Platelets’ Nanomechanics and Morphology in Neurodegenerative Pathologies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Studied Individuals
2.2. Isolation and Immobilization of Platelets
2.3. AFM Imaging and Force Mapping
2.4. Correlation Analysis
3. Results
3.1. Topography and Morphology of Human Platelets from PD, AD and ALS Patients and Healthy Individuals
3.2. Nanomechanical Characteristics of Platelets from NDD Patients
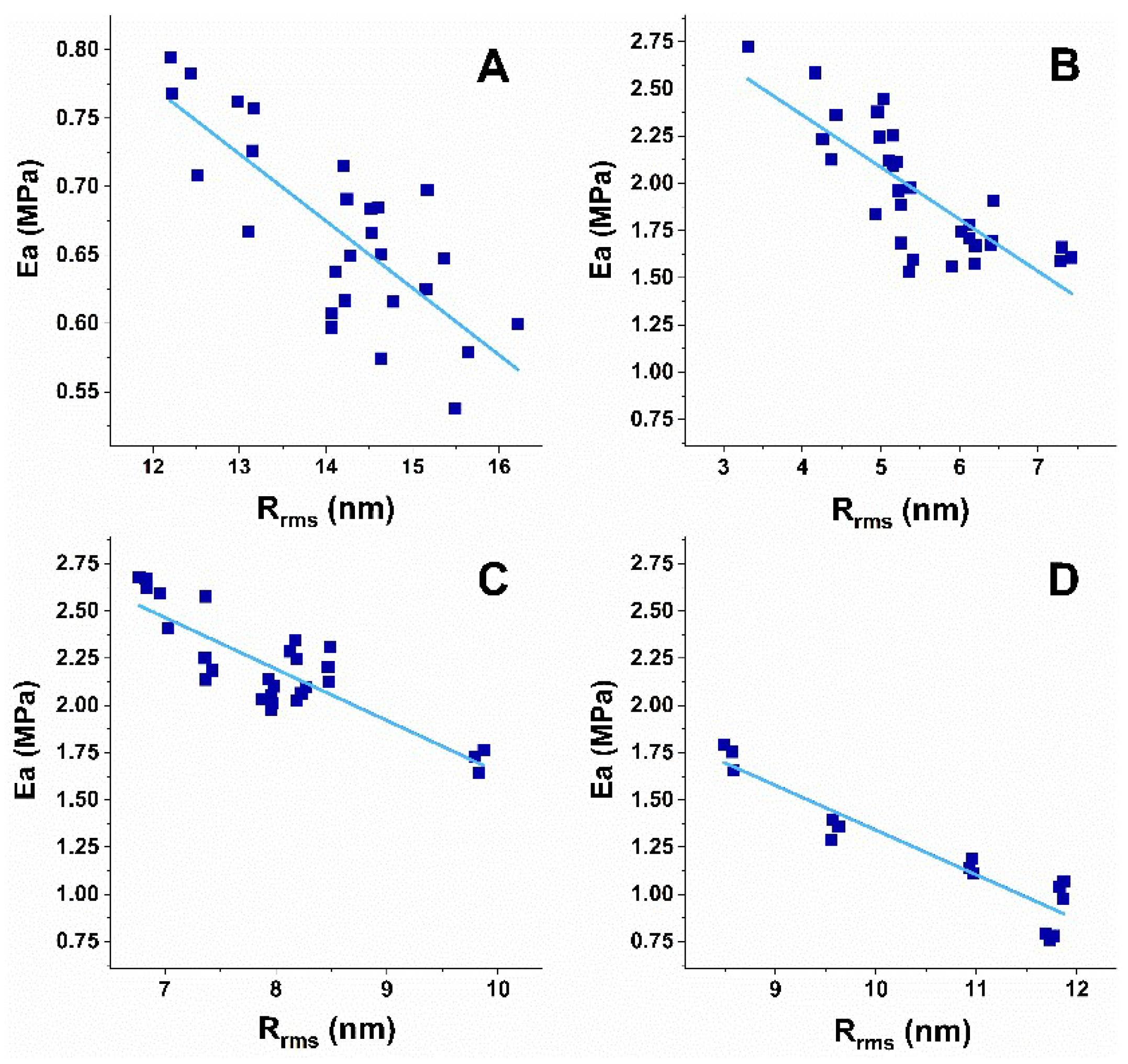
3.3. Correlation Analysis of Platelets’ Nanomechanical and Morphological Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Behari, M.; Shrivastava, M. Role of platelets in neurodegenerative diseases: A universal pathophysiology. Int. J. Neurosci. 2013, 123, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Periayah, M.H.; Halim, A.S.; Mat Saad, A.Z. Mechanism Action of Platelets and Crucial Blood Coagulation Pathways in Hemostasis. Int. J. Hematol. Oncol. Stem Cell Res. 2017, 11, 319–327. [Google Scholar] [PubMed]
- Holinstat, M. Normal platelet function. Cancer Metastasis Rev. 2017, 36, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Gianazza, E.; Brioschi, M.; Baetta, R.; Mallia, A.; Banfi, C.; Tremoli, E. Platelets in Healthy and Disease States: From Biomarkers Discovery to Drug Targets Identification by Proteomics. Int. J. Mol. Sci. 2020, 21, 4541. [Google Scholar] [CrossRef]
- Locatelli, L.; Colciago, A.; Castiglioni, S.; Maier, J.A. Platelets in Wound Healing: What Happens in Space? Front. Bioeng. Biotechnol. 2021, 9, 716184. [Google Scholar] [CrossRef]
- Ambrosio, A.L.; Di Pietro, S.M. Storage pool diseases illuminate platelet dense granule biogenesis. Platelets 2017, 8, 138–146. [Google Scholar] [CrossRef]
- Chen, Y.; Yuan, Y.; Li, W. Sorting machineries: How platelet-dense granules differ from α-granules. Biosci. Rep. 2018, 38, BSR20180458. [Google Scholar] [CrossRef]
- Talib, L.L.; Joaquim, H.P.; Forlenza, O.V. Platelet biomarkers in Alzheimer’s disease. World J. Psychiatry 2012, 2, 95–101. [Google Scholar] [CrossRef]
- Perello, M.; Stuart, R.; Nillni, E.A. Prothyrotropin-releasing hormone targets its processing products to different vesicles of the secretory pathway. J. Biol. Chem. 2008, 283, 19936–19947. [Google Scholar] [CrossRef]
- Italiano, J.E., Jr.; Richardson, J.L.; Patel-Hett, S.; Battinelli, E.; Zaslavsky, A.; Short, S.; Ryeom, S.; Folkman, J.; Klement, G.L. Angiogenesis is regulated by a novel mechanism: Pro- and antiangiogenic proteins are organized into separate platelet a-granules and differentially released. Blood 2008, 111, 1227–1233. [Google Scholar] [CrossRef] [Green Version]
- Gowert, N.S.; Donner, L.; Chatterjee, M.; Eisele, Y.S.; Towhid, S.T.; Münzer, P.; Walker, B.; Ogorek, I.; Borst, O.; Grandoch, M.; et al. Blood platelets in the progression of Alzheimer’s disease. PLoS ONE 2014, 9, e90523. [Google Scholar] [CrossRef]
- Veitinger, M.; Varga, B.; Guterres, S.B.; Zellner, M. Platelets, a reliable source for peripheral Alzheimer’s disease biomarkers? Acta Neuropathol. Commun. 2014, 2, 65. [Google Scholar] [CrossRef]
- Stellos, K.; Panagiota, V.; Kögel, A.; Leyhe, T.; Gawaz, M.; Laske, C. Predictive value of platelet activation for the rate of cognitive decline in Alzheimer’s disease patients. J. Cereb. Blood Flow Metab. 2010, 30, 1817–1820. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Parrilla, Y.; Gonzalez-Billault, C.; Fuentes, E.; Palomo, I.; Alarcón, M. Decoding the Role of Platelets and Related MicroRNAs in Aging and Neurodegenerative Disorders. Front. Aging Neurosci. 2019, 11, 151. [Google Scholar] [CrossRef]
- Kiktenko, A.I.; Zlobina, G.P.; Brusov, O.S.; Zakharova, M.N. Structure of peripheral blood platelets surface in patients with amyotrophic lateral sclerosis and multiple sclerosis. Zh. Nevrol. Psikhiatr. Im. S.S. Korsakova 2005, 105, 40–42. [Google Scholar]
- Lim, K.M.; Kim, H.H.; Bae, O.N.; Noh, J.Y.; Kim, K.Y.; Kim, S.H.; Chung, S.M.; Shin, S.; Kim, H.Y.; Chung, J.H. Inhibition of platelet aggregation by 1-methyl-4-phenyl pyridinium ion (MPP+) through ATP depletion: Evidence for the reduced platelet activities in Parkinson’s disease. Platelets 2009, 20, 163–170. [Google Scholar] [CrossRef]
- Wojsiat, J.; Laskowska-Kaszub, K.; Mietelska-Porowska, A.; Wojda, U. Search for Alzheimer’s disease biomarkers in blood cells: Hypotheses-driven approach. Biomark. Med. 2017, 11, 917–931. [Google Scholar] [CrossRef]
- Ferrer-Raventós, P.; Beyer, K. Alternative platelet activation pathways and their role in neurodegenerative diseases. Neurobiol. Dis. 2021, 159, 105512. [Google Scholar] [CrossRef]
- Catricala, S.; Torti, M.; Ricevuti, G. Alzheimer disease and platelets: How’s that relevant. Immun. Ageing 2012, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Donner, L.; Elvers, M. Platelets and Neurodegenerative Diseases. In Platelets in Thrombotic and Non-Thrombotic Disorders; Gresele, P., Kleiman, N., Lopez, J., Page, C., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1209–1224. [Google Scholar]
- Li, Q.X.; Berndt, M.C.; Bush, A.I.; Rumble, B.; Mackenzie, I.; Friedhuber, A.; Beyreuther, K.; Masters, C.L. Membrane-associated forms of the beta A4 amyloid protein precursor of Alzheimer’s disease in human platelet and brain: Surface expression on the activated human platelet. Blood 1994, 84, 133–142. [Google Scholar] [CrossRef]
- Van Nostrand, W.E.; Schmaier, A.H.; Farrow, J.S.; Cunningham, D.D. Protease Nexin-II(amyloid β-protein Precursor): A Platelet α-Granule Protein. Science 1990, 248, 745–748. [Google Scholar] [CrossRef]
- Pluta, R.; Ułamek-Kozioł, M. Lymphocytes, Platelets, Erythrocytes, and Exosomes as Possible Biomarkers for Alzheimer’s Disease Clinical Diagnosis. Adv. Exp. Med. Biol. 2019, 1118, 71–82. [Google Scholar] [PubMed]
- Kniewallner, K.M.; Ehrlich, D.; Kiefer, A.; Marksteiner, J.; Humpel, C. Platelets in the Alzheimer’s disease brain: Do they play a role in cerebral amyloid angiopathy? Curr. Neurovasc. Res. 2015, 12, 4–14. [Google Scholar] [CrossRef]
- Kucheryavykh, L.Y.; Davila-Rodriguez, J.; Rivera-Aponte, D.E.; Zueva, L.V.; Washington, A.V.; Sanabria, P.; Inyushin, M.Y. Platelets are responsible for the accumulation of β-amyloid in blood clots inside and around blood vessels in mouse brain after thrombosis. Brain Res. Bull. 2017, 128, 98–105. [Google Scholar] [CrossRef]
- Di Luca, M.; Colciaghi, F.; Pastorino, L.; Borroni, B.; Padovani, A.; Cattabeni, F. Platelets as a peripheral district where to study pathogenetic mechanisms of alzheimer disease: The case of amyloid precursor protein. Eur. J. Pharmacol. 2000, 405, 277–283. [Google Scholar] [CrossRef]
- Nesteruk, M.; Nesteruk, T.; Styczyńska, M.; Mandecka, M.; Barczak, A.; Barcikowska, M. Combined use of biochemical and volumetric biomarkers to assess the risk of conversion of mild cognitive impairment to Alzheimer’s disease. Folia Neuropathol. 2016, 54, 369. [Google Scholar] [CrossRef] [PubMed]
- Mietelska-Porowska, A.; Wojda, U. T Lymphocytes and Inflammatory Mediators in the Interplay between Brain and Blood in Alzheimer’s Disease: Potential Pools of New Biomarkers. J. Immunol. Res. 2017, 2017, 4626540. [Google Scholar] [CrossRef] [PubMed]
- Pienimaeki-Roemer, A.; Kuhlmann, K.; Böttcher, A.; Konovalova, T.; Black, A.; Orsõ, E.; Liebisch, G.; Ahrens, M.; Eisenacher, M.; Meyer, H.E.; et al. Lipidomic and proteomic characterization of platelet extracellular vesicle subfractions from senescent platelets. Transfusion 2015, 55, 507–521. [Google Scholar] [CrossRef]
- Pei, Y.; Maitta, R.W. Alpha synuclein in hematopoiesis and immunity. Heliyon 2019, 5, e02590. [Google Scholar] [CrossRef]
- Carrim, N.; Arthur, J.F.; Hamilton, J.R.; Gardiner, E.E.; Andrews, R.K.; Moran, N.; Berndt, M.C.; Metharom, P. Thrombin-induced reactive oxygen species generation in platelets: A novel role for protease-activated receptor 4 and GPIbα. Redox Biol. 2015, 6, 640–647. [Google Scholar] [CrossRef]
- Kean, P.C.; Kurzawa, M.; Blain, P.G.; Morris, C.M. Mitochondrial dysfunction in Parkinson’s disease. Parkinsons Dis. 2011, 2011, 716871. [Google Scholar] [CrossRef] [Green Version]
- Hishizawa, M.; Yamashita, H.; Akizuki, M.; Urushitani, M.; Takahashi, R. TDP-43 levels are higher in platelets from patients with sporadic amyotrophic lateral sclerosis than in healthy controls. Neurochem. Int. 2019, 124, 41–45. [Google Scholar] [CrossRef]
- Shrivastava, M.; Das, T.K.; Behari, M.; Pati, U.; Vivekanandhan, S. Ultrastructural Variations in Platelets and Platelet Mitochondria: A Novel Feature in Amyotrophic Lateral Sclerosis. Ultrastruct. Pathol. 2011, 35, 52–59. [Google Scholar] [CrossRef]
- Shrivastava, M.; Vivekanandhan, S.; Pati, U.; Behari, M.; Das, T.K. Mitochondrial perturbance and execution of apoptosis in platelet mitochondria of patients with amyotrophic lateral sclerosis. Int. J. Neurosci. 2011, 121, 149–158. [Google Scholar] [CrossRef]
- Briones, M.R.S.; Snyder, A.M.; Ferreira, R.C.; Neely, E.B.; Connor, J.R.; Broach, J.R. Activating Factor Receptor in Amyotrophic Lateral Sclerosis Treatment. Front. Neurol. 2018, 9, 39. [Google Scholar] [CrossRef]
- Dantzer, R. Neuroimmune interactions: From the brain to the immune system and vice versa. Physiol. Rev. 2018, 98, 477–504. [Google Scholar] [CrossRef]
- Dupuis, L.; Spreux-Varoquaux, O.; Bensimon, G.; Jullien, P.; Lacomblez, L.; Salachas, F.; Bruneteau, G.; Pradat, P.F.; Loeffler, J.P.; Meininger, V. Platelet serotonin level predicts survival in amyotrophic lateral sclerosis. PLoS ONE 2010, 5, e13346. [Google Scholar] [CrossRef]
- Kumar, A.M.; Sevush, S.; Kumar, M.; Ruiz, J.; Eisdorfer, C. Peripheral serotonin in Alzheimer’s disease. Neuropsychobiology 1995, 32, 9–12. [Google Scholar] [CrossRef]
- Kumar, A.M.; Kumar, M.; Sevush, S.; Ruiz, J.; Eisdorfer, C. Serotonin uptake and its kinetics in platelets of women with Alzheimer’s Disease. Psychiatry Res. 1995, 59, 145–150. [Google Scholar] [CrossRef]
- Postuma, R.B.; Berg, D.; Stern, M.; Poewe, W.; Olanow, C.W.; Oertel, W.; Obeso, J.; Marek, K.; Litvan, I.; Lang, A.; et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 2015, 30, 1591–1601. [Google Scholar] [CrossRef]
- Hoehn, M.M.; Yahr, M.D. Parkinsonism: Onset, progression and mortality. Neurology 1967, 17, 427–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brooks, B.R.; Miller, R.G.; Swash, M.; Munsat, T.L. World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Other Mot. Neuron. Disord. 2000, 1, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Cedarbaum, J.M.; Stambler, N.; Malta, E.; Fuller, C.; Hilt, D.; Thurmond, B.; Nakanishi, A. The ALSFRS-R: A revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J. Neurol. Sci. 1999, 169, 13–21. [Google Scholar] [CrossRef]
- Mckhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.; Kawas, C.; Klunk, W.; Koroshetz, W.; Manly, J.; Mayeux, R.; Mohs, R.C.; et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging? Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. Mini-mental status. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Dagur, P.K.; McCoy, J.P., Jr. Collection, Storage, and Preparation of Human Blood Cells. Curr. Protoc. Cytom. 2015, 73, 5.1.1–5.1.16. [Google Scholar] [CrossRef]
- Gadelmawla, E.S.; Koura, M.M.; Macsoud, T.M.A.; Elewa, I.M.; Soliman, H.H. Roughness parameters. J. Mater. Process. Technol. 2002, 123, 133–145. [Google Scholar] [CrossRef]
- Girasole, M.; Dinarelli, S.; Boumis, G. Structure and function in native and pathological erythrocytes: A quantitative view from the nanoscale. Micron 2012, 43, 1273–1286. [Google Scholar] [CrossRef]
- Sneddon, I.N. The relation between load and penetration in the axisymmetric boussinesq problem for a punch of arbitrary profile. Int. J. Eng. Sci. 1965, 3, 47–57. [Google Scholar] [CrossRef]
- Bilodeau, G.G. Regular Pyramid Punch Problem. J. Appl. Mech. 1992, 59, 519–523. [Google Scholar] [CrossRef]
- Hartwig, J.H. The platelet: Form and function. Semin. Hematol. 2006, 43, S94–S100. [Google Scholar] [CrossRef]
- Sorrentino, S.; Studt, J.-D.; Horev, M.B.; Medalia, O.; Sapra, K.T. Toward correlating structure and mechanics of platelets. Cell Adh. Migr. 2016, 10, 568–575. [Google Scholar] [CrossRef]
- Li, A.; Chen, J.; Liang, Z.-H.; Caid, J.; Cai, H.-H.; Chen, M. Comparison of ultrastructural and nanomechanical signature of platelets from acute myocardial infarction and platelet activation. Biochem. Biophys. Res. Commun. 2017, 486, 245–251. [Google Scholar] [CrossRef]
- Radmacher, M.; Fritz, M.; Kacher, C.; Cleveland, J.; Hansma, P. Measuring the viscoelastic properties of human platelets with the atomic force microscope. Biophys. J. 1996, 70, 556–567. [Google Scholar] [CrossRef]
- Lee, I.I.; Marchant, R.E. Force measurements on platelet surfaces with high spatial resolution under physiological conditions. Colloids Surf. B Biointerfaces 2000, 19, 357–365. [Google Scholar] [CrossRef]
- Walch, M.; Ziegler, U.; Groscurth, P. Effect of streptolysin O on the microelasticity of human platelets analyzed by atomic force microscopy. Ultramicroscopy 2000, 82, 259–267. [Google Scholar] [CrossRef]
- Fujita, Y.; Fujii, T.; Nishio, A.; Tuboi, K.; Tsuji, K.; Nakamura, M. Familial case of May-Hegglin anomaly associated with familial spastic paraplegia. Am. J. Hematol. 1990, 35, 219–221. [Google Scholar] [CrossRef]
- Shribman, S.; Reid, E.; Crosby, A.H.; Houlden, H.; Warner, T.T. Hereditary spastic paraplegia: From diagnosis to emerging therapeutic approaches. Lancet Neurol. 2019, 18, 1136–1146. [Google Scholar] [CrossRef]
- Harding, A.E. Hereditary spastic paraplegias. Semin. Neurol. 1993, 13, 333–336. [Google Scholar] [CrossRef]
- Lallemant-Dudek, P.; Darios, F.; Durr, A. Recent advances in understanding hereditary spastic paraplegias and emerging therapies. Fac. Rev. 2021, 10, 27. [Google Scholar] [CrossRef]
- Novarino, G.; Fenstermaker, A.G.; Zaki, M.S.; Hofree, M.; Silhavy, J.L.; Heiberg, A.D.; Abdellateef, M.; Rosti, B.; Scott, E.; Mansour, L.; et al. Exome sequencing links corticospinal motor neuron disease to common neurodegenerative disorders. Science 2014, 343, 506–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strong, M.J.; Gordon, P.H. Primary lateral sclerosis, hereditary spastic paraplegia and amyotrophic lateral sclerosis: Discrete entities or spectrum? Amyotroph. Lateral. Scler. Other Motor Neuron. Disord. 2005, 6, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Denora, P.S.; Smets, K.; Zolfanelli, F.; Ceuterick-de Groote, C.; Casali, C.; Deconinck, T.; Sieben, A.; Gonzales, M.; Zucher, S.; Darios, F.; et al. Motor neuron degeneration in spastic paraplegia 11 mimics amyotrophic lateral sclerosis lesions. Brain 2016, 139, 1723–1734. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kim, J.M.; Kim, Y.K.; Kim, S.E.; Yun, J.Y.; Jeon, B.S. Striatal dopaminergic functioning in patients with sporadic and hereditary spastic paraplegias with parkinsonism. J. Korean Med. Sci. 2013, 28, 1661–1666. [Google Scholar] [CrossRef]
- Hsu, L.J.; Sagara, Y.; Arroyo, A.; Rockenstein, E.; Sisk, A.; Mallory, M.; Wong, J.; Takenouchi, T.; Hashimoto, M.; Masliah, E. α-Synuclein promotes mitochondrial deficit and oxidative stress. Am. J. Pathol. 2000, 157, 401–410. [Google Scholar] [CrossRef]
- Herczenik, E.; Bouma, B.; Korporaal, S.J.; Strangi, R.; Zeng, Q.; Gros, P.; Van Eck, M.; Van Berkel, T.J.; Gebbink, M.F.; Akkerman, J.W. Activation of human platelets by misfolded proteins. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1657–1665. [Google Scholar] [CrossRef]
- Fritz, M.; Radmacher, M.; Gaub, H.E. Granula Motion and Membrane Spreading During Activation of Human Platelets Imaged by Atomic Force Microscopy. Biophys. J. 1994, 66, 1328–1334. [Google Scholar] [CrossRef]
- Shamova, E.V.; Gorudko, I.V.; Drozd, E.S.; Chizhik, S.A.; Martinovich, G.G.; Cherenkevich, S.N.; Timoshenko, A.V. Redox regulation of morphology, cell stiffness, and lectin-induced aggregation of human platelets. Eur. Biophys. J. 2011, 40, 195–208. [Google Scholar] [CrossRef]
- Posch, S.; Neundlinger, I.; Leitner, M.; Siostrzonek, P.; Panzer, S.; Hinterdorfer, P.; Ebner, A. Activation induced morphological changes and integrin αIIbβ3 activity of living platelets. Methods 2013, 60, 179–185. [Google Scholar] [CrossRef]
- Du Plooy, J.N.; Buys, A.; Duim, W.; Pretorius, E. Comparison of platelet ultrastructure and elastic properties in thrombo-embolic ischemic stroke and smoking using atomic force and scanning electron microscopy. PLoS ONE 2013, 8, 124–130. [Google Scholar] [CrossRef]
- Cross, S.E.; Jin, Y.S.; Tondre, J.; Wong, R.; Rao, J.; Gimzewski, J.K. AFM-based analysis of human metastatic cancer cells. Nanotechnology 2008, 19, 384003. [Google Scholar] [CrossRef]
- Lekka, M.; Laidler, P.; Gil, D.; Lekki, J.; Stachura, Z.; Hrynkiewicz, A.Z. Elasticity of normal and cancerous human bladder cells studied by scanning force microscopy. Eur. Biophys. J. 1999, 28, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Mezencev, R.; Kim, B.; Wang, L.; McDonald, J.; Sulchek, T. Cell stiffness is a biomarker of the metastatic potential of ovarian cancer cells. PLoS ONE 2012, 7, e46609. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Iwata, M. Stiffness of cancer cells measured with an AFM indentation method. J. Mech. Behav. Biomed. Mater. 2015, 49, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Kuang, D.; Zhang, B.; Song, G. Cell stiffness determined by atomic force microscopy and its correlation with cell motility. Biochim. Biophys. Acta 2016, 1860, 1953–1960. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Xiong, F.; Li, X.; Xiang, B.; Li, Z.; Wu, X.; Guo, C.; Li, X.; Li, Y.; Li, G.; et al. Application of atomic force microscopy in cancer research. J. Nanobiotechnol. 2018, 16, 102. [Google Scholar] [CrossRef] [PubMed]
- Iyer, K.S.; Dayal, S. Modulators of platelet function in aging. Platelets 2020, 31, 474–482. [Google Scholar] [CrossRef]
- Gleerup, G.; Winther, K. The effect of ageing on platelet function and fibrinolytic activity. Angiology 1995, 46, 715–718. [Google Scholar] [CrossRef]
- Jones, C.I. Platelet function and ageing. Mammal. Genome 2016, 27, 358–366. [Google Scholar] [CrossRef]
- Le Blanc, J.; Lordkipanidzé, M. Platelet Function in Aging. Front. Cardiovasc. Med. 2019, 6, 109. [Google Scholar] [CrossRef]
- Montenont, E.; Rondina, M.T.; Campbell, R.A. Altered functions of platelets during aging. Curr. Opin Hematol. 2019, 26, 336–342. [Google Scholar] [CrossRef]
- Chan, M.V.; Chen, M.-H.; Barwari, T.; Huffman, J.E.; Armstrong, P.C.; Hwang, S.-J.; Santer, P.; Wierer, B.; Mayr, M.; Kiechl, S.; et al. Platelet Reactivity in Individuals Over 65 Years Old Is Not Modulated by Age. Circ. Res. 2020, 127, 394–396. [Google Scholar] [CrossRef]
- Koçer, A.; Yaman, A.; Niftaliyev, E.; Dürüyen, H.; Eryılmaz, M.; Koçer, E. Assessment of platelet indices in patients with neurodegenerative diseases: Mean platelet volume was increased in patients with Parkinson’s disease. Curr. Gerontol. Geriatr. Res. 2013, 2013, 986254. [Google Scholar] [CrossRef]
- Sevush, S.; Jy, W.; Horstman, L.L.; Mao, W.W.; Kolodny, L.; Ahn, Y.S. Platelet activation in Alzheimer’s disease. Arch. Neurol. 1998, 55, 530–536. [Google Scholar] [CrossRef]
- Tian, J.; Cheng, L.H.; Cui, X.; Lei, X.X.; Tang, J.B.; Cheng, B. Investigating the effect of age on platelet ultrastructure using transmission electron microscopy. Int. Wound J. 2019, 16, 1457–1463. [Google Scholar] [CrossRef]
- Lippi, G.; Meschi, T.; Borghi, L. Mean platelet volume increases with aging in a large population study. Thromb. Res. 2012, 129, e159–e160. [Google Scholar] [CrossRef]
- Strijkova-Kenderova, V.; Todinova, S.; Andreeva, T.; Bogdanova, D.; Langari, A.; Danailova, A.; Krumova, S.; Zlatareva, E.; Kalaydzhiev, N.; Milanov, I.; et al. Morphometry and Stiffness of Red Blood Cells—Signatures of Neurodegenerative Diseases and Aging. Int. J. Mol. Sci. 2022, 23, 227. [Google Scholar] [CrossRef]
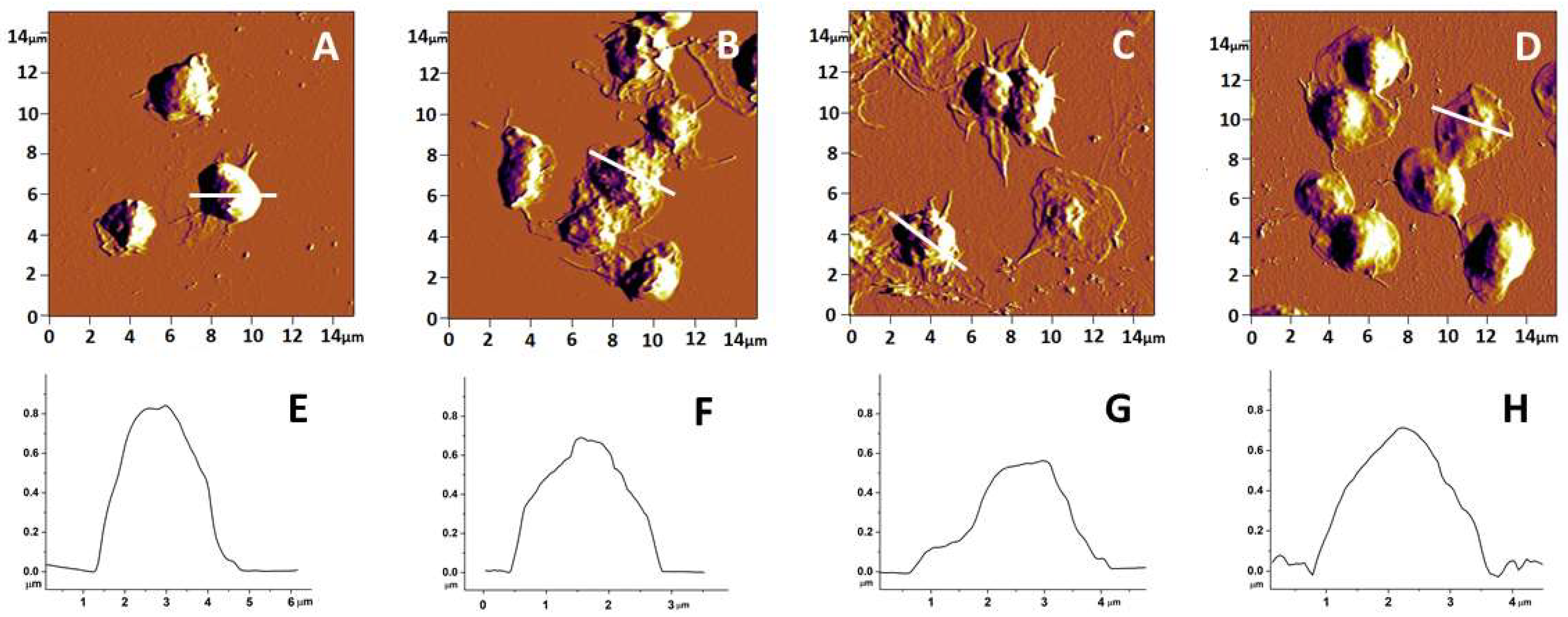



| Subject of Investigation | Area (μm2) | Height (μm) | Roughness (nm) | Ea (MPa) |
|---|---|---|---|---|
| Healthy | 4.8 ± 0.5 | 1.00 ± 0.18 | 14.3 ± 2.2 | 0.60 ± 0.21 |
| PD | 4.2 ± 0.6 | 0.53 ± 0.12 ** | 5.4 ± 1.2 ** | 2.04 ± 0.36 * |
| ALS | 3.4 ± 0.9 * | 0.50 ± 0.17 ** | 8.0 ± 0.9 ** | 2.22 ± 0.33 * |
| AD | 4.0 ± 1.1 | 0.82 ± 0.10 | 10.5 ± 1.4 | 1.25 ± 0.29 * |
| Subject of Investigation | Ea/Rrms | CI | Area/Rrms | CI | ||
|---|---|---|---|---|---|---|
| r | Lower Limit | Upper Limit | r | Lower Limit | Upper Limit | |
| Healthy | −0.75 | −0.8794 | −0.5175 | 0.88 | 0.7512 | 0.9442 |
| PD | −0.78 | −0.8887 | −0.5882 | 0.76 | 0.5552 | 0.8779 |
| ALS | −0.84 | −0.9248 | −0.6757 | 0.81 | 0.6212 | 0.9099 |
| AD | −0.93 | −0.9769 | −0.7978 | 0.85 | 0.5982 | 0.949 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strijkova, V.; Todinova, S.; Andreeva, T.; Langari, A.; Bogdanova, D.; Zlatareva, E.; Kalaydzhiev, N.; Milanov, I.; Taneva, S.G. Platelets’ Nanomechanics and Morphology in Neurodegenerative Pathologies. Biomedicines 2022, 10, 2239. https://doi.org/10.3390/biomedicines10092239
Strijkova V, Todinova S, Andreeva T, Langari A, Bogdanova D, Zlatareva E, Kalaydzhiev N, Milanov I, Taneva SG. Platelets’ Nanomechanics and Morphology in Neurodegenerative Pathologies. Biomedicines. 2022; 10(9):2239. https://doi.org/10.3390/biomedicines10092239
Chicago/Turabian StyleStrijkova, Velichka, Svetla Todinova, Tonya Andreeva, Ariana Langari, Desislava Bogdanova, Elena Zlatareva, Nikolay Kalaydzhiev, Ivan Milanov, and Stefka G. Taneva. 2022. "Platelets’ Nanomechanics and Morphology in Neurodegenerative Pathologies" Biomedicines 10, no. 9: 2239. https://doi.org/10.3390/biomedicines10092239
APA StyleStrijkova, V., Todinova, S., Andreeva, T., Langari, A., Bogdanova, D., Zlatareva, E., Kalaydzhiev, N., Milanov, I., & Taneva, S. G. (2022). Platelets’ Nanomechanics and Morphology in Neurodegenerative Pathologies. Biomedicines, 10(9), 2239. https://doi.org/10.3390/biomedicines10092239







