Methods for Assessing Oocyte Quality: A Review of Literature
Abstract
:1. Introduction
2. Materials and Methods
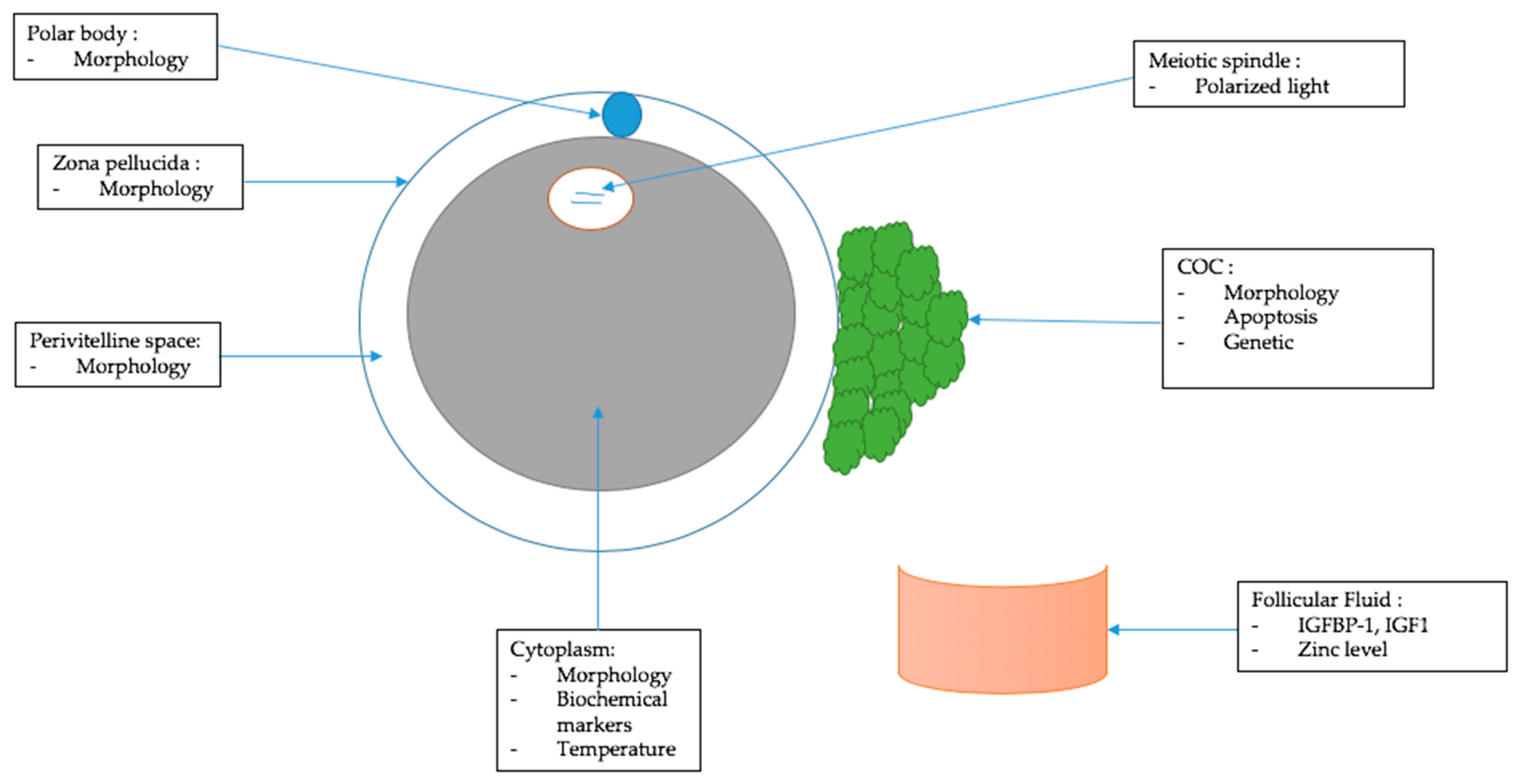
3. Morphological Criteria of Oocyte Quality
3.1. Cumulus–Oocyte Complex (COC)
3.2. Polar Body
3.3. Zona Pellucida
3.4. Perivitelline Space
3.5. Cytoplasm
3.6. Morphometric Parameter
4. Other Criteria to Assess Oocyte Quality
4.1. Follicular Fluid
4.2. Cumulus–Oocyte Complex (COC)
4.3. Cytoplasm
4.4. Meiotic Spindle
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ozturk, S. Selection of competent oocytes by morphological criteria for assisted reproductive technologies. Mol. Reprod. Dev. 2020, 87, 1021–1036. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Sun, Q.-Y. Evaluation of oocyte quality: Morphological, cellular and molecular predictors. Reprod. Fertil. Dev. 2007, 19, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lasiene, K.; Vitkus, A.; Valanciūte, A.; Lasys, V. Morphological criteria of oocyte quality. Medicina 2009, 45, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, Y. Updating the markers for oocyte quality evaluation: Intracellular temperature as a new index. Reprod. Med. Biol. 2018, 17, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Balaban, B.; Urman, B. Effect of oocyte morphology on embryo development and implantation. Reprod. Biomed. Online 2006, 12, 608–615. [Google Scholar] [CrossRef]
- Patrizio, P.; Fragouli, E.; Bianchi, V.; Borini, A.; Wells, D. Molecular methods for selection of the ideal oocyte. Reprod. Biomed. Online 2007, 15, 346–353. [Google Scholar] [CrossRef]
- Wang, X.; Xiao, Y.; Sun, Z.; Zhen, J.; Yu, Q. Smooth Endoplasmic Reticulum Clusters in Oocytes from Patients Who Received Intracytoplasmic Sperm Injections Negatively Affect Blastocyst Quality and Speed of Blastocyst Development. Front. Physiol. 2021, 12, 732547. [Google Scholar] [CrossRef] [PubMed]
- Bassil, R.; Casper, R.F.; Meriano, J.; Smith, R.; Haas, J.; Mehta, C.; Orvieto, R.; Zilberberg, E. Can Oocyte Diameter Predict Embryo Quality? Reprod. Sci. 2021, 28, 904–908. [Google Scholar] [CrossRef] [PubMed]
- Janati, S.; Behmanesh, M.A.; Najafzadehvarzi, H.; Akhundzade, Z.; Poormoosavi, S.M. Follicular Fluid Zinc Level and Oocyte Maturity and Embryo Quality in Women with Polycystic Ovary Syndrome. Int. J. Fertil. Steril. 2021, 15, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Ruvolo, G.; Fattouh, R.R.; Bosco, L.; Brucculeri, A.M.; Cittadini, E. New molecular markers for the evaluation of gamete quality. J. Assist. Reprod. Genet. 2013, 30, 207–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.S.; Joo, B.S.; Na, Y.J.; Yoon, M.S.; Choi, O.H.; Kim, W.W. Cumulus cells apoptosis as an indicator to predict the quality of oocytes and the outcome of IVF-ET. J. Assist. Reprod. Genet. 2001, 18, 490–498. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, L.J.; Pangas, S.A.; Carson, S.A.; Kovanci, E.; Cisneros, P.; Buster, J.E.; Amato, P.; Matzuk, M.M. Human cumulus granulosa cell gene expression: A predictor of fertilization and embryo selection in women undergoing IVF. Hum. Reprod. 2004, 19, 2869–2874. [Google Scholar] [CrossRef] [PubMed]
- Cheng, E.-H.; Chen, S.-U.; Lee, T.-H.; Pai, Y.-P.; Huang, L.-S.; Huang, C.-C.; Lee, M.-S. Evaluation of telomere length in cumulus cells as a potential biomarker of oocyte and embryo quality. Hum. Reprod. 2013, 28, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Fesahat, F.; Sheikhha, M.H.; Kalantar, S.M.; Tabibnejad, N.; Firouzabadi, R.D.; Saeedi, H.; Khalili, M.A. Developmental competence and apoptotic gene expression patterns of mature and immature human oocytes retrieved from controlled ovarian stimulation cycles. Reprod. Biol. 2018, 18, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Kirillova, A.; Smitz, J.E.J.; Sukhikh, G.T.; Mazunin, I. The Role of Mitochondria in Oocyte Maturation. Cells 2021, 10, 2484. [Google Scholar] [CrossRef] [PubMed]
- Dib, L.A.; Broi, M.G.D.; Navarro, P.A. Comparative Analysis of the Spindle of Fresh In Vivo-Matured Human Oocytes Through Polarized Light and Confocal Microscopy: A Pilot Study. Reprod. Sci. 2014, 21, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Bogliolo, L.; Leoni, G.G.; Ledda, S. Raman spectroscopy-based approach to study the female gamete. Theriogenology 2020, 150, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Bennabi, I.; Crozet, F.; Nikalayevich, E.; Chaigne, A.; Letort, G.; Manil-Ségalen, M.; Campillo, C.; Cadart, C.; Othmani, A.; Attia, R.; et al. Artificially decreasing cortical tension generates aneuploidy in mouse oocytes. Nat. Commun. 2020, 11, 1649. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Criteria | Outcome | Strength | Weakness | |
|---|---|---|---|---|
| COC | Compactness and clarity [2,3] | ↗ developmental potential | Non-invasive | Subjective Bovine oocyte |
| Presence of blood clots [1] | ↘ oocyte quality; fertility, cleavage, and blastulation rates | Non-invasive Easy to analyze under microscope | Not reproducible | |
| Expanded corona cells [1] | ↗ fertilization rates and pregnancy | Non-invasive | Subjective Not reproducible | |
| Number of layers of cells [3] | ↗ oocyte quality | Non-invasive | Not quantifiable and not reproducible | |
| Higher apoptosis rate [1,3,9] | ↘ oocyte quality; fertilization | Objective Reproducible | Difficult to use in daily routine | |
| Gene expression of gremlin [2] | ↗ oocyte quality, fertilization rates, and embryo of good quality | Objective Reproducible | Difficult to use in daily routine | |
| Higher length of telomeres [10] | ↗ oocyte quality, embryo of good quality | Objective Reproducible | Difficult to use in daily routine | |
| Expression of pro and anti-apoptotic genes | No significant difference | None | Useless | |
| Polar body | Integrity, correct shape, and size [2,3,4,5,6] | ↗ fertilization rates ↗ embryo quality ↗ oocyte quality | Non-invasive Easy to analyze under microscope | Subjective Controversial |
| Zona Pellucida | Thick zona pellucida [1,3] | Better oocyte development ↗ fertilization rates | Non-invasive | Subjective Controversial |
| Perivitelline space | Size and content [1,3] | No link found | None | Subjective Non-standardized |
| Cytoplasm | Vacuoles, granulations, and inclusions [1,2,5,6] | No link found | None | Contradictory studies Preliminary |
| Biochemical markers [2,4,8] | Oocyte quality | Could be objective | Not applicable in daily routine Invasive | |
| Intracellular temperature [4] | Higher temperature in fresh mature oocyte | Objective | Invasive Not applicable in daily routine | |
| Morphometric | MOD | ↗ quality of day 5 blastocyst | Non-invasive Easy to use with time laps | Does not help to choose the oocyte |
| Follicular fluid | Higher level IGFBP-1 and IGF1 [2] | Mature oocyte | Non-invasive | Needs to be standardized |
| Level of zinc < 35 µg/mL [7] | Lower number of mature oocyte | Non-invasive Reproducible | Needs to be standardized | |
| Meiotic spindle | Birefringent [2,3] | Better embryonic development | Non-invasive Reproducible | Costly Experience |
| No MS seen in polarized light [2] | ↘ rate of fertilization and blastocyst formation | Non-invasive Reproducible | Costly experience |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lemseffer, Y.; Terret, M.-E.; Campillo, C.; Labrune, E. Methods for Assessing Oocyte Quality: A Review of Literature. Biomedicines 2022, 10, 2184. https://doi.org/10.3390/biomedicines10092184
Lemseffer Y, Terret M-E, Campillo C, Labrune E. Methods for Assessing Oocyte Quality: A Review of Literature. Biomedicines. 2022; 10(9):2184. https://doi.org/10.3390/biomedicines10092184
Chicago/Turabian StyleLemseffer, Yassir, Marie-Emilie Terret, Clément Campillo, and Elsa Labrune. 2022. "Methods for Assessing Oocyte Quality: A Review of Literature" Biomedicines 10, no. 9: 2184. https://doi.org/10.3390/biomedicines10092184





