eller, L.A.; Kroon, E.; Tovanabutra, S.; Bose, M.; Nau, M.; Fletcher, J.L.K.; Tipsuk, S.; Vandergeeten, C.; et al. HIV DNA Set Point is Rapidly Established in Acute HIV Infection and Dramatically Reduced by Early ART. EBioMedicine 2016, 11, 68–72. [Google Scholar] [CrossRef]
- Robb, M.L.; Ananworanich, J. Lessons from acute HIV infection. Curr. Opin. HIV AIDS 2016, 11, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Quinn, T.C.; Wawer, M.J.; Sewankambo, N.; Serwadda, D.; Li, C.; Wabwire-Mangen, F.; Meehan, M.O.; Lutalo, T.; Gray, R.H. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N. Engl. J. Med. 2000, 342, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Wawer, M.J.; Gray, R.H.; Sewankambo, N.K.; Serwadda, D.; Li, X.; Laeyendecker, O.; Kiwanuka, N.; Kigozi, G.; Kiddugavu, M.; Lutalo, T.; et al. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J. Infect. Dis. 2005, 191, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.S.; Pilcher, C.D. Amplified HIV transmission and new approaches to HIV prevention. J. Infect. Dis. 2005, 191, 1391–1393. [Google Scholar] [CrossRef] [PubMed]
- Mellors, J.W.; Rinaldo, C.R., Jr.; Gupta, P.; White, R.M.; Todd, J.A.; Kingsley, L.A. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science 1996, 272, 1167–1170. [Google Scholar] [CrossRef]
- Gurdasani, D.; Iles, L.; Dillon, D.G.; Young, E.H.; Olson, A.D.; Naranbhai, V.; Fidler, S.; Gkrania-Klotsas, E.; Post, F.A.; Kellam, P.; et al. A systematic review of definitions of extreme phenotypes of HIV control and progression. AIDS 2014, 28, 149–162. [Google Scholar] [CrossRef]
- Casado, C.; Colombo, S.; Rauch, A.; Martinez, R.; Gunthard, H.F.; Garcia, S.; Rodriguez, C.; Del Romero, J.; Telenti, A.; Lopez-Galindez, C. Host and viral genetic correlates of clinical definitions of HIV-1 disease progression. PLoS ONE 2010, 5, e11079. [Google Scholar] [CrossRef]
- Beyrer, C.; Sullivan, P.; Sanchez, J.; Baral, S.D.; Collins, C.; Wirtz, A.L.; Altman, D.; Trapence, G.; Mayer, K. The increase in global HIV epidemics in MSM. AIDS 2013, 27, 2665–2678. [Google Scholar] [CrossRef]
- Fontaine, J.; Coutlee, F.; Tremblay, C.; Routy, J.P.; Poudrier, J.; Roger, M.; Montreal Primary, H.I.V.I.; Long-Term Nonprogressor Study, G. HIV infection affects blood myeloid dendritic cells after successful therapy and despite nonprogressing clinical disease. J. Infect. Dis. 2009, 199, 1007–1018. [Google Scholar] [CrossRef]
- Lajoie, J.; Fontaine, J.; Tremblay, C.; Routy, J.P.; Poudrier, J.; Roger, M. Persistence of high levels of blood soluble human leukocyte antigen-G is associated with rapid progression of HIV infection. AIDS 2009, 23, 1437–1440. [Google Scholar] [CrossRef]
- Deeks, S.G.; Walker, B.D. Human immunodeficiency virus controllers: Mechanisms of durable virus control in the absence of antiretroviral therapy. Immunity 2007, 27, 406–416. [Google Scholar] [CrossRef]
- Buchbinder, S.P.; Katz, M.H.; Hessol, N.A.; O’Malley, P.M.; Holmberg, S.D. Long-term HIV-1 infection without immunologic progression. AIDS 1994, 8, 1123–1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okulicz, J.F.; Marconi, V.C.; Landrum, M.L.; Wegner, S.; Weintrob, A.; Ganesan, A.; Hale, B.; Crum-Cianflone, N.; Delmar, J.; Barthel, V.; et al. Clinical outcomes of elite controllers, viremic controllers, and long-term nonprogressors in the US Department of Defense HIV natural history study. J. Infect. Dis. 2009, 200, 1714–1723. [Google Scholar] [CrossRef] [PubMed]
- Grabar, S.; Selinger-Leneman, H.; Abgrall, S.; Pialoux, G.; Weiss, L.; Costagliola, D. Prevalence and comparative characteristics of long-term nonprogressors and HIV controller patients in the French Hospital Database on HIV. AIDS 2009, 23, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Canducci, F.; Marinozzi, M.C.; Sampaolo, M.; Berre, S.; Bagnarelli, P.; Degano, M.; Gallotta, G.; Mazzi, B.; Lemey, P.; Burioni, R.; et al. Dynamic features of the selective pressure on the human immunodeficiency virus type 1 (HIV-1) gp120 CD4-binding site in a group of long term non progressor (LTNP) subjects. Retrovirology 2009, 6, 4. [Google Scholar] [CrossRef]
- Diop, G.; Hirtzig, T.; Do, H.; Coulonges, C.; Vasilescu, A.; Labib, T.; Spadoni, J.L.; Therwath, A.; Lathrop, M.; Matsuda, F.; et al. Exhaustive genotyping of the interferon alpha receptor 1 (IFNAR1) gene and association of an IFNAR1 protein variant with AIDS progression or susceptibility to HIV-1 infection in a French AIDS cohort. Biomed. Pharmacother. 2006, 60, 569–577. [Google Scholar] [CrossRef]
- Limou, S.; Le Clerc, S.; Coulonges, C.; Carpentier, W.; Dina, C.; Delaneau, O.; Labib, T.; Taing, L.; Sladek, R.; Deveau, C.; et al. Genomewide association study of an AIDS-nonprogression cohort emphasizes the role played by HLA genes (ANRS Genomewide Association Study 02). J. Infect. Dis. 2009, 199, 419–426. [Google Scholar] [CrossRef]
- Rotger, M.; Dang, K.K.; Fellay, J.; Heinzen, E.L.; Feng, S.; Descombes, P.; Shianna, K.V.; Ge, D.; Gunthard, H.F.; Goldstein, D.B.; et al. Genome-wide mRNA expression correlates of viral control in CD4+ T-cells from HIV-1-infected individuals. PLoS Pathog. 2010, 6, e1000781. [Google Scholar] [CrossRef]
- Kamya, P.; Boulet, S.; Tsoukas, C.M.; Routy, J.P.; Thomas, R.; Cote, P.; Boulassel, M.R.; Baril, J.G.; Kovacs, C.; Migueles, S.A.; et al. Receptor-ligand requirements for increased NK cell polyfunctional potential in slow progressors infected with HIV-1 coexpressing KIR3DL1*h/*y and HLA-B*57. J. Virol. 2011, 85, 5949–5960. [Google Scholar] [CrossRef]
- Gillespie, G.M.; Kaul, R.; Dong, T.; Yang, H.B.; Rostron, T.; Bwayo, J.J.; Kiama, P.; Peto, T.; Plummer, F.A.; McMichael, A.J.; et al. Cross-reactive cytotoxic T lymphocytes against a HIV-1 p24 epitope in slow progressors with B*57. AIDS 2002, 16, 961–972. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, Y.; Zhang, M.; Liu, J.; Sun, G.; Shi, W.; Wang, Y.; Shang, H. Alterations of CD4(+) CD25(+) Foxp3(+) regulatory T cells in HIV-infected slow progressors of former blood donors in China. Microbiol. Immunol. 2010, 54, 625–633. [Google Scholar] [CrossRef]
- Lambotte, O.; Boufassa, F.; Madec, Y.; Nguyen, A.; Goujard, C.; Meyer, L.; Rouzioux, C.; Venet, A.; Delfraissy, J.F.; Group, S.-H.S. HIV controllers: A homogeneous group of HIV-1-infected patients with spontaneous control of viral replication. Clin. Infect. Dis. 2005, 41, 1053–1056. [Google Scholar] [CrossRef] [Green Version]
- Blankson, J.N. Control of HIV-1 replication in elite suppressors. Discov. Med. 2010, 9, 261–266. [Google Scholar]
- Shaw, J.M.; Hunt, P.W.; Critchfield, J.W.; McConnell, D.H.; Garcia, J.C.; Pollard, R.B.; Somsouk, M.; Deeks, S.G.; Shacklett, B.L. Increased frequency of regulatory T cells accompanies increased immune activation in rectal mucosae of HIV-positive noncontrollers. J. Virol. 2011, 85, 11422–11434. [Google Scholar] [CrossRef]
- Casado, C.; Galvez, C.; Pernas, M.; Tarancon-Diez, L.; Rodriguez, C.; Sanchez-Merino, V.; Vera, M.; Olivares, I.; De Pablo-Bernal, R.; Merino-Mansilla, A.; et al. Permanent control of HIV-1 pathogenesis in exceptional elite controllers: A model of spontaneous cure. Sci. Rep. 2020, 10, 1902. [Google Scholar] [CrossRef]
- Samson, M.; Libert, F.; Doranz, B.J.; Rucker, J.; Liesnard, C.; Farber, C.M.; Saragosti, S.; Lapoumeroulie, C.; Cognaux, J.; Forceille, C.; et al. Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature 1996, 382, 722–725. [Google Scholar] [CrossRef]
- Hutter, G.; Nowak, D.; Mossner, M.; Ganepola, S.; Mussig, A.; Allers, K.; Schneider, T.; Hofmann, J.; Kucherer, C.; Blau, O.; et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N. Engl. J. Med. 2009, 360, 692–698. [Google Scholar] [CrossRef]
- Gupta, R.K.; Abdul-Jawad, S.; McCoy, L.E.; Mok, H.P.; Peppa, D.; Salgado, M.; Martinez-Picado, J.; Nijhuis, M.; Wensing, A.M.J.; Lee, H.; et al. HIV-1 remission following CCR5Δ32/Δ32 haematopoietic stem-cell transplantation. Nature 2019, 568, 244–248. [Google Scholar] [CrossRef]
- Dean, M.; Carrington, M.; Winkler, C.; Huttley, G.A.; Smith, M.W.; Allikmets, R.; Goedert, J.J.; Buchbinder, S.P.; Vittinghoff, E.; Gomperts, E.; et al. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Hemophilia Growth and Development Study, Multicenter AIDS Cohort Study, Multicenter Hemophilia Cohort Study, San Francisco City Cohort, ALIVE Study. Science 1996, 273, 1856–1862. [Google Scholar] [CrossRef]
- Liu, R.; Paxton, W.A.; Choe, S.; Ceradini, D.; Martin, S.R.; Horuk, R.; MacDonald, M.E.; Stuhlmann, H.; Koup, R.A.; Landau, N.R. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell 1996, 86, 367–377. [Google Scholar] [CrossRef]
- Masquelier, C.; Servais, J.Y.; Rusanganwa, E.; Roman, F.; Havuga, E.; Servais, J.; Tuyizere, S.; Omes, C.; Karasi, J.C.; Coruteille, O.; et al. A novel 24-base pair deletion in the coding region of CCR5 in an African population. AIDS 2007, 21, 111–113. [Google Scholar] [CrossRef]
- Arendt, V.; Amand, M.; Iserentant, G.; Lemaire, M.; Masquelier, C.; Ndayisaba, G.F.; Verhofstede, C.; Karita, E.; Allen, S.; Chevigne, A.; et al. Predominance of the heterozygous CCR5 delta-24 deletion in African individuals resistant to HIV infection might be related to a defect in CCR5 addressing at the cell surface. J. Int. AIDS Soc. 2019, 22, e25384. [Google Scholar] [CrossRef]
- Huang, Y.; Paxton, W.A.; Wolinsky, S.M.; Neumann, A.U.; Zhang, L.; He, T.; Kang, S.; Ceradini, D.; Jin, Z.; Yazdanbakhsh, K.; et al. The role of a mutant CCR5 allele in HIV-1 transmission and disease progression. Nat. Med. 1996, 2, 1240–1243. [Google Scholar] [CrossRef]
- Gonzalez, E.; Bamshad, M.; Sato, N.; Mummidi, S.; Dhanda, R.; Catano, G.; Cabrera, S.; McBride, M.; Cao, X.H.; Merrill, G.; et al. Race-specific HIV-1 disease-modifying effects associated with CCR5 haplotypes. Proc. Natl. Acad. Sci. USA 1999, 96, 12004–12009. [Google Scholar] [CrossRef]
- Ioannidis, J.P.; Rosenberg, P.S.; Goedert, J.J.; Ashton, L.J.; Benfield, T.L.; Buchbinder, S.P.; Coutinho, R.A.; Eugen-Olsen, J.; Gallart, T.; Katzenstein, T.L.; et al. Effects of CCR5-Delta32, CCR2-64I, and SDF-1 3′A alleles on HIV-1 disease progression: An international meta-analysis of individual-patient data. Ann. Intern. Med. 2001, 135, 782–795. [Google Scholar] [CrossRef]
- Misrahi, M.; Teglas, J.P.; N’Go, N.; Burgard, M.; Mayaux, M.J.; Rouzioux, C.; Delfraissy, J.F.; Blanche, S. CCR5 chemokine receptor variant in HIV-1 mother-to-child transmission and disease progression in children. French Pediatric HIV Infection Study Group. JAMA 1998, 279, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Mateos, E.; Tarancon-Diez, L.; Alvarez-Rios, A.I.; Dominguez-Molina, B.; Genebat, M.; Pulido, I.; Abad, M.A.; Munoz-Fernandez, M.A.; Leal, M. Association of heterozygous CCR5Delta32 deletion with survival in HIV-infection: A cohort study. Antiviral Res. 2018, 150, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Liu, Y.; Lai, Y. Knowledge From London and Berlin: Finding Threads to a Functional HIV Cure. Front. Immunol. 2021, 12, 688747. [Google Scholar] [CrossRef] [PubMed]
- Autran, B.; Descours, B.; Avettand-Fenoel, V.; Rouzioux, C. Elite controllers as a model of functional cure. Curr. Opin. HIV AIDS 2011, 6, 181–187. [Google Scholar] [CrossRef]
- Canoui, E.; Lecuroux, C.; Avettand-Fenoel, V.; Gousset, M.; Rouzioux, C.; Saez-Cirion, A.; Meyer, L.; Boufassa, F.; Lambotte, O.; Noel, N.; et al. A Subset of Extreme Human Immunodeficiency Virus (HIV) Controllers Is Characterized by a Small HIV Blood Reservoir and a Weak T-Cell Activation Level. Open Forum Infect. Dis. 2017, 4, ofx064. [Google Scholar] [CrossRef]
- Mendoza, D.; Johnson, S.A.; Peterson, B.A.; Natarajan, V.; Salgado, M.; Dewar, R.L.; Burbelo, P.D.; Doria-Rose, N.A.; Graf, E.H.; Greenwald, J.H.; et al. Comprehensive analysis of unique cases with extraordinary control over HIV replication. Blood 2012, 119, 4645–4655. [Google Scholar] [CrossRef]
- International, H.I.V.C.S.; Pereyra, F.; Jia, X.; McLaren, P.J.; Telenti, A.; de Bakker, P.I.; Walker, B.D.; Ripke, S.; Brumme, C.J.; Pulit, S.L.; et al. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science 2010, 330, 1551–1557. [Google Scholar] [CrossRef]
- Fellay, J.; Shianna, K.V.; Ge, D.; Colombo, S.; Ledergerber, B.; Weale, M.; Zhang, K.; Gumbs, C.; Castagna, A.; Cossarizza, A.; et al. A whole-genome association study of major determinants for host control of HIV-1. Science 2007, 317, 944–947. [Google Scholar] [CrossRef] [Green Version]
- Migueles, S.A.; Sabbaghian, M.S.; Shupert, W.L.; Bettinotti, M.P.; Marincola, F.M.; Martino, L.; Hallahan, C.W.; Selig, S.M.; Schwartz, D.; Sullivan, J.; et al. HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc. Natl. Acad. Sci. USA 2000, 97, 2709–2714. [Google Scholar] [CrossRef]
- Genovese, L.; Nebuloni, M.; Alfano, M. Cell-Mediated Immunity in Elite Controllers Naturally Controlling HIV Viral Load. Front. Immunol. 2013, 4, 86. [Google Scholar] [CrossRef]
- Migueles, S.A.; Laborico, A.C.; Shupert, W.L.; Sabbaghian, M.S.; Rabin, R.; Hallahan, C.W.; Van Baarle, D.; Kostense, S.; Miedema, F.; McLaughlin, M.; et al. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat. Immunol. 2002, 3, 1061–1068. [Google Scholar] [CrossRef]
- Saez-Cirion, A.; Lacabaratz, C.; Lambotte, O.; Versmisse, P.; Urrutia, A.; Boufassa, F.; Barre-Sinoussi, F.; Delfraissy, J.F.; Sinet, M.; Pancino, G.; et al. HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation phenotype. Proc. Natl. Acad. Sci. USA 2007, 104, 6776–6781. [Google Scholar] [CrossRef]
- Martin, M.P.; Naranbhai, V.; Shea, P.R.; Qi, Y.; Ramsuran, V.; Vince, N.; Gao, X.; Thomas, R.; Brumme, Z.L.; Carlson, J.M.; et al. Killer cell immunoglobulin-like receptor 3DL1 variation modifies HLA-B*57 protection against HIV-1. J. Clin. Investig. 2018, 128, 1903–1912. [Google Scholar] [CrossRef]
- Jiang, C.; Lian, X.; Gao, C.; Sun, X.; Einkauf, K.B.; Chevalier, J.M.; Chen, S.M.Y.; Hua, S.; Rhee, B.; Chang, K.; et al. Distinct viral reservoirs in individuals with spontaneous control of HIV-1. Nature 2020, 585, 261–267. [Google Scholar] [CrossRef]
- Abdel-Mohsen, M.; Raposo, R.A.; Deng, X.; Li, M.; Liegler, T.; Sinclair, E.; Salama, M.S.; Ghanem Hel, D.; Hoh, R.; Wong, J.K.; et al. Expression profile of host restriction factors in HIV-1 elite controllers. Retrovirology 2013, 10, 106. [Google Scholar] [CrossRef]
- Perez-Yanes, S.; Pernas, M.; Marfil, S.; Cabrera-Rodriguez, R.; Ortiz, R.; Urrea, V.; Rovirosa, C.; Estevez-Herrera, J.; Olivares, I.; Casado, C.; et al. The Characteristics of the HIV-1 Env Glycoprotein Are Linked With Viral Pathogenesis. Front. Microbiol. 2022, 13, 763039. [Google Scholar] [CrossRef]
- Cabrera-Rodriguez, R.; Perez-Yanes, S.; Estevez-Herrera, J.; Marquez-Arce, D.; Cabrera, C.; Espert, L.; Blanco, J.; Valenzuela-Fernandez, A. The Interplay of HIV and Autophagy in Early Infection. Front. Microbiol. 2021, 12, 661446. [Google Scholar] [CrossRef]
- Cabrera-Rodriguez, R.; Hebmann, V.; Marfil, S.; Pernas, M.; Marrero-Hernandez, S.; Cabrera, C.; Urrea, V.; Casado, C.; Olivares, I.; Marquez-Arce, D.; et al. HIV-1 envelope glycoproteins isolated from Viremic Non-Progressor individuals are fully functional and cytopathic. Sci. Rep. 2019, 9, 5544. [Google Scholar] [CrossRef] [Green Version]
- Casado, C.; Pernas, M.; Sandonis, V.; Alvaro-Cifuentes, T.; Olivares, I.; Fuentes, R.; Martinez-Prats, L.; Grau, E.; Ruiz, L.; Delgado, R.; et al. Identification of a cluster of HIV-1 controllers infected with low replicating viruses. PLoS ONE 2013, 8, e77663. [Google Scholar] [CrossRef]
- Murakoshi, H.; Koyanagi, M.; Akahoshi, T.; Chikata, T.; Kuse, N.; Gatanaga, H.; Rowland-Jones, S.L.; Oka, S.; Takiguchi, M. Impact of a single HLA-A*24:02-associated escape mutation on the detrimental effect of HLA-B*35:01 in HIV-1 control. EBioMedicine 2018, 36, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Kuse, N.; Murakoshi, H.; Akahoshi, T.; Chikata, T.; James, K.L.; Gatanaga, H.; Rowland-Jones, S.L.; Oka, S.; Takiguchi, M. Collaboration of a Detrimental HLA-B*35:01 Allele with HLA-A*24:02 in Coevolution of HIV-1 with T Cells Leading to Poorer Clinical Outcomes. J. Virol. 2021, 95, e0125921. [Google Scholar] [CrossRef]
- Nehete, P.N.; Lewis, D.E.; Tang, D.N.; Pollack, M.S.; Sastry, K.J. Presence of HLA-C-restricted cytotoxic T-lymphocyte responses in long-term nonprogressors infected with human immunodeficiency virus. Viral Immunol. 1998, 11, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Malnati, M.S.; Ugolotti, E.; Monti, M.C.; Battista, D.; Vanni, I.; Bordo, D.; Sironi, F.; Larghero, P.; Marco, E.D.; Biswas, P.; et al. Activating Killer Immunoglobulin Receptors and HLA-C: A successful combination providing HIV-1 control. Sci. Rep. 2017, 7, 42470. [Google Scholar] [CrossRef]
- Kyobe, S.; Mwesigwa, S.; Kisitu, G.P.; Farirai, J.; Katagirya, E.; Mirembe, A.N.; Ketumile, L.; Wayengera, M.; Katabazi, F.A.; Kigozi, E.; et al. Exome Sequencing Reveals a Putative Role for HLA-C*03:02 in Control of HIV-1 in African Pediatric Populations. Front. Genet. 2021, 12, 720213. [Google Scholar] [CrossRef] [PubMed]
- Alexander, L.; Weiskopf, E.; Greenough, T.C.; Gaddis, N.C.; Auerbach, M.R.; Malim, M.H.; O’Brien, S.J.; Walker, B.D.; Sullivan, J.L.; Desrosiers, R.C. Unusual polymorphisms in human immunodeficiency virus type 1 associated with nonprogressive infection. J. Virol. 2000, 74, 4361–4376. [Google Scholar] [CrossRef]
- Lassen, K.G.; Lobritz, M.A.; Bailey, J.R.; Johnston, S.; Nguyen, S.; Lee, B.; Chou, T.; Siliciano, R.F.; Markowitz, M.; Arts, E.J. Elite suppressor-derived HIV-1 envelope glycoproteins exhibit reduced entry efficiency and kinetics. PLoS Pathog. 2009, 5, e1000377. [Google Scholar] [CrossRef]
- Curriu, M.; Fausther-Bovendo, H.; Pernas, M.; Massanella, M.; Carrillo, J.; Cabrera, C.; López-Galíndez, C.; Clotet, B.; Debré, P.; Vieillard, V.; et al. Viremic HIV infected individuals with high CD4 T cells and functional envelope proteins show anti-gp41 antibodies with unique specificity and function. PLoS ONE 2012, 7, e30330. [Google Scholar] [CrossRef]
- Miura, T.; Brumme, Z.L.; Brockman, M.A.; Rosato, P.; Sela, J.; Brumme, C.J.; Pereyra, F.; Kaufmann, D.E.; Trocha, A.; Block, B.L.; et al. Impaired replication capacity of acute/early viruses in persons who become HIV controllers. J. Virol. 2010, 84, 7581–7591. [Google Scholar] [CrossRef] [Green Version]
- Sandonis, V.; Casado, C.; Alvaro, T.; Pernas, M.; Olivares, I.; Garcia, S.; Rodriguez, C.; del Romero, J.; Lopez-Galindez, C. A combination of defective DNA and protective host factors are found in a set of HIV-1 ancestral LTNPs. Virology 2009, 391, 73–82. [Google Scholar] [CrossRef]
- Sáez-Cirión, A.; Bacchus, C.; Hocqueloux, L.; Avettand-Fenoel, V.; Girault, I.; Lecuroux, C.; Potard, V.; Versmisse, P.; Melard, A.; Prazuck, T.; et al. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog 2013, 9, e1003211. [Google Scholar] [CrossRef]
- Blankson, J.N.; Bailey, J.R.; Thayil, S.; Yang, H.C.; Lassen, K.; Lai, J.; Gandhi, S.K.; Siliciano, J.D.; Williams, T.M.; Siliciano, R.F. Isolation and characterization of replication-competent human immunodeficiency virus type 1 from a subset of elite suppressors. J. Virol. 2007, 81, 2508–2518. [Google Scholar] [CrossRef]
- Rhodes, D.I.; Ashton, L.; Solomon, A.; Carr, A.; Cooper, D.; Kaldor, J.; Deacon, N. Characterization of three nef-defective human immunodeficiency virus type 1 strains associated with long-term nonprogression. Australian Long-Term Nonprogressor Study Group. J. Virol. 2000, 74, 10581–10588. [Google Scholar] [CrossRef]
- Sluis-Cremer, N.; Tachedjian, G. Mechanisms of inhibition of HIV replication by non-nucleoside reverse transcriptase inhibitors. Virus Res. 2008, 134, 147–156. [Google Scholar] [CrossRef]
- Hu, W.S.; Hughes, S.H. HIV-1 reverse transcription. Cold Spring Harb. Perspect. Med. 2012, 2, a006882. [Google Scholar] [CrossRef]
- Rawal, R.K.; Murugesan, V.; Katti, S.B. Structure-activity relationship studies on clinically relevant HIV-1 NNRTIs. Curr. Med. Chem. 2012, 19, 5364–5380. [Google Scholar] [CrossRef]
- De Clercq, E. The role of non-nucleoside reverse transcriptase inhibitors (NNRTIs) in the therapy of HIV-1 infection. Antivir. Res. 1998, 38, 153–179. [Google Scholar] [CrossRef]
- De Clercq, E. Perspectives of non-nucleoside reverse transcriptase inhibitors (NNRTIs) in the therapy of HIV-1 infection. Farmaco 1999, 54, 26–45. [Google Scholar] [CrossRef]
- Ren, J.; Milton, J.; Weaver, K.L.; Short, S.A.; Stuart, D.I.; Stammers, D.K. Structural basis for the resilience of efavirenz (DMP-266) to drug resistance mutations in HIV-1 reverse transcriptase. Structure 2000, 8, 1089–1094. [Google Scholar] [CrossRef]
- Das, K.; Arnold, E. HIV-1 reverse transcriptase and antiviral drug resistance. Part 1. Curr. Opin. Virol. 2013, 3, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Arnold, E. HIV-1 reverse transcriptase and antiviral drug resistance. Part 2. Curr. Opin. Virol. 2013, 3, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Sarafianos, S.G.; Sonnerborg, A. Long-Acting Anti-HIV Drugs Targeting HIV-1 Reverse Transcriptase and Integrase. Pharmaceuticals 2019, 12, 62. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Kang, D.; Nguyen, L.A.; Smithline, Z.B.; Pannecouque, C.; Zhan, P.; Liu, X.; Steitz, T.A. Structural basis for potent and broad inhibition of HIV-1 RT by thiophene [3,2-d]pyrimidine non-nucleoside inhibitors. eLife 2018, 7, e36340. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.X.; Xue, P.; Ju, X.L.; Zhu, Y.Y. Advances in rationally designed dual inhibitors of HIV-1 reverse transcriptase and integrase. Bioorg. Med. Chem. 2016, 24, 5007–5016. [Google Scholar] [CrossRef]
- Boyer, P.L.; Smith, S.J.; Zhao, X.Z.; Das, K.; Gruber, K.; Arnold, E.; Burke, T.R., Jr.; Hughes, S.H. Developing and Evaluating Inhibitors against the RNase H Active Site of HIV-1 Reverse Transcriptase. J. Virol. 2018, 92, e02203-17. [Google Scholar] [CrossRef]
- Saccon, E.; Mikaeloff, F.; Figueras Ivern, P.; Végvári, Á.; Sönnerborg, A.; Neogi, U.; van Domselaar, R. Cytotoxic Lymphocytes Target HIV-1 Gag Through Granzyme M-Mediated Cleavage. Front. Immunol. 2021, 12, 669347. [Google Scholar] [CrossRef]
- Salgado, M.; Garcia-Minambres, A.; Dalmau, J.; Jimenez-Moyano, E.; Viciana, P.; Alejos, B.; Clotet, B.; Prado, J.G.; Martinez-Picado, J. Control of HIV-1 Pathogenesis in Viremic Nonprogressors Is Independent of Gag-Specific Cytotoxic T Lymphocyte Responses. J. Virol. 2018, 92, e00346-18. [Google Scholar] [CrossRef]
- Laher, F.; Ranasinghe, S.; Porichis, F.; Mewalal, N.; Pretorius, K.; Ismail, N.; Buus, S.; Stryhn, A.; Carrington, M.; Walker, B.D.; et al. HIV Controllers Exhibit Enhanced Frequencies of Major Histocompatibility Complex Class II Tetramer(+) Gag-Specific CD4(+) T Cells in Chronic Clade C HIV-1 Infection. J. Virol. 2017, 91, e02477-16. [Google Scholar] [CrossRef] [PubMed]
- Dyer, W.B.; Zaunders, J.J.; Yuan, F.F.; Wang, B.; Learmont, J.C.; Geczy, A.F.; Saksena, N.K.; McPhee, D.A.; Gorry, P.R.; Sullivan, J.S. Mechanisms of HIV non-progression; robust and sustained CD4+ T-cell proliferative responses to p24 antigen correlate with control of viraemia and lack of disease progression after long-term transfusion-acquired HIV-1 infection. Retrovirology 2008, 5, 112. [Google Scholar] [CrossRef] [Green Version]
- Tarosso, L.F.; Vieira, V.A.; Sauer, M.M.; Tomiyama, H.I.; Kalil, J.; Kallas, E.G. Conserved HIV-1 Gag p24 Epitopes Elicit Cellular Immune Responses That Impact Disease Outcome. AIDS Res. Hum. Retroviruses 2017, 33, 832–842. [Google Scholar] [CrossRef]
- French, M.A.; Abudulai, L.N.; Fernandez, S. Isotype Diversification of IgG Antibodies to HIV Gag Proteins as a Therapeutic Vaccination Strategy for HIV Infection. Vaccines 2013, 1, 328–342. [Google Scholar] [CrossRef]
- Pitcher, C.J.; Quittner, C.; Peterson, D.M.; Connors, M.; Koup, R.A.; Maino, V.C.; Picker, L.J. HIV-1-specific CD4+ T cells are detectable in most individuals with active HIV-1 infection, but decline with prolonged viral suppression. Nat. Med. 1999, 5, 518–525. [Google Scholar] [CrossRef]
- Rosenberg, E.S.; Billingsley, J.M.; Caliendo, A.M.; Boswell, S.L.; Sax, P.E.; Kalams, S.A.; Walker, B.D. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science 1997, 278, 1447–1450. [Google Scholar] [CrossRef]
- Valentine, F.T.; Paolino, A.; Saito, A.; Holzman, R.S. Lymphocyte-proliferative responses to HIV antigens as a potential measure of immunological reconstitution in HIV disease. AIDS Res. Hum. Retroviruses 1998, 14 (Suppl. 2), S161–S166. [Google Scholar]
- Quillay, H.; El Costa, H.; Duriez, M.; Marlin, R.; Cannou, C.; Madec, Y.; de Truchis, C.; Rahmati, M.; Barre-Sinoussi, F.; Nugeyre, M.T.; et al. NK cells control HIV-1 infection of macrophages through soluble factors and cellular contacts in the human decidua. Retrovirology 2016, 13, 39. [Google Scholar] [CrossRef]
- Quaranta, M.G.; Napolitano, A.; Sanchez, M.; Giordani, L.; Mattioli, B.; Viora, M. HIV-1 Nef impairs the dynamic of DC/NK crosstalk: Different outcome of CD56(dim) and CD56(bright) NK cell subsets. FASEB J. 2007, 21, 2323–2334. [Google Scholar] [CrossRef]
- Rydyznski, C.; Daniels, K.A.; Karmele, E.P.; Brooks, T.R.; Mahl, S.E.; Moran, M.T.; Li, C.; Sutiwisesak, R.; Welsh, R.M.; Waggoner, S.N. Generation of cellular immune memory and B-cell immunity is impaired by natural killer cells. Nat. Commun. 2015, 6, 6375. [Google Scholar] [CrossRef]
- Castellano, P.; Prevedel, L.; Eugenin, E.A. HIV-infected macrophages and microglia that survive acute infection become viral reservoirs by a mechanism involving Bim. Sci. Rep. 2017, 7, 12866. [Google Scholar] [CrossRef] [PubMed]
- Moanna, A.; Dunham, R.; Paiardini, M.; Silvestri, G. CD4+ T-cell depletion in HIV infection: Killed by friendly fire? Curr. HIV/AIDS Rep. 2005, 2, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Okoye, A.A.; Picker, L.J. CD4(+) T-cell depletion in HIV infection: Mechanisms of immunological failure. Immunol. Rev. 2013, 254, 54–64. [Google Scholar] [CrossRef] [Green Version]
- Denizot, M.; Varbanov, M.; Espert, L.; Robert-Hebmann, V.; Sagnier, S.; Garcia, E.; Curriu, M.; Mamoun, R.; Blanco, J.; Biard-Piechaczyk, M. HIV-1 gp41 fusogenic function triggers autophagy in uninfected cells. Autophagy 2008, 4, 998–1008. [Google Scholar] [CrossRef]
- Cunyat, F.; Curriu, M.; Marfil, S.; Garcia, E.; Clotet, B.; Blanco, J.; Cabrera, C. Evaluation of the cytopathicity (fusion/hemifusion) of patient-derived HIV-1 envelope glycoproteins comparing two effector cell lines. J. Biomol. Screen. 2012, 17, 727–737. [Google Scholar] [CrossRef]
- Cunyat, F.; Marfil, S.; García, E.; Svicher, V.; Pérez-Alvárez, N.; Curriu, M.; Perno, C.F.; Clotet, B.; Blanco, J.; Cabrera, C. The HR2 polymorphism N140I in the HIV-1 gp41 combined with the HR1 V38A mutation is associated with a less cytopathic phenotype. Retrovirology 2012, 9, 15. [Google Scholar] [CrossRef]
- Blanco, J.; Barretina, J.; Ferri, K.F.; Jacotot, E.; Gutierrez, A.; Armand-Ugon, M.; Cabrera, C.; Kroemer, G.; Clotet, B.; Este, J.A. Cell-surface-expressed HIV-1 envelope induces the death of CD4 T cells during GP41-mediated hemifusion-like events. Virology 2003, 305, 318–329. [Google Scholar] [CrossRef]
- Barretina, J.; Blanco, J.; Armand-Ugon, M.; Gutierrez, A.; Clotet, B.; Este, J.A. Anti-HIV-1 activity of enfuvirtide (T-20) by inhibition of bystander cell death. Antivir. Ther. 2003, 8, 155–161. [Google Scholar] [CrossRef]
- Blanco, J.; Barretina, J.; Clotet, B.; Este, J.A. R5 HIV gp120-mediated cellular contacts induce the death of single CCR5-expressing CD4 T cells by a gp41-dependent mechanism. J. Leukoc. Biol. 2004, 76, 804–811. [Google Scholar] [CrossRef]
- Lian, X.; Gao, C.; Sun, X.; Jiang, C.; Einkauf, K.B.; Seiger, K.W.; Chevalier, J.M.; Yuki, Y.; Martin, M.; Hoh, R.; et al. Signatures of immune selection in intact and defective proviruses distinguish HIV-1 elite controllers. Sci. Transl. Med. 2021, 13, eabl4097. [Google Scholar] [CrossRef]
- Casado, C.; Marrero-Hernandez, S.; Marquez-Arce, D.; Pernas, M.; Marfil, S.; Borras-Granana, F.; Olivares, I.; Cabrera-Rodriguez, R.; Valera, M.S.; de Armas-Rillo, L.; et al. Viral Characteristics Associated with the Clinical Nonprogressor Phenotype Are Inherited by Viruses from a Cluster of HIV-1 Elite Controllers. mBio 2018, 9, e02338-17. [Google Scholar] [CrossRef]
- Serena, M.; Parolini, F.; Biswas, P.; Sironi, F.; Blanco Miranda, A.; Zoratti, E.; Scupoli, M.T.; Ziglio, S.; Valenzuela-Fernandez, A.; Gibellini, D.; et al. HIV-1 Env associates with HLA-C free-chains at the cell membrane modulating viral infectivity. Sci. Rep. 2017, 7, 40037. [Google Scholar] [CrossRef]
- Garcia-Exposito, L.; Ziglio, S.; Barroso-Gonzalez, J.; de Armas-Rillo, L.; Valera, M.S.; Zipeto, D.; Machado, J.D.; Valenzuela-Fernandez, A. Gelsolin activity controls efficient early HIV-1 infection. Retrovirology 2013, 10, 39. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Exposito, L.; Barroso-Gonzalez, J.; Puigdomenech, I.; Machado, J.D.; Blanco, J.; Valenzuela-Fernandez, A. HIV-1 requires Arf6-mediated membrane dynamics to efficiently enter and infect T lymphocytes. Mol. Biol. Cell 2011, 22, 1148–1166. [Google Scholar] [CrossRef] [PubMed]
- Barrero-Villar, M.; Cabrero, J.R.; Gordon-Alonso, M.; Barroso-Gonzalez, J.; Alvarez-Losada, S.; Munoz-Fernandez, M.A.; Sanchez-Madrid, F.; Valenzuela-Fernandez, A. Moesin is required for HIV-1-induced CD4-CXCR4 interaction, F-actin redistribution, membrane fusion and viral infection in lymphocytes. J. Cell Sci. 2009, 122, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela-Fernandez, A.; Cabrero, J.R.; Serrador, J.M.; Sanchez-Madrid, F. HDAC6: A key regulator of cytoskeleton, cell migration and cell-cell interactions. Trends Cell Biol. 2008, 18, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Rodríguez, R.; Pérez-Yanes, S.; Montelongo, R.; Lorenzo-Salazar, J.M.; Estévez-Herrera, J.; García-Luis, J.; Íñigo-Campos, A.; Rubio-Rodríguez, L.A.; Muñoz-Barrera, A.; Trujillo-González, R.; et al. Transactive Response DNA-Binding Protein (TARDBP/TDP-43) Regulates Cell Permissivity to HIV-1 Infection by Acting on HDAC6. Int. J. Mol. Sci. 2022, 23, 6180. [Google Scholar] [CrossRef]
- McDougal, J.S.; Kennedy, M.S.; Sligh, J.M.; Cort, S.P.; Mawle, A.; Nicholson, J.K. Binding of HTLV-III/LAV to T4+ T cells by a complex of the 110K viral protein and the T4 molecule. Science 1986, 231, 382–385. [Google Scholar] [CrossRef]
- Dalgleish, A.G.; Beverley, P.C.; Clapham, P.R.; Crawford, D.H.; Greaves, M.F.; Weiss, R.A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature 1984, 312, 763–767. [Google Scholar] [CrossRef]
- Klatzmann, D.; Barre-Sinoussi, F.; Nugeyre, M.T.; Danquet, C.; Vilmer, E.; Griscelli, C.; Brun-Veziret, F.; Rouzioux, C.; Gluckman, J.C.; Chermann, J.C.; et al. Selective tropism of lymphadenopathy associated virus (LAV) for helper-inducer T lymphocytes. Science 1984, 225, 59–63. [Google Scholar] [CrossRef]
- Klatzmann, D.; Champagne, E.; Chamaret, S.; Gruest, J.; Guetard, D.; Hercend, T.; Gluckman, J.C.; Montagnier, L. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature 1984, 312, 767–768. [Google Scholar] [CrossRef]
- Maddon, P.J.; Dalgleish, A.G.; McDougal, J.S.; Clapham, P.R.; Weiss, R.A.; Axel, R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell 1986, 47, 333–348. [Google Scholar] [CrossRef]
- Kwong, P.D.; Wyatt, R.; Robinson, J.; Sweet, R.W.; Sodroski, J.; Hendrickson, W.A. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature 1998, 393, 648–659. [Google Scholar] [CrossRef] [Green Version]
- Bleul, C.C.; Farzan, M.; Choe, H.; Parolin, C.; Clark-Lewis, I.; Sodroski, J.; Springer, T.A. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature 1996, 382, 829–833. [Google Scholar] [CrossRef]
- Oberlin, E.; Amara, A.; Bachelerie, F.; Bessia, C.; Virelizier, J.L.; Arenzana-Seisdedos, F.; Schwartz, O.; Heard, J.M.; Clark-Lewis, I.; Legler, D.F.; et al. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature 1996, 382, 833–835. [Google Scholar] [CrossRef]
- Choe, H.; Farzan, M.; Sun, Y.; Sullivan, N.; Rollins, B.; Ponath, P.D.; Wu, L.; Mackay, C.R.; LaRosa, G.; Newman, W.; et al. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell 1996, 85, 1135–1148. [Google Scholar] [CrossRef]
- Chanel, C.; Staropoli, I.; Baleux, F.; Amara, A.; Valenzuela-Fernandez, A.; Virelizier, J.L.; Arenzana-Seisdedos, F.; Altmeyer, R. Low levels of co-receptor CCR5 are sufficient to permit HIV envelope-mediated fusion with resting CD4 T cells. AIDS 2002, 16, 2337–2340. [Google Scholar] [CrossRef]
- Valenzuela-Fernández, A.; Palanche, T.; Amara, A.; Magerus, A.; Altmeyer, R.; Delaunay, T.; Virelizier, J.L.; Baleux, F.; Galzi, J.L.; Arenzana-Seisdedos, F. Optimal inhibition of X4 HIV isolates by the CXC chemokine stromal cell-derived factor 1 alpha requires interaction with cell surface heparan sulfate proteoglycans. J. Biol. Chem. 2001, 276, 26550–26558. [Google Scholar] [CrossRef]
- Puigdomènech, I.; Massanella, M.; Cabrera, C.; Clotet, B.; Blanco, J. On the steps of cell-to-cell HIV transmission between CD4 T cells. Retrovirology 2009, 6, 89. [Google Scholar] [CrossRef]
- Santos, G.; Valenzuela-Fernandez, A.; Torres, N.V. Quantitative analysis of the processes and signaling events involved in early HIV-1 infection of T cells. PLoS ONE 2014, 9, e103845. [Google Scholar] [CrossRef]
- Liu, Y.; Belkina, N.V.; Shaw, S. HIV infection of T cells: Actin-in and actin-out. Sci. Signal. 2009, 2, pe23. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela-Fernandez, A.; Alvarez, S.; Gordon-Alonso, M.; Barrero, M.; Ursa, A.; Cabrero, J.R.; Fernandez, G.; Naranjo-Suarez, S.; Yanez-Mo, M.; Serrador, J.M.; et al. Histone deacetylase 6 regulates human immunodeficiency virus type 1 infection. Mol. Biol. Cell 2005, 16, 5445–5454. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Woodward, A.; Zhu, H.; Andrus, T.; McNevin, J.; Lee, J.; Mullins, J.I.; Corey, L.; McElrath, M.J.; Zhu, T. Preinfection human immunodeficiency virus (HIV)-specific cytotoxic T lymphocytes failed to prevent HIV type 1 infection from strains genetically unrelated to viruses in long-term exposed partners. J. Virol. 2009, 83, 10821–10829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jimenez-Baranda, S.; Gomez-Mouton, C.; Rojas, A.; Martinez-Prats, L.; Mira, E.; Ana Lacalle, R.; Valencia, A.; Dimitrov, D.S.; Viola, A.; Delgado, R.; et al. Filamin-A regulates actin-dependent clustering of HIV receptors. Nat. Cell Biol. 2007, 9, 838–846. [Google Scholar] [CrossRef]
- Yoder, A.; Yu, D.; Dong, L.; Iyer, S.R.; Xu, X.; Kelly, J.; Liu, J.; Wang, W.; Vorster, P.J.; Agulto, L.; et al. HIV envelope-CXCR4 signaling activates cofilin to overcome cortical actin restriction in resting CD4 T cells. Cell 2008, 134, 782–792. [Google Scholar] [CrossRef]
- Almeida, J.R.; Price, D.A.; Papagno, L.; Arkoub, Z.A.; Sauce, D.; Bornstein, E.; Asher, T.E.; Samri, A.; Schnuriger, A.; Theodorou, I.; et al. Superior control of HIV-1 replication by CD8+ T cells is reflected by their avidity, polyfunctionality, and clonal turnover. J. Exp. Med. 2007, 204, 2473–2485. [Google Scholar] [CrossRef]
- Overbaugh, J.; Bangham, C.R. Selection forces and constraints on retroviral sequence variation. Science 2001, 292, 1106–1109. [Google Scholar] [CrossRef] [PubMed]
- Shankarappa, R.; Margolick, J.B.; Gange, S.J.; Rodrigo, A.G.; Upchurch, D.; Farzadegan, H.; Gupta, P.; Rinaldo, C.R.; Learn, G.H.; He, X.; et al. Consistent viral evolutionary changes associated with the progression of human immunodeficiency virus type 1 infection. J. Virol. 1999, 73, 10489–10502. [Google Scholar] [CrossRef]
- Piantadosi, A.; Chohan, B.; Panteleeff, D.; Baeten, J.M.; Mandaliya, K.; Ndinya-Achola, J.O.; Overbaugh, J. HIV-1 evolution in gag and env is highly correlated but exhibits different relationships with viral load and the immune response. AIDS 2009, 23, 579–587. [Google Scholar] [CrossRef]
- Gao, F.; Weaver, E.A.; Lu, Z.; Li, Y.; Liao, H.X.; Ma, B.; Alam, S.M.; Scearce, R.M.; Sutherland, L.L.; Yu, J.S.; et al. Antigenicity and immunogenicity of a synthetic human immunodeficiency virus type 1 group m consensus envelope glycoprotein. J. Virol. 2005, 79, 1154–1163. [Google Scholar] [CrossRef]
- Rathore, U.; Purwar, M.; Vignesh, V.S.; Das, R.; Kumar, A.A.; Bhattacharyya, S.; Arendt, H.; DeStefano, J.; Wilson, A.; Parks, C.; et al. Bacterially expressed HIV-1 gp120 outer-domain fragment immunogens with improved stability and affinity for CD4-binding site neutralizing antibodies. J. Biol. Chem. 2018, 293, 15002–15020. [Google Scholar] [CrossRef] [PubMed]
- Korber, B.; Gaschen, B.; Yusim, K.; Thakallapally, R.; Kesmir, C.; Detours, V. Evolutionary and immunological implications of contemporary HIV-1 variation. Br. Med. Bull. 2001, 58, 19–42. [Google Scholar] [CrossRef]
- Kulkarni, P.S.; Butera, S.T.; Duerr, A.C. Resistance to HIV-1 infection: Lessons learned from studies of highly exposed persistently seronegative (HEPS) individuals. AIDS Rev. 2003, 5, 87–103. [Google Scholar] [PubMed]
- Ng’uni, T.; Chasara, C.; Ndhlovu, Z.M. Major Scientific Hurdles in HIV Vaccine Development: Historical Perspective and Future Directions. Front. Immunol. 2020, 11, 590780. [Google Scholar] [CrossRef]
- Griffith, S.A.; McCoy, L.E. To bnAb or Not to bnAb: Defining Broadly Neutralising Antibodies Against HIV-1. Front. Immunol. 2021, 12, 708227. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Y.; Tian, M.; Gao, Y. Development of an anti-HIV vaccine eliciting broadly neutralizing antibodies. AIDS Res. Ther. 2017, 14, 50. [Google Scholar] [CrossRef]
- Rudometov, A.P.; Chikaev, A.N.; Rudometova, N.B.; Antonets, D.V.; Lomzov, A.A.; Kaplina, O.N.; Ilyichev, A.A.; Karpenko, L.I. Artificial Anti-HIV-1 Immunogen Comprising Epitopes of Broadly Neutralizing Antibodies 2F5, 10E8, and a Peptide Mimic of VRC01 Discontinuous Epitope. Vaccines 2019, 7, 83. [Google Scholar] [CrossRef] [PubMed]
- Marusic, C.; Vitale, A.; Pedrazzini, E.; Donini, M.; Frigerio, L.; Bock, R.; Dix, P.J.; McCabe, M.S.; Bellucci, M.; Benvenuto, E. Plant-based strategies aimed at expressing HIV antigens and neutralizing antibodies at high levels. Nef as a case study. Transgenic Res. 2009, 18, 499–512. [Google Scholar] [CrossRef]
- Henrich, T.J. Second example reported of a stem-cell transplant in the clinic leading to HIV remission. Nature 2019, 568, 175–176. [Google Scholar] [CrossRef]
- Peterson, C.W.; Kiem, H.P. Lessons from London and Berlin: Designing A Scalable Gene Therapy Approach for HIV Cure. Cell Stem Cell 2019, 24, 685–687. [Google Scholar] [CrossRef]
- Duarte, R.F.; Salgado, M.; Sánchez-Ortega, I.; Arnan, M.; Canals, C.; Domingo-Domenech, E.; Fernández-de-Sevilla, A.; González-Barca, E.; Morón-López, S.; Nogues, N.; et al. CCR5 Δ32 homozygous cord blood allogeneic transplantation in a patient with HIV: A case report. Lancet HIV 2015, 2, e236–e242. [Google Scholar] [CrossRef]
- Derdeyn, C.A.; Decker, J.M.; Bibollet-Ruche, F.; Mokili, J.L.; Muldoon, M.; Denham, S.A.; Heil, M.L.; Kasolo, F.; Musonda, R.; Hahn, B.H.; et al. Envelope-constrained neutralization-sensitive HIV-1 after heterosexual transmission. Science 2004, 303, 2019–2022. [Google Scholar] [CrossRef] [PubMed]
- Chohan, B.; Lang, D.; Sagar, M.; Korber, B.; Lavreys, L.; Richardson, B.; Overbaugh, J. Selection for human immunodeficiency virus type 1 envelope glycosylation variants with shorter V1-V2 loop sequences occurs during transmission of certain genetic subtypes and may impact viral RNA levels. J. Virol. 2005, 79, 6528–6531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gnanakaran, S.; Bhattacharya, T.; Daniels, M.; Keele, B.F.; Hraber, P.T.; Lapedes, A.S.; Shen, T.; Gaschen, B.; Krishnamoorthy, M.; Li, H.; et al. Recurrent signature patterns in HIV-1 B clade envelope glycoproteins associated with either early or chronic infections. PLoS Pathog. 2011, 7, e1002209. [Google Scholar] [CrossRef]
- Bello, G.; Casado, C.; Garcia, S.; Rodriguez, C.; Del Romero, J.; Lopez-Galindez, C. Co-existence of recent and ancestral nucleotide sequences in viral quasispecies of human immunodeficiency virus type 1 patients. J. Gen. Virol. 2004, 85, 399–407. [Google Scholar] [CrossRef]
- Sagar, M.; Wu, X.; Lee, S.; Overbaugh, J. Human immunodeficiency virus type 1 V1-V2 envelope loop sequences expand and add glycosylation sites over the course of infection, and these modifications affect antibody neutralization sensitivity. J. Virol. 2006, 80, 9586–9598. [Google Scholar] [CrossRef]
- Curlin, M.E.; Zioni, R.; Hawes, S.E.; Liu, Y.; Deng, W.; Gottlieb, G.S.; Zhu, T.; Mullins, J.I. HIV-1 envelope subregion length variation during disease progression. PLoS Pathog. 2010, 6, e1001228. [Google Scholar] [CrossRef]
- Yuan, T.; Li, J.; Zhang, M.Y. HIV-1 envelope glycoprotein variable loops are indispensable for envelope structural integrity and virus entry. PLoS ONE 2013, 8, e69789. [Google Scholar] [CrossRef]
- Rong, R.; Bibollet-Ruche, F.; Mulenga, J.; Allen, S.; Blackwell, J.L.; Derdeyn, C.A. Role of V1V2 and other human immunodeficiency virus type 1 envelope domains in resistance to autologous neutralization during clade C infection. J. Virol. 2007, 81, 1350–1359. [Google Scholar] [CrossRef]
- Castro, E.; Belair, M.; Rizzardi, G.P.; Bart, P.A.; Pantaleo, G.; Graziosi, C. Independent evolution of hypervariable regions of HIV-1 gp120: V4 as a swarm of N-Linked glycosylation variants. AIDS Res. Hum. Retroviruses 2008, 24, 106–113. [Google Scholar] [CrossRef]
- Moore, P.L.; Gray, E.S.; Choge, I.A.; Ranchobe, N.; Mlisana, K.; Abdool Karim, S.S.; Williamson, C.; Morris, L.; Team, C.S. The c3-v4 region is a major target of autologous neutralizing antibodies in human immunodeficiency virus type 1 subtype C infection. J. Virol. 2008, 82, 1860–1869. [Google Scholar] [CrossRef]
- Van Gils, M.J.; Bunnik, E.M.; Boeser-Nunnink, B.D.; Burger, J.A.; Terlouw-Klein, M.; Verwer, N.; Schuitemaker, H. Longer V1V2 region with increased number of potential N-linked glycosylation sites in the HIV-1 envelope glycoprotein protects against HIV-specific neutralizing antibodies. J. Virol. 2011, 85, 6986–6995. [Google Scholar] [CrossRef]
- Davenport, Y.W.; West, A.P., Jr.; Bjorkman, P.J. Structure of an HIV-2 gp120 in Complex with CD4. J. Virol. 2016, 90, 2112–2118. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Izadmehr, S.; Kamau, E.; Kong, X.P.; Chen, B.K. Sequential trafficking of Env and Gag to HIV-1 T cell virological synapses revealed by live imaging. Retrovirology 2019, 16, 2. [Google Scholar] [CrossRef]
- Cocchi, F.; DeVico, A.L.; Garzino-Demo, A.; Cara, A.; Gallo, R.C.; Lusso, P. The V3 domain of the HIV-1 gp120 envelope glycoprotein is critical for chemokine-mediated blockade of infection. Nat. Med. 1996, 2, 1244–1247. [Google Scholar] [CrossRef]
- Wu, L.; Gerard, N.P.; Wyatt, R.; Choe, H.; Parolin, C.; Ruffing, N.; Borsetti, A.; Cardoso, A.A.; Desjardin, E.; Newman, W.; et al. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature 1996, 384, 179–183. [Google Scholar] [CrossRef]
- Bieniasz, P.D.; Fridell, R.A.; Aramori, I.; Ferguson, S.S.; Caron, M.G.; Cullen, B.R. HIV-1-induced cell fusion is mediated by multiple regions within both the viral envelope and the CCR-5 co-receptor. EMBO J 1997, 16, 2599–2609. [Google Scholar] [CrossRef]
- Speck, R.F.; Wehrly, K.; Platt, E.J.; Atchison, R.E.; Charo, I.F.; Kabat, D.; Chesebro, B.; Goldsmith, M.A. Selective employment of chemokine receptors as human immunodeficiency virus type 1 coreceptors determined by individual amino acids within the envelope V3 loop. J. Virol. 1997, 71, 7136–7139. [Google Scholar] [CrossRef]
- Isaka, Y.; Sato, A.; Miki, S.; Kawauchi, S.; Sakaida, H.; Hori, T.; Uchiyama, T.; Adachi, A.; Hayami, M.; Fujiwara, T.; et al. Small amino acid changes in the V3 loop of human immunodeficiency virus type 2 determines the coreceptor usage for CXCR4 and CCR5. Virology 1999, 264, 237–243. [Google Scholar] [CrossRef]
- Valenzuela, A.; Blanco, J.; Krust, B.; Franco, R.; Hovanessian, A.G. Neutralizing antibodies against the V3 loop of human immunodeficiency virus type 1 gp120 block the CD4-dependent and -independent binding of virus to cells. J. Virol. 1997, 71, 8289–8298. [Google Scholar] [CrossRef]
- Trkola, A.; Dragic, T.; Arthos, J.; Binley, J.M.; Olson, W.C.; Allaway, G.P.; Cheng-Mayer, C.; Robinson, J.; Maddon, P.J.; Moore, J.P. CD4-dependent, antibody-sensitive interactions between HIV-1 and its co-receptor CCR-5. Nature 1996, 384, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Pollakis, G.; Baan, E.; van Werkhoven, M.B.; Berkhout, B.; Bakker, M.; Jurriaans, S.; Paxton, W.A. Association between gp120 envelope V1V2 and V4V5 variable loop profiles in a defined HIV-1 transmission cluster. AIDS 2015, 29, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Curlin, M.E.; Diem, K.; Zhao, H.; Ghosh, A.K.; Zhu, H.; Woodward, A.S.; Maenza, J.; Stevens, C.E.; Stekler, J.; et al. Env length and N-linked glycosylation following transmission of human immunodeficiency virus Type 1 subtype B viruses. Virology 2008, 374, 229–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keele, B.F.; Giorgi, E.E.; Salazar-Gonzalez, J.F.; Decker, J.M.; Pham, K.T.; Salazar, M.G.; Sun, C.; Grayson, T.; Wang, S.; Li, H.; et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc. Natl. Acad. Sci. USA 2008, 105, 7552–7557. [Google Scholar] [CrossRef] [PubMed]
- Quan, Y.; Liang, C.; Brenner, B.G.; Wainberg, M.A. Multidrug-resistant variants of HIV type 1 (HIV-1) can exist in cells as defective quasispecies and be rescued by superinfection with other defective HIV-1 variants. J. Infect. Dis. 2009, 200, 1479–1483. [Google Scholar] [CrossRef]
- Fischer, W.; Ganusov, V.V.; Giorgi, E.E.; Hraber, P.T.; Keele, B.F.; Leitner, T.; Han, C.S.; Gleasner, C.D.; Green, L.; Lo, C.C.; et al. Transmission of single HIV-1 genomes and dynamics of early immune escape revealed by ultra-deep sequencing. PLoS ONE 2010, 5, e12303. [Google Scholar] [CrossRef]
- Roche, M.; Jakobsen, M.R.; Sterjovski, J.; Ellett, A.; Posta, F.; Lee, B.; Jubb, B.; Westby, M.; Lewin, S.R.; Ramsland, P.A.; et al. HIV-1 escape from the CCR5 antagonist maraviroc associated with an altered and less-efficient mechanism of gp120-CCR5 engagement that attenuates macrophage tropism. J. Virol. 2011, 85, 4330–4342. [Google Scholar] [CrossRef] [PubMed]
- Fraser, C.; Lythgoe, K.; Leventhal, G.E.; Shirreff, G.; Hollingsworth, T.D.; Alizon, S.; Bonhoeffer, S. Virulence and pathogenesis of HIV-1 infection: An evolutionary perspective. Science 2014, 343, 1243727. [Google Scholar] [CrossRef]
- Dang, L.V.P.; Pham, H.V.; Dinh, T.T.; Nguyen, T.H.; Vu, Q.T.H.; Vu, N.T.P.; Le, P.T.B.; Nguyen, L.V.; Le, H.T.; Vu, P.T.; et al. Characterization of envelope sequence of HIV virus in children infected with HIV in Vietnam. SAGE Open Med. 2020, 8, 2050312120937198. [Google Scholar] [CrossRef]
- Koot, M.; Keet, I.P.; Vos, A.H.; de Goede, R.E.; Roos, M.T.; Coutinho, R.A.; Miedema, F.; Schellekens, P.T.; Tersmette, M. Prognostic value of HIV-1 syncytium-inducing phenotype for rate of CD4+ cell depletion and progression to AIDS. Ann. Intern. Med. 1993, 118, 681–688. [Google Scholar] [CrossRef]
- Kitrinos, K.M.; Nelson, J.A.; Resch, W.; Swanstrom, R. Effect of a protease inhibitor-induced genetic bottleneck on human immunodeficiency virus type 1 env gene populations. J. Virol. 2005, 79, 10627–10637. [Google Scholar] [CrossRef]
- Kitchen, C.M.; Philpott, S.; Burger, H.; Weiser, B.; Anastos, K.; Suchard, M.A. Evolution of human immunodeficiency virus type 1 coreceptor usage during antiretroviral Therapy: A Bayesian approach. J. Virol. 2004, 78, 11296–11302. [Google Scholar] [CrossRef]
- Hunt, P.W.; Harrigan, P.R.; Huang, W.; Bates, M.; Williamson, D.W.; McCune, J.M.; Price, R.W.; Spudich, S.S.; Lampiris, H.; Hoh, R.; et al. Prevalence of CXCR4 tropism among antiretroviral-treated HIV-1-infected patients with detectable viremia. J. Infect. Dis. 2006, 194, 926–930. [Google Scholar] [CrossRef] [Green Version]
- Duenas-Decamp, M.J.; Peters, P.; Burton, D.; Clapham, P.R. Natural resistance of human immunodeficiency virus type 1 to the CD4bs antibody b12 conferred by a glycan and an arginine residue close to the CD4 binding loop. J. Virol. 2008, 82, 5807–5814. [Google Scholar] [CrossRef]
- Salazar-Gonzalez, J.F.; Bailes, E.; Pham, K.T.; Salazar, M.G.; Guffey, M.B.; Keele, B.F.; Derdeyn, C.A.; Farmer, P.; Hunter, E.; Allen, S.; et al. Deciphering human immunodeficiency virus type 1 transmission and early envelope diversification by single-genome amplification and sequencing. J. Virol. 2008, 82, 3952–3970. [Google Scholar] [CrossRef]
- Kassaye, S.; Johnston, E.; McColgan, B.; Kantor, R.; Zijenah, L.; Katzenstein, D. Envelope coreceptor tropism, drug resistance, and viral evolution among subtype C HIV-1-infected individuals receiving nonsuppressive antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 2009, 50, 9–18. [Google Scholar] [CrossRef]
- Moore, P.L.; Gray, E.S.; Morris, L. Specificity of the autologous neutralizing antibody response. Curr. Opin. HIV AIDS 2009, 4, 358–363. [Google Scholar] [CrossRef]
- Shi, B.; Kitchen, C.; Weiser, B.; Mayers, D.; Foley, B.; Kemal, K.; Anastos, K.; Suchard, M.; Parker, M.; Brunner, C.; et al. Evolution and recombination of genes encoding HIV-1 drug resistance and tropism during antiretroviral therapy. Virology 2010, 404, 5–20. [Google Scholar] [CrossRef]
- Sterjovski, J.; Churchill, M.J.; Ellett, A.; Gray, L.R.; Roche, M.J.; Dunfee, R.L.; Purcell, D.F.; Saksena, N.; Wang, B.; Sonza, S.; et al. Asn 362 in gp120 contributes to enhanced fusogenicity by CCR5-restricted HIV-1 envelope glycoprotein variants from patients with AIDS. Retrovirology 2007, 4, 89. [Google Scholar] [CrossRef]
- Coffin, J.; Swanstrom, R. HIV pathogenesis: Dynamics and genetics of viral populations and infected cells. Cold Spring Harb. Perspect. Med. 2013, 3, a012526. [Google Scholar] [CrossRef]
- Mishra, N.; Makhdoomi, M.A.; Sharma, S.; Kumar, S.; Dobhal, A.; Kumar, D.; Chawla, H.; Singh, R.; Kanga, U.; Das, B.K.; et al. Viral Characteristics Associated with Maintenance of Elite Neutralizing Activity in Chronically HIV-1 Clade C-Infected Monozygotic Pediatric Twins. J. Virol. 2019, 93, e00654-19. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.; Gibson, R.M.; Sacka, L.; Strunin, D.; Hodek, J.; Weberova, J.; Pavova, M.; Alouani, D.J.; Asaad, R.; Rodriguez, B.; et al. Impaired human immunodeficiency virus type 1 replicative fitness in atypical viremic non-progressor individuals. AIDS Res. Ther. 2017, 14, 15. [Google Scholar] [CrossRef] [PubMed]
- Armand-Ugon, M.; Quinones-Mateu, M.E.; Gutierez, A.; Barretina, J.; Blanco, J.; Schols, D.; De Clercq, E.; Clotet, B.; Este, J.A. Reduced fitness of HIV-1 resistant to CXCR4 antagonists. Antivir. Ther. 2003, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pietzsch, J.; Scheid, J.F.; Mouquet, H.; Klein, F.; Seaman, M.S.; Jankovic, M.; Corti, D.; Lanzavecchia, A.; Nussenzweig, M.C. Human anti-HIV-neutralizing antibodies frequently target a conserved epitope essential for viral fitness. J. Exp. Med. 2010, 207, 1995–2002. [Google Scholar] [CrossRef] [PubMed]
- Valera, M.S.; de Armas-Rillo, L.; Barroso-Gonzalez, J.; Ziglio, S.; Batisse, J.; Dubois, N.; Marrero-Hernandez, S.; Borel, S.; Garcia-Exposito, L.; Biard-Piechaczyk, M.; et al. The HDAC6/APOBEC3G complex regulates HIV-1 infectiveness by inducing Vif autophagic degradation. Retrovirology 2015, 12, 53. [Google Scholar] [CrossRef] [PubMed]
- Rosas-Umbert, M.; Llano, A.; Bellido, R.; Olvera, A.; Ruiz-Riol, M.; Rocafort, M.; Fernandez, M.A.; Cobarsi, P.; Crespo, M.; Dorrell, L.; et al. Mechanisms of Abrupt Loss of Virus Control in a Cohort of Previous HIV Controllers. J. Virol. 2019, 93, e01436-18. [Google Scholar] [CrossRef]
- Borrell, M.; Fernandez, I.; Etcheverrry, F.; Ugarte, A.; Plana, M.; Leal, L.; Garcia, F. High rates of long-term progression in HIV-1-positive elite controllers. J. Int. AIDS Soc. 2021, 24, e25675. [Google Scholar] [CrossRef]
- Balasubramaniam, M.; Pandhare, J.; Dash, C. Immune Control of HIV. J. Life Sci. 2019, 1, 4–37. [Google Scholar] [CrossRef]
- Kitrinos, K.M.; Hoffman, N.G.; Nelson, J.A.; Swanstrom, R. Turnover of env variable region 1 and 2 genotypes in subjects with late-stage human immunodeficiency virus type 1 infection. J. Virol. 2003, 77, 6811–6822. [Google Scholar] [CrossRef]
- Silver, Z.A.; Dickinson, G.M.; Seaman, M.S.; Desrosiers, R.C. A Highly Unusual V1 Region of Env in an Elite Controller of HIV Infection. J. Virol. 2019, 93, e00094-19. [Google Scholar] [CrossRef]
- Muenchhoff, M.; Adland, E.; Karimanzira, O.; Crowther, C.; Pace, M.; Csala, A.; Leitman, E.; Moonsamy, A.; McGregor, C.; Hurst, J.; et al. Nonprogressing HIV-infected children share fundamental immunological features of nonpathogenic SIV infection. Sci. Transl. Med. 2016, 8, 358ra125. [Google Scholar] [CrossRef]
- Rowland-Jones, S.L.; Whittle, H.C. Out of Africa: What can we learn from HIV-2 about protective immunity to HIV-1? Nat. Immunol. 2007, 8, 329–331. [Google Scholar] [CrossRef]
- Turk, G.; Seiger, K.; Lian, X.; Sun, W.; Parsons, E.M.; Gao, C.; Rassadkina, Y.; Polo, M.L.; Czernikier, A.; Ghiglione, Y.; et al. A Possible Sterilizing Cure of HIV-1 Infection Without Stem Cell Transplantation. Ann. Intern. Med. 2022, 175, 95–100. [Google Scholar] [CrossRef]
- Kirchhoff, F.; Greenough, T.C.; Brettler, D.B.; Sullivan, J.L.; Desrosiers, R.C. Brief report: Absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N. Engl. J. Med. 1995, 332, 228–232. [Google Scholar] [CrossRef]
- Fenwick, C.; Joo, V.; Jacquier, P.; Noto, A.; Banga, R.; Perreau, M.; Pantaleo, G. T-cell exhaustion in HIV infection. Immunol. Rev. 2019, 292, 149–163. [Google Scholar] [CrossRef]
- Bansal, A.; Sterrett, S.; Erdmann, N.; Westfall, A.O.; Dionne-Odom, J.; Overton, E.T.; Goepfert, P.A. Normal T-cell activation in elite controllers with preserved CD4+ T-cell counts. AIDS 2015, 29, 2245–2254. [Google Scholar] [CrossRef]
- Bruyand, M.; Thiébaut, R.; Lawson-Ayayi, S.; Joly, P.; Sasco, A.J.; Mercié, P.; Pellegrin, J.L.; Neau, D.; Dabis, F.; Morlat, P.; et al. Role of uncontrolled HIV RNA level and immunodeficiency in the occurrence of malignancy in HIV-infected patients during the combination antiretroviral therapy era: Agence Nationale de Recherche sur le Sida (ANRS) CO3 Aquitaine Cohort. Clin. Infect. Dis. 2009, 49, 1109–1116. [Google Scholar] [CrossRef]
- Patel, P.; Borkowf, C.B.; Brooks, J.T.; Lasry, A.; Lansky, A.; Mermin, J. Estimating per-act HIV transmission risk: A systematic review. AIDS 2014, 28, 1509–1519. [Google Scholar] [CrossRef]
- Gonzalo-Gil, E.; Ikediobi, U.; Sutton, R.E. Mechanisms of Virologic Control and Clinical Characteristics of HIV+ Elite/Viremic Controllers. Yale J. Biol. Med. 2017, 90, 245–259. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valenzuela-Fernández, A.; Cabrera-Rodríguez, R.; Casado, C.; Pérez-Yanes, S.; Pernas, M.; García-Luis, J.; Marfil, S.; Olivares, I.; Estévez-Herrera, J.; Trujillo-González, R.; et al. Contribution of the HIV-1 Envelope Glycoprotein to AIDS Pathogenesis and Clinical Progression. Biomedicines 2022, 10, 2172. https://doi.org/10.3390/biomedicines10092172
Valenzuela-Fernández A, Cabrera-Rodríguez R, Casado C, Pérez-Yanes S, Pernas M, García-Luis J, Marfil S, Olivares I, Estévez-Herrera J, Trujillo-González R, et al. Contribution of the HIV-1 Envelope Glycoprotein to AIDS Pathogenesis and Clinical Progression. Biomedicines. 2022; 10(9):2172. https://doi.org/10.3390/biomedicines10092172
Chicago/Turabian StyleValenzuela-Fernández, Agustín, Romina Cabrera-Rodríguez, Concha Casado, Silvia Pérez-Yanes, María Pernas, Jonay García-Luis, Silvia Marfil, Isabel Olivares, Judith Estévez-Herrera, Rodrigo Trujillo-González, and et al. 2022. "Contribution of the HIV-1 Envelope Glycoprotein to AIDS Pathogenesis and Clinical Progression" Biomedicines 10, no. 9: 2172. https://doi.org/10.3390/biomedicines10092172
APA StyleValenzuela-Fernández, A., Cabrera-Rodríguez, R., Casado, C., Pérez-Yanes, S., Pernas, M., García-Luis, J., Marfil, S., Olivares, I., Estévez-Herrera, J., Trujillo-González, R., Blanco, J., & Lopez-Galindez, C. (2022). Contribution of the HIV-1 Envelope Glycoprotein to AIDS Pathogenesis and Clinical Progression. Biomedicines, 10(9), 2172. https://doi.org/10.3390/biomedicines10092172





