The Anti-Aggregative Peptide KLVFF Mimics Aβ1-40 in the Modulation of Nicotinic Receptors: Implications for Peptide-Based Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Brain Tissue Preparation
2.2. Preparation of Aβ Solutions
2.3. Release Experiments from Synaptosomes
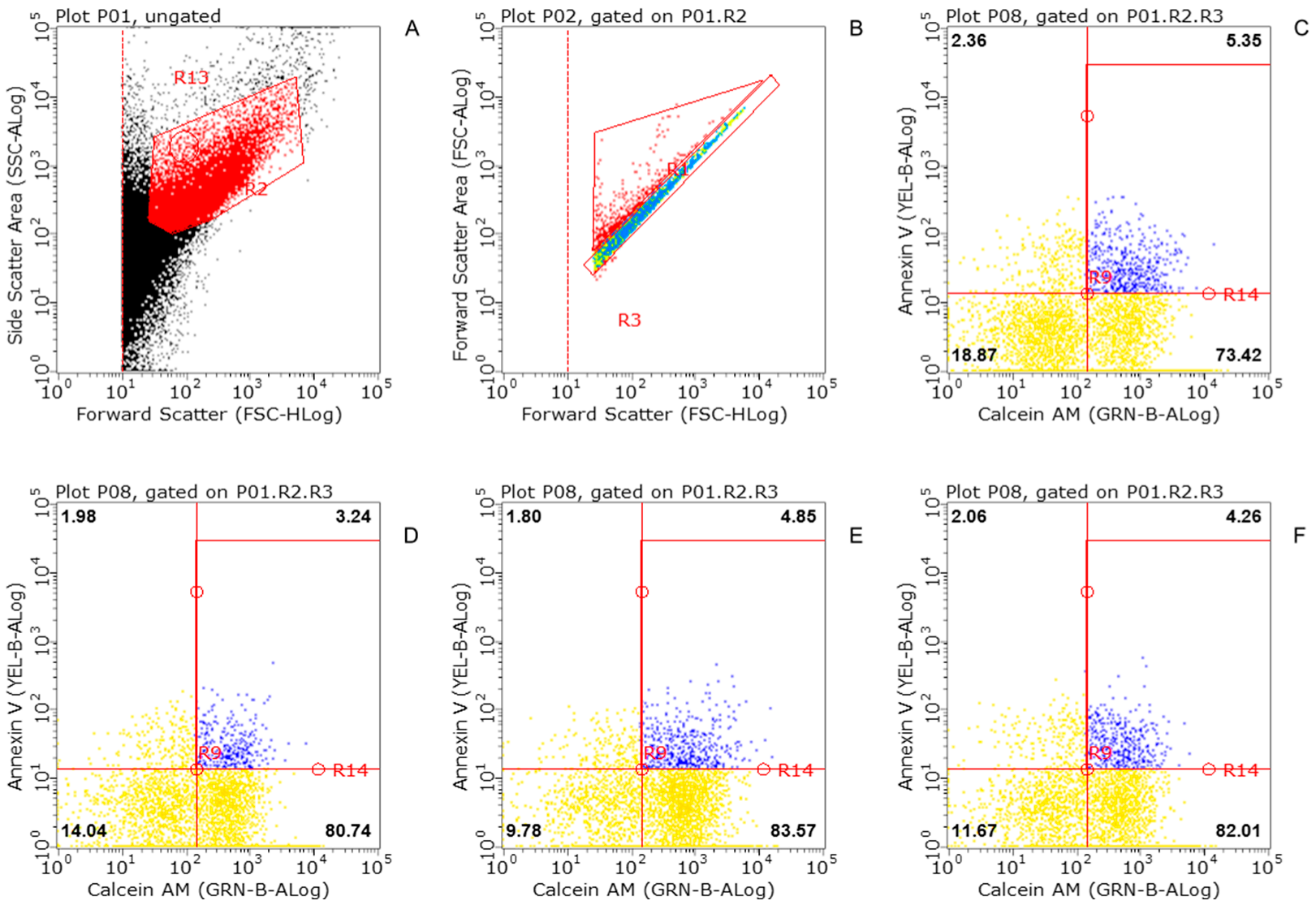
2.4. Flow Cytometric Analysis (FACS)
2.5. Statistical Analysis
2.6. Chemicals
3. Results
3.1. Flow Cytometry of Striatal Synaptosomes Exposed to KLVFF
3.2. KLVFF-Mimics Aβ1-40 in the Regulation of nAChRs Modulating Dopamine Release
3.3. Subtype-Selective Modulation of Nicotinic-Induced Dopamine Release
3.4. KLVFF Effects on nAChRs Modulating Noradrenaline Release from Hippocampal Nerve Terminals
3.5. Effects of Entrapped KLVFF on Nicotinic and Muscarinic Receptors
3.6. Desformylflutrabromine Counteracts KLVFF on Dopaminergic nAChRs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cummings, J.L.; Goldman, D.P.; Simmons-Stern, N.R.; Ponton, E. The Costs of Developing Treatments for Alzheimer’s Disease: A Retrospective Exploration. Alzheimer’s Dement. 2022, 18, 469–477. [Google Scholar] [CrossRef]
- Karran, E.; de Strooper, B. The Amyloid Hypothesis in Alzheimer Disease: New Insights from New Therapeutics. Nat. Rev. Drug Discov. 2022, 21, 306–318. [Google Scholar] [CrossRef]
- Alexander, G.C.; Emerson, S.; Kesselheim, A.S. Evaluation of Aducanumab for Alzheimer Disease: Scientific Evidence and Regulatory Review Involving Efficacy, Safety, and Futility. JAMA 2021, 325, 1717–1718. [Google Scholar] [CrossRef] [PubMed]
- Musiek, E.S.; Gomez-Isla, T.; Holtzman, D.M. Aducanumab for Alzheimer Disease: The Amyloid Hypothesis Moves from Bench to Bedside. J. Clin. Investig. 2021, 131, 20. [Google Scholar] [CrossRef] [PubMed]
- Caballero, E.; Hernando-Pérez, E.; Tapias, V.; Calvo-Rodríguez, M.; Villalobos, C.; Núñez, L. Amyloid Beta Oligomers-Induced Ca2+ Entry Pathways: Role of Neuronal Networks, NMDA Receptors and Amyloid Channel Formation. Biomedicines 2022, 10, 1153. [Google Scholar] [CrossRef]
- Fagiani, F.; Lanni, C.; Racchi, M.; Govoni, S. (Dys)Regulation of Synaptic Activity and Neurotransmitter Release by β-Amyloid: A Look Beyond Alzheimer’s Disease Pathogenesis. Front. Mol. Neurosci. 2021, 14, 12. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Shin, H.; Hong, S.; Kim, Y. Physiological Roles of Monomeric Amyloid-β and Implications for Alzheimer’s Disease Therapeutics. Exp. Neurobiol. 2022, 31, 65–88. [Google Scholar] [CrossRef]
- Evin, G.; Barakat, A. Critical Analysis of the Use of β-Site Amyloid Precursor Protein-Cleaving Enzyme 1 Inhibitors in the Treatment of Alzheimer’s Disease. Degener. Neurol. Neuromuscul. Dis. 2014, 4, 1. [Google Scholar] [CrossRef]
- Stefani, M.; Rigacci, S. Protein Folding and Aggregation into Amyloid: The Interference by Natural Phenolic Compounds. Int. J. Mol. Sci. 2013, 14, 12411. [Google Scholar] [CrossRef]
- Soto, C.; Pritzkow, S. Protein Misfolding, Aggregation, and Conformational Strains in Neurodegenerative Diseases. Nat. Neurosci. 2018, 21, 1332. [Google Scholar] [CrossRef]
- Mitra, A.; Sarkar, N. Sequence and Structure-Based Peptides as Potent Amyloid Inhibitors: A Review. Arch. Biochem. Biophys. 2020, 695, 108614. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Fu, Z.; Meng, L.; He, M.; Zhang, Z. The Early Events That Initiate β-Amyloid Aggregation in Alzheimer’s Disease. Front. Aging Neurosci. 2018, 10, 359. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.N.; Liu, C.; Zhao, M.; Eisenberg, D.; Nowick, J.S. Amyloid β-Sheet Mimics That Antagonize Protein Aggregation and Reduce Amyloid Toxicity. Nat. Chem. 2012, 4, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Finder, V.H.; Glockshuber, R. Amyloid-β Aggregation. Neurodegener. Dis. 2007, 4, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Jokar, S.; Khazaei, S.; Behnammanesh, H.; Shamloo, A.; Erfani, M.; Beiki, D.; Bavi, O. Recent Advances in the Design and Applications of Amyloid-β Peptide Aggregation Inhibitors for Alzheimer’s Disease Therapy. Biophys. Rev. 2019, 11, 901–925. [Google Scholar] [CrossRef]
- Phongpradist, R.; Thongchai, W.; Thongkorn, K.; Lekawanvijit, S.; Chittasupho, C. Surface Modification of Curcumin Microemulsions by Coupling of KLVFF Peptide: A Prototype for Targeted Bifunctional Microemulsions. Polymers 2022, 14, 443. [Google Scholar] [CrossRef]
- Chafekar, S.M.; Malda, H.; Merkx, M.; Meijer, E.W.; Viertl, D.; Lashuel, H.A.; Baas, F.; Scheper, W. Branched KLVFF Tetramers Strongly Potentiate Inhibition of β-Amyloid Aggregation. ChemBioChem 2007, 8, 1857–1864. [Google Scholar] [CrossRef]
- Lowe, T.L.; Strzelec, A.; Kiessling, L.L.; Murphy, R.M. Structure—Function Relationships for Inhibitors of β-Amyloid Toxicity Containing the Recognition Sequence KLVFF. Biochemistry 2001, 40, 7882–7889. [Google Scholar] [CrossRef]
- Olivero, G.; Grilli, M.; Chen, J.; Preda, S.; Mura, E.; Govoni, S.; Marchi, M. Effects of Soluble β-Amyloid on the Release of Neurotransmitters from Rat Brain Synaptosomes. Front. Aging Neurosci. 2014, 6, 166. [Google Scholar] [CrossRef]
- Gutiérrez, I.L.; Dello Russo, C.; Novellino, F.; Caso, J.R.; García-Bueno, B.; Leza, J.C.; Madrigal, J.L.M. Noradrenaline in Alzheimer’s Disease: A New Potential Therapeutic Target. Int. J. Mol. Sci. 2022, 23, 6143. [Google Scholar] [CrossRef]
- Henjum, K.; Watne, L.O.; Godang, K.; Halaas, N.B.; Eldholm, R.S.; Blennow, K.; Zetterberg, H.; Saltvedt, I.; Bollerslev, J.; Knapskog, A.B. Cerebrospinal Fluid Catecholamines in Alzheimer’s Disease Patients with and without Biological Disease. Transl. Psychiatry 2022, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gallo, A.; Pillet, L.E.; Verpillot, R. New Frontiers in Alzheimer’s Disease Diagnostic: Monoamines and Their Derivatives in Biological Fluids. Exp. Gerontol. 2021, 152, 111452. [Google Scholar] [CrossRef] [PubMed]
- Mura, E.; Zappettini, S.; Preda, S.; Biundo, F.; Lanni, C.; Grilli, M.; Cavallero, A.; Olivero, G.; Salamone, A.; Govoni, S.; et al. Dual Effect of Beta-Amyloid on A7 and A4β2 Nicotinic Receptors Controlling the Release of Glutamate, Aspartate and GABA in Rat Hippocampus. PLoS ONE 2012, 7, e29661. [Google Scholar] [CrossRef] [PubMed]
- Grilli, M.; Lagomarsino, F.; Zappettini, S.; Preda, S.; Mura, E.; Govoni, S.; Marchi, M. Specific Inhibitory Effect of Amyloid-β on Presynaptic Muscarinic Receptor Subtypes Modulating Neurotransmitter Release in the Rat Nucleus Accumbens. Neuroscience 2010, 167, 482–489. [Google Scholar] [CrossRef]
- Patti, L.; Raiteri, L.; Grilli, M.; Parodi, M.; Raiteri, M.; Marchi, M. P2X7 Receptors Exert a Permissive Role on the Activation of Release-Enhancing Presynaptic A7 Nicotinic Receptors Co-Existing on Rat Neocortex Glutamatergic Terminals. Neuropharmacology 2006, 50, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Grilli, M.; Patti, L.; Robino, F.; Zappettini, S.; Raiteri, M.; Marchi, M. Release-Enhancing Pre-Synaptic Muscarinic and Nicotinic Receptors Co-Exist and Interact on Dopaminergic Nerve Endings of Rat Nucleus Accumbens. J. Neurochem. 2008, 105, 2205–2213. [Google Scholar] [CrossRef]
- Raiteri, M.; Sala, R.; Fassio, A.; Rossetto, O.; Bonanno, G. Entrapping of Impermeant Probes of Different Size into Nonpermeabilized Synaptosomes as a Method to Study Presynaptic Mechanisms. J. Neurochem. 2000, 74, 423–431. [Google Scholar] [CrossRef]
- Grilli, M.; Summa, M.; Salamone, A.; Olivero, G.; Zappettini, S.; Di Prisco, S.; Feligioni, M.; Usai, C.; Pittaluga, A.; Marchi, M. In Vitro Exposure to Nicotine Induces Endocytosis of Presynaptic AMPA Receptors Modulating Dopamine Release in Rat Nucleus Accumbens Nerve Terminals. Neuropharmacology 2012, 63, 916–926. [Google Scholar] [CrossRef]
- Gylys, K.H.; Fein, J.A.; Wiley, D.J.; Cole, G.M. Rapid Annexin-V Labeling in Synaptosomes. Neurochem. Int. 2004, 44, 125–131. [Google Scholar] [CrossRef]
- Logue, S.E.; Elgendy, M.; Martin, S.J. Expression, Purification and Use of Recombinant Annexin V for the Detection of Apoptotic Cells. Nat. Protoc. 2009, 4, 1383–1395. [Google Scholar] [CrossRef]
- Neri, S.; Mariani, E.; Meneghetti, A.; Cattini, L.; Facchini, A. Calcein-Acetyoxymethyl Cytotoxicity Assay: Standardization of a Method Allowing Additional Analyses on Recovered Effector Cells and Supernatants. Clin. Diagn. Lab. Immunol. 2001, 8, 1131–1135. [Google Scholar] [CrossRef] [PubMed]
- Centonze, D.; Muzio, L.; Rossi, S.; Cavasinni, F.; de Chiara, V.; Bergami, A.; Musella, A.; D’Amelio, M.; Cavallucci, V.; Martorana, A.; et al. Inflammation Triggers Synaptic Alteration and Degeneration in Experimental Autoimmune Encephalomyelitis. J. Neurosci. 2009, 29, 3442–3452. [Google Scholar] [CrossRef]
- Gylys, K.H.; Bilousova, T. Flow Cytometry Analysis and Quantitative Characterization of Tau in Synaptosomes from Alzheimer’s Disease Brains. Methods Mol. Biol. 2017, 1523, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Everhart, D.; Cartier, G.E.; Malhotra, A.; Gomes, A.V.; Mcintosh, J.M.; Luetje, C.W. Determinants of Potency on R-Conotoxin MII, a Peptide Antagonist of Neuronal Nicotinic Receptors †. Biochemistry 2004, 43, 2732–2737. [Google Scholar] [CrossRef] [PubMed]
- Kulak, J.M.; Nguyen, T.A.; Olivera, B.M.; McIntosh, J.M. α-Conotoxin MII Blocks Nicotine-Stimulated Dopamine Release in Rat Striatal Synaptosomes. J. Neurosci. 1997, 17, 5263–5270. [Google Scholar] [CrossRef] [PubMed]
- Clarke, P.B.S.; Reuben, M. Release of [3H]-Noradrenaline from Rat Hippocampal Synaptosomes by Nicotine: Mediation by Different Nicotinic Receptor Subtypes from Striatal [3H]-Dopamine Release. Br. J. Pharmacol. 1996, 117, 595–606. [Google Scholar] [CrossRef]
- Nehls, M. Unified Theory of Alzheimer’s Disease (UTAD): Implications for Prevention and Curative Therapy. J. Mol. Psychiatry 2016, 4, 3. [Google Scholar] [CrossRef]
- Robinson, M.; Lou, J.; Mehrazma, B.; Rauk, A.; Beazely, M.; Leonenko, Z. Pseudopeptide Amyloid Aggregation Inhibitors: In Silico, Single Molecule and Cell Viability Studies. Int. J. Mol. Sci. 2021, 22, 1051. [Google Scholar] [CrossRef]
- Novo, M.; Freire, S.; Al-Soufi, W. Critical Aggregation Concentration for the Formation of Early Amyloid-β (1–42) Oligomers. Sci. Rep. 2018, 8, 1783. [Google Scholar] [CrossRef]
- Giuffrida, M.L.; Caraci, F.; de Bona, P.; Pappalardo, G.; Nicoletti, F.; Rizzarelli, E.; Copani, A. The Monomer State of Beta-Amyloid: Where the Alzheimer’s Disease Protein Meets Physiology. Rev. Neurosci. 2010, 21, 83–93. [Google Scholar] [CrossRef]
- Cárdenas-Aguayo, M.C.; Silva-Lucero, M.C.; García, U. Physiological Role of Amyloid Beta in Neural Cells: The Cellular Trophic Activity. In Neurochemistry; IntechOpen: London, UK, 2014; pp. 1–26. [Google Scholar] [CrossRef]
- Ruz, C.; Alcantud, J.L.; Montero, F.V.; Duran, R.; Bandres-Ciga, S. Proteotoxicity and Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 5646. [Google Scholar] [CrossRef] [PubMed]
- Puzzo, D.; Privitera, L.; Fa’, M.; Staniszewski, A.; Hashimoto, G.; Aziz, F.; Sakurai, M.; Ribe, E.M.; Troy, C.M.; Mercken, M.; et al. Endogenous Amyloid-β Is Necessary for Hippocampal Synaptic Plasticity and Memory. Ann. Neurol. 2011, 69, 819–830. [Google Scholar] [CrossRef] [PubMed]
- Palmeri, A.; Ricciarelli, R.; Gulisano, W.; Rivera, D.; Rebosio, C.; Calcagno, E.; Tropea, M.R.; Conti, S.; Das, U.; Roy, S.; et al. Amyloid-β Peptide Is Needed for CGMP-Induced Long-Term Potentiation and Memory. J. Neurosci. 2017, 37, 6926–6937. [Google Scholar] [CrossRef] [PubMed]
- Gulisano, W.; Melone, M.; Ripoli, C.; Tropea, M.R.; Li Puma, D.D.; Giunta, S.; Cocco, S.; Marcotulli, D.; Origlia, N.; Palmeri, A.; et al. Neuromodulatory Action of Picomolar Extracellular Aβ42 Oligomers on Presynaptic and Postsynaptic Mechanisms Underlying Synaptic Function and Memory. J. Neurosci. 2019, 39, 5986–6000. [Google Scholar] [CrossRef]
- Sadigh-Eteghad, S.; Majdi, A.; Talebi, M.; Mahmoudi, J.; Babri, S. Regulation of Nicotinic Acetylcholine Receptors in Alzheimer’s Disease: A Possible Role of Chaperones. Eur. J. Pharmacol. 2015, 755, 35–41. [Google Scholar] [CrossRef]
- Morales, I.; Guzmán-MartÃnez, L.; Cerda-Troncoso, C.; FarÃas, G.A.; Maccioni, R.B. Neuroinflammation in the Pathogenesis of Alzheimerâ€TMs Disease. A Rational Framework for the Search of Novel Therapeutic Approaches. Front. Cell. Neurosci. 2014, 8, 112. [Google Scholar] [CrossRef]
- Griffiths, J.; Grant, S.G.N. Synapse Pathology in Alzheimer’s Disease. Semin. Cell Dev. Biol. 2022. [Google Scholar] [CrossRef]
- Hillen, H. The Beta Amyloid Dysfunction (BAD) Hypothesis for Alzheimer’s Disease. Front. Neurosci. 2019, 13, 1154. [Google Scholar] [CrossRef]
- Preda, S.; Govoni, S.; Lanni, C.; Racchi, M.; Mura, E.; Grilli, M.; Marchi, M. Acute Beta-Amyloid Administration Disrupts the Cholinergic Control of Dopamine Release in the Nucleus Accumbens. Neuropsychopharmacology 2008, 33, 1062–1070. [Google Scholar] [CrossRef] [Green Version]
- Castillo, C.A.; Ballesteros-Yáñez, I.; León-Navarro, D.A.; Albasanz, J.L.; Martín, M. Early Effects of the Soluble Amyloid Β25-35 Peptide in Rat Cortical Neurons: Modulation of Signal Transduction Mediated by Adenosine and Group I Metabotropic Glutamate Receptors. Int. J. Mol. Sci. 2021, 22, 6577. [Google Scholar] [CrossRef]
- Castelletto, V.; Ryumin, P.; Cramer, R.; Hamley, I.W.; Taylor, M.; Allsop, D.; Reza, M.; Ruokolainen, J.; Arnold, T.; Hermida-Merino, D.; et al. Self-Assembly and Anti-Amyloid Cytotoxicity Activity of Amyloid Beta Peptide Derivatives. Sci. Rep. 2017, 7, 43637. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Zhao, Q.; Peng, J.; Yu, Y.; Wang, C.; Zou, Y.; Su, Y.; Zhu, L.; Wang, C.; Yang, Y. Peptide-Polyphenol (KLVFF/EGCG) Binary Modulators for Inhibiting Aggregation and Neurotoxicity of Amyloid-β Peptide. ACS Omega 2019, 4, 4233–4242. [Google Scholar] [CrossRef]
- Arai, T.; Sasaki, D.; Araya, T.; Sato, T.; Sohma, Y.; Kanai, M. A Cyclic KLVFF-Derived Peptide Aggregation Inhibitor Induces the Formation of Less-Toxic off-Pathway Amyloid-β Oligomers. ChemBioChem 2014, 15, 2577–2583. [Google Scholar] [CrossRef]
- Arispe, N.; Rojas, E.; Pollard, H.B. Alzheimer Disease Amyloid β Protein Forms Calcium Channels in Bilayer Membranes: Blockade by Tromethamine and Aluminum. Proc. Natl. Acad. Sci. USA 1993, 90, 567–571. [Google Scholar] [CrossRef]
- Fein, J.A.; Sokolow, S.; Miller, C.A.; Vinters, H.v.; Yang, F.; Cole, G.M.; Gylys, K.H. Co-Localization of Amyloid Beta and Tau Pathology in Alzheimer’s Disease Synaptosomes. Am. J. Pathol. 2008, 172, 1683–1692. [Google Scholar] [CrossRef] [PubMed]
- Pym, L.J.; Buckingham, S.D.; Tsetlin, V.; Boyd, C.A.R.; Sattelle, D.B. The Abeta1-42M35C Mutated Amyloid Peptide Abeta1-42 and the 25-35 Fragment Fail to Mimic the Subtype-Specificity of Actions on Recombinant Human Nicotinic Acetylcholine Receptors (Alpha7, Alpha4beta2, Alpha3beta4). Neurosci. Lett. 2007, 427, 28–33. [Google Scholar] [CrossRef]
- Chatzidaki, A.; Millar, N.S. Allosteric Modulation of Nicotinic Acetylcholine Receptors. Biochem. Pharmacol. 2015, 97, 408–417. [Google Scholar] [CrossRef]
- Nguyen, P.V.; Connor, S.A. Noradrenergic Regulation of Hippocampus-Dependent Memory. Cent. Nerv. Syst. Agents Med. Chem. 2019, 19, 187–196. [Google Scholar] [CrossRef]
- Song, S.; Wang, Q.; Jiang, L.; Oyarzabal, E.; Riddick, N.V.; Wilson, B.; Moy, S.S.; Shih, Y.Y.I.; Hong, J.S. Noradrenergic Dysfunction Accelerates LPS-Elicited Inflammation-Related Ascending Sequential Neurodegeneration and Deficits in Non-Motor/Motor Functions. Brain Behav. Immun. 2019, 81, 374. [Google Scholar] [CrossRef]
- Marien, M.R.; Colpaert, F.C.; Rosenquist, A.C. Noradrenergic Mechanisms in Neurodegenerative Diseases: A Theory. Brain Res. Rev. 2004, 45, 38–78. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trebesova, H.; Olivero, G.; Marchi, M.; Grilli, M. The Anti-Aggregative Peptide KLVFF Mimics Aβ1-40 in the Modulation of Nicotinic Receptors: Implications for Peptide-Based Therapy. Biomedicines 2022, 10, 2231. https://doi.org/10.3390/biomedicines10092231
Trebesova H, Olivero G, Marchi M, Grilli M. The Anti-Aggregative Peptide KLVFF Mimics Aβ1-40 in the Modulation of Nicotinic Receptors: Implications for Peptide-Based Therapy. Biomedicines. 2022; 10(9):2231. https://doi.org/10.3390/biomedicines10092231
Chicago/Turabian StyleTrebesova, Hanna, Guendalina Olivero, Mario Marchi, and Massimo Grilli. 2022. "The Anti-Aggregative Peptide KLVFF Mimics Aβ1-40 in the Modulation of Nicotinic Receptors: Implications for Peptide-Based Therapy" Biomedicines 10, no. 9: 2231. https://doi.org/10.3390/biomedicines10092231
APA StyleTrebesova, H., Olivero, G., Marchi, M., & Grilli, M. (2022). The Anti-Aggregative Peptide KLVFF Mimics Aβ1-40 in the Modulation of Nicotinic Receptors: Implications for Peptide-Based Therapy. Biomedicines, 10(9), 2231. https://doi.org/10.3390/biomedicines10092231





