Postoperative Radiotherapy of Prostate Cancer: Adjuvant versus Early Salvage
Abstract
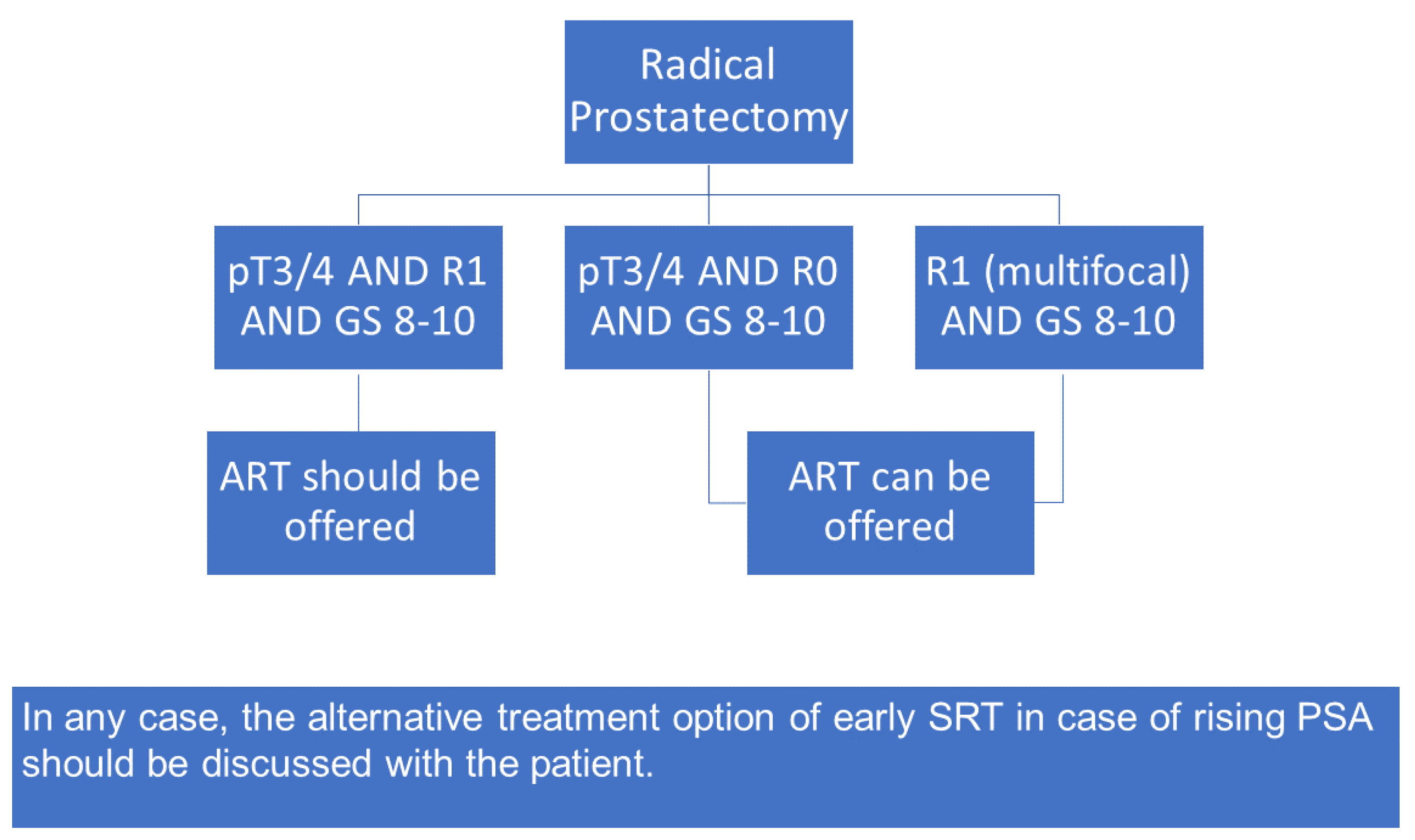
:1. Introduction
2. Methods
3. Results
4. Discussion
4.1. Follow-Up Time Not Sufficient to Conclude on Oncological Outcome
4.2. Immortal Time Bias
4.3. High-Risk Subgroup Underpowered
4.4. Impact of ADT on Trial Results
4.5. Toxicity Possibly Just Delayed
4.6. RT Technique (IMRT vs. 3DRT) Might Have Influenced Outcomes
4.7. Heterogeneity and Inconsistencies in Radiation Dose and Target Volume Definition among the Three RCTs in the Meta-Analysis
4.8. Radiation Dose and Fractionation Not Considered Evidence-Based Standard of Care
4.9. ART Was Performed within Less Than 6 Months after Surgery
4.10. Potential Inclusion of Patients with Persisting PSA after RP
4.11. Risk of Delayed Referral to SRT
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Mottet, N.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2021, 79, 243–262. [Google Scholar] [CrossRef] [PubMed]
- Wiegel, T.; Bartkowiak, D.; Bottke, D.; Bronner, C.; Steiner, U.; Siegmann, A.; Golz, R.; Störkel, S.; Willich, N.; Semjonow, A.; et al. Adjuvant radiotherapy versus wait-and-see after radical prostatectomy: 10-year follow-up of the ARO 96-02/AUO AP 09/95 trial. Eur. Urol. 2014, 66, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Bolla, M.; van Poppel, H.; Tombal, B.; Vekemans, K.; Da Pozzo, L.; de Reijke, T.M.; Verbaeys, A.; Bosset, J.F.; van Velthoven, R.; Colombel, M.; et al. Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: Long-term results of a randomised controlled trial (EORTC trial 22911). Lancet 2012, 380, 2018–2027. [Google Scholar] [CrossRef]
- Thompson, I.M.; Tangen, C.M.; Paradelo, J.; Lucia, M.S.; Miller, G.; Troyer, D.; Messing, E.; Forman, J.; Chin, J.; Swanson, G.; et al. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: Long-term followup of a randomized clinical trial. J. Urol. 2009, 181, 956–962. [Google Scholar] [CrossRef] [PubMed]
- Kneebone, A.; Fraser-Browne, C.; Duchesne, G.M.; Fisher, R.; Frydenberg, M.; Herschtal, A.; Williams, S.G.; Brown, C.; Delprado, W.; Haworth, A.; et al. Adjuvant radiotherapy versus early salvage radiotherapy following radical prostatectomy (TROG 08.03/ANZUP RAVES): A randomised, controlled, phase 3, non-inferiority trial. Lancet Oncol. 2020, 21, 1331–1340. [Google Scholar] [CrossRef]
- Sargos, P.; Chabaud, S.; Latorzeff, I.; Magné, N.; Benyoucef, A.; Supiot, S.; Pasquier, D.; Abdiche, M.S.; Gilliot, O.; Graff-Cailleaud, P.; et al. Adjuvant radiotherapy versus early salvage radiotherapy plus short-term androgen deprivation therapy in men with localised prostate cancer after radical prostatectomy (GETUG-AFU 17): A randomised, phase 3 trial. Lancet Oncol. 2020, 21, 1341–1352. [Google Scholar] [CrossRef]
- Parker, C.C.; Clarke, N.W.; Cook, A.D.; Kynaston, H.G.; Petersen, P.M.; Catton, C.; Cross, W.; Logue, J.; Parulekar, W.; Payne, H.; et al. Timing of radiotherapy after radical prostatectomy (RADICALS-RT): A randomised, controlled phase 3 trial. Lancet 2020, 396, 1413–1421. [Google Scholar] [CrossRef]
- Vale, C.L.; Fisher, D.; Kneebone, A.; Parker, C.; Pearse, M.; Richaud, P.; Sargos, P.; Sydes, M.R.; Brawley, C.; Brihoum, M.; et al. Adjuvant or early salvage radiotherapy for the treatment of localised and locally advanced prostate cancer: A prospectively planned systematic review and meta-analysis of aggregate data. Lancet 2020, 396, 1422–1431. [Google Scholar] [CrossRef]
- Hackman, G.; Taari, K.; Tammela, T.L.; Matikainen, M.; Kouri, M.; Joensuu, T.; Luukkaala, T.; Salonen, A.; Isotalo, T.; Petas, A.; et al. Randomised Trial of Adjuvant Radiotherapy Following Radical Prostatectomy Versus Radical Prostatectomy Alone in Prostate Cancer Patients with Positive Margins or Extracapsular Extension. Eur. Urol. 2019, 76, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Tilki, D.; D’Amico, A.V. Timing of radiotherapy after radical prostatectomy. Lancet 2020, 396, 1374–1375. [Google Scholar] [CrossRef]
- Tilki, D.; Chen, M.H.; Wu, J.; Huland, H.; Graefen, M.; Wiegel, T.; Böhmer, D.; Mohamad, O.; Cowan, J.E.; Feng, F.Y.; et al. Adjuvant Versus Early Salvage Radiation Therapy for Men at High Risk for Recurrence Following Radical Prostatectomy for Prostate Cancer and the Risk of Death. J. Clin. Oncol. 2021, 39, 2284–2293. [Google Scholar] [CrossRef] [PubMed]
- Bottke, D.; Golz, R.; Störkel, S.; Hinke, A.; Siegmann, A.; Hertle, L.; Miller, K.; Hinkelbein, W.; Wiegel, T. Phase 3 study of adjuvant radiotherapy versus wait and see in pT3 prostate cancer: Impact of pathology review on analysis. Eur. Urol. 2013, 64, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Ghadjar, P.; Höcht, S.; Wiegel, T. Postoperative radiotherapy in prostate cancer. Lancet 2021, 397, 1623. [Google Scholar] [CrossRef]
- Chen, R.C.; Choudhury, A. Adjuvant Versus Early Salvage Radiation Therapy After Radical Prostatectomy for Men with Adverse Pathologic Features-The Debate Continues. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 839–843. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.R.; Tom, M.C.; Agrawal, S.; Efstathiou, J.A.; Michalski, J.M.; Abramowitz, M.C.; Pollack, A.; Spratt, D.E.; Hearn, J.W.D.; Stephans, K.L.; et al. Integrating Prostate-specific Antigen Kinetics into Contemporary Predictive Nomograms of Salvage Radiotherapy After Radical Prostatectomy. Eur. Urol. Oncol. 2021, 5, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Bartkowiak, D.; Thamm, R.; Bottke, D.; Siegmann, A.; Böhmer, D.; Budach, V.; Wiegel, T. Prostate-specific antigen after salvage radiotherapy for postprostatectomy biochemical recurrence predicts long-term outcome including overall survival. Acta Oncol. 2018, 57, 362–367. [Google Scholar] [CrossRef] [PubMed]

| RADICALS-RT | GETUG-AFU 17 | RAVES | ARTISTIC Meta-Analysis | |
|---|---|---|---|---|
| Trial design | Superiority (two-sided, 80% power to detect increase from 90% to 95% 10-year FFDM, α = 5%) | Superiority of ART (one-sided, 80% power to detect increase of 10% 5-year EFS for ART over SRT, α = 5%) | Non-inferiority of SRT (one-sided, 80% power to detect 5-year FFbP difference of <10% for SRT, α = 5%) | Superiority (two-sided, 90% power to detect a 5% difference in 5-year EFS, 99% power to detect 10% difference) |
| Primary Outcome | FFDM | EFS | FFbP | EFS |
| Accrual period | 2007–2016 | 2008–2016 | 2009–2015 | |
| Planned accrual (n) | 2600 | 718 | 470 | (>120 events per group) |
| Actual inclusion (n, %) | 1396 (53.7%) | 424 (59.1%) | 333 (70.9%) | 2153 (events: 138 SRT, 126 ART) |
| Main inclusion criteria | One or more of: pT3/pT4, R+, Gleason Score 7–10, PSA > 10 ng/mL (including pT2) | pT3/pT4a and R+ | pT3/4 or R+ (including pT2) | |
| RT dose for ART and eSRT | 66 Gy in 33 Fx both groups (61%) or 52.5 Gy in 20 Fx (29%) | 66 Gy in 33 Fx both groups (lymph nodes median 46 Gy) | 64 Gy in 32 Fx both groups | |
| RT field | Prostate bed, additionally pelvic lymph nodes in 7% of ART arm and 3 % of SRT arm | Prostate bed, additionally pelvic lymph nodes in 18% of ART arm and 24 % of SRT arm | Prostate bed | |
| ADT | Randomized to 6 or 24 months of LHRH- analogue or Bicalutamid (RADICALS-HT double randomization) or as clinically indicated for 24% of ART arm and 27% of SRT arm. | All patients: 6 months of triptoreline | none | |
| ART timing | ≤6 months after RP | ≤6 months after RP | ≤6 months after RP | |
| Trigger for eSRT | PSA > 0.1 ng/mL and rising or 3 × consecutive rising below 0.1 ng/mL | PSA > 0.2 ng/mL | PSA > 0.2 ng/mL | |
| eSRT timing | ≤2 months of trigger PSA | ASAP after trigger PSA, before PSA of 1 ng/mL | ≤4 months of trigger PSA | |
| Number of patients who received SRT | 228/699 (32.6%) | 115/212 (54.2%) | 84/167 (50.3%) | 421/1078 (39.1%) * |
| Median follow-up (months) | 60 | 75 | 78 | 60–78 months |
| Main result (ART vs. eSRT) | 5-year FFbP: 85% vs. 88% | 5-year EFS: 92% vs. 90% | 5-year FFbP: 86% vs. 87%. | 5-year EFS: 89% vs. 88% |
| GU toxicity (ART vs. eSRT) | G3+: 9% (54/599) vs. 5.1% (32/621) # | G2+: 59% (125/212) vs. 22% (46/212) | G2+: 70% (116/166) vs. 54% (90/167) | |
| GI toxicity (ART vs. eSRT) | G3+: 2% (12/599) vs. 0.5% (3/621) ## | G2+: 8% (17/212) vs. 5% (11/212) | G2+: 14% (24/116) vs. 10% (16/167) | |
| % of IMRT (ART vs. eSRT) | 30% vs. 47% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wegener, D.; Aebersold, D.M.; Grimm, M.-O.; Hammerer, P.; Froehner, M.; Graefen, M.; Boehmer, D.; Zips, D.; Wiegel, T. Postoperative Radiotherapy of Prostate Cancer: Adjuvant versus Early Salvage. Biomedicines 2022, 10, 2256. https://doi.org/10.3390/biomedicines10092256
Wegener D, Aebersold DM, Grimm M-O, Hammerer P, Froehner M, Graefen M, Boehmer D, Zips D, Wiegel T. Postoperative Radiotherapy of Prostate Cancer: Adjuvant versus Early Salvage. Biomedicines. 2022; 10(9):2256. https://doi.org/10.3390/biomedicines10092256
Chicago/Turabian StyleWegener, Daniel, Daniel M. Aebersold, Marc-Oliver Grimm, Peter Hammerer, Michael Froehner, Markus Graefen, Dirk Boehmer, Daniel Zips, and Thomas Wiegel. 2022. "Postoperative Radiotherapy of Prostate Cancer: Adjuvant versus Early Salvage" Biomedicines 10, no. 9: 2256. https://doi.org/10.3390/biomedicines10092256
APA StyleWegener, D., Aebersold, D. M., Grimm, M.-O., Hammerer, P., Froehner, M., Graefen, M., Boehmer, D., Zips, D., & Wiegel, T. (2022). Postoperative Radiotherapy of Prostate Cancer: Adjuvant versus Early Salvage. Biomedicines, 10(9), 2256. https://doi.org/10.3390/biomedicines10092256






