Association of Hypernatremia with Immune Profiles and Clinical Outcomes in Adult Intensive Care Unit Patients with Sepsis
Abstract
:1. Introduction
2. Materials and Methods
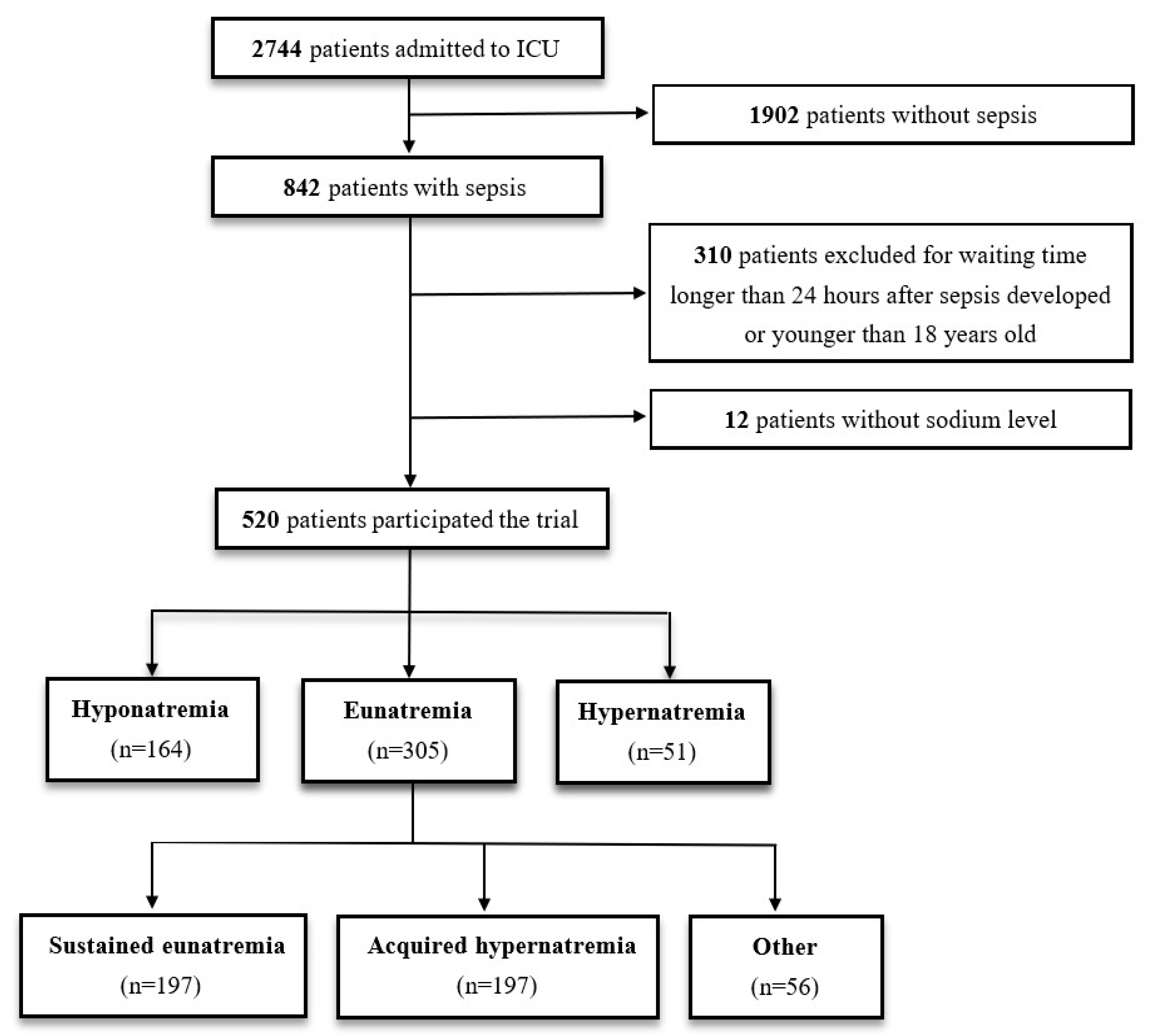
2.1. Patient Enrollment
2.2. Study Design
2.3. Definitions
2.4. PBMC Preparation
2.5. HLA-DR-Expressing Monocyte Percentage Measurement through Flow Cytometry
2.6. Cell Culture and LPS-Stimulated Cytokine Response
2.7. Milliplex Assay
2.8. Statistical Analyses
3. Results
3.1. Patient Characteristics
3.2. Baseline Clinical Parameters and Immune Profiles of Patients with Sepsis
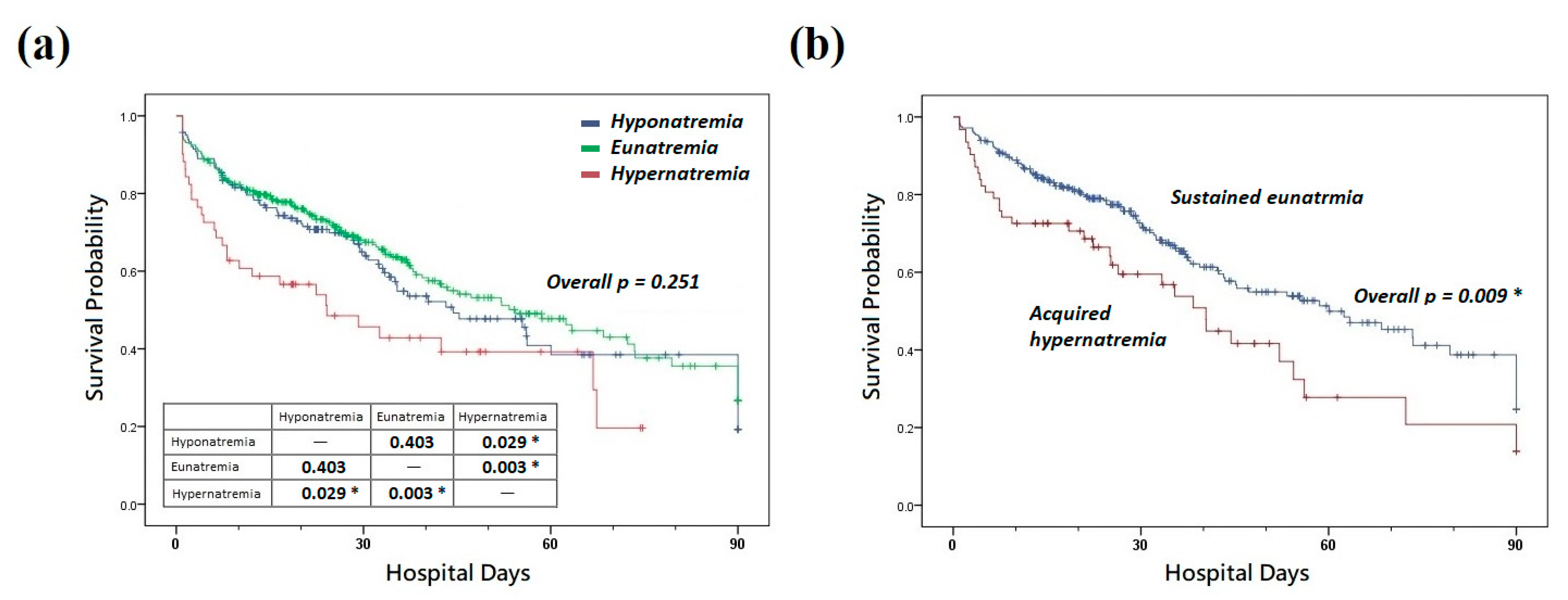
3.3. Effects of Dysnatremia on Clinical Outcomes
3.4. LPS-Stimulated Cytokine Release and HLA-DR-Expressing Monocyte Percentage
3.5. Validation Using a Validation Cohort
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salahudeen, A.K.; Doshi, S.M.; Shah, P. The frequency, cost, and clinical outcomes of hypernatremia in patients hospitalized to a comprehensive cancer center. Support. Care Cancer 2013, 21, 1871–1878. [Google Scholar] [CrossRef]
- Lansink-Hartgring, A.O.; Hessels, L.; Weigel, J.; De Smet, A.M.G.A.; Gommers, D.; Panday, P.V.N.; Hoorn, E.J.; Nijsten, M.W. Long-term changes in dysnatremia incidence in the ICU: A shift from hyponatremia to hypernatremia. Ann. Intensive Care 2016, 6, 22. [Google Scholar] [CrossRef]
- Stelfox, H.T.; Ahmed, S.B.; Khandwala, F.; Zygun, D.; Shahpori, R.; Laupland, K. The epidemiology of intensive care unit-acquired hyponatraemia and hypernatraemia in medical-surgical intensive care units. Crit. Care 2008, 12, R162. [Google Scholar] [CrossRef]
- Sterns, R.H. Disorders of plasma sodium—Causes, consequences, and correction. N. Engl. J. Med. 2015, 372, 55–65. [Google Scholar] [CrossRef]
- Van De Louw, A.; Shaffer, C.; Schaefer, E. Early intensive care unit-acquired hypernatremia in severe sepsis patients receiving 0.9% saline fluid resuscitation. Acta Anaesthesiol. Scand. 2014, 58, 1007–1014. [Google Scholar] [CrossRef]
- Shirazy, M.; Omar, I.; Abduljabbar, D.; Bousselmi, K.; Alkhaja, M.; Chaari, A.; Kauts, V.; Hakim, K. Prevalence and Prognostic Impact of Hypernatremia in Sepsis and Septic Shock Patients in the Intensive Care Unit: A Single Centre Experience. J. Crit. Care Med. 2020, 6, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.-B.; Hu, X.-X.; Huang, X.-F.; Liu, K.-Q.; Yu, C.-B.; Wang, X.-M.; Ke, L. Risk Factors and Outcomes in Patients With Hypernatremia and Sepsis. Am. J. Med. Sci. 2016, 351, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Arens, C.; Bajwa, S.A.; Koch, C.; Siegler, B.H.; Schneck, E.; Hecker, A.; Weiterer, S.; Lichtenstern, C.; Weigand, M.A.; Uhle, F. Sepsis-induced long-term immune paralysis—Results of a descriptive, explorative study. Crit. Care 2016, 20, 93. [Google Scholar] [CrossRef] [PubMed]
- Sundar, K.M.; Sires, M. Sepsis induced immunosuppression: Implications for secondary infections and complications. Indian J. Crit. Care Med. 2013, 17, 162–169. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Monneret, G.; Payen, D. Sepsis-induced immunosuppression: From cellular dysfunctions to immunotherapy. Nat. Rev. Immunol. 2013, 13, 862–874. [Google Scholar] [CrossRef]
- Abolhassani, M.; Wertz, X.; Pooya, M.; Chaumet-Riffaud, P.; Guais, A.; Schwartz, L. Hyperosmolarity causes inflammation through the methylation of protein phosphatase 2A. Inflamm. Res. 2008, 57, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Alberdi, M.; Iglesias, M.; Tejedor, S.; Merino, R.; López-Rodríguez, C.; Aramburu, J. Context-dependent regulation of Th17-associated genes and IFNγ expression by the transcription factor NFAT5. Immunol. Cell Biol. 2017, 95, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Rugg, C.; Ströhle, M.; Treml, B.; Bachler, M.; Schmid, S.; Kreutziger, J. ICU-Acquired Hypernatremia Is Associated with Persistent Inflammation, Immunosuppression and Catabolism Syndrome. J. Clin. Med. 2020, 9, 3017. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.-F.; Douglas, I.S.; Chen, Y.-M.; Lin, C.-Y.; Kao, H.-C.; Fang, Y.-T.; Huang, C.-H.; Chang, Y.-T.; Huang, K.-T.; Wang, Y.-H.; et al. Development and validation of immune dysfunction score to predict 28-day mortality of sepsis patients. PLoS ONE 2017, 12, e0187088. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Huang, K.-T.; Chen, Y.-M.; Wang, C.-C.; Wang, Y.-H.; Tseng, C.-C.; Lin, M.-C.; Fang, W.-F. Ventilator Dependence Risk Score for the Prediction of Prolonged Mechanical Ventilation in Patients Who Survive Sepsis/Septic Shock with Respiratory Failure. Sci. Rep. 2018, 8, 5650. [Google Scholar] [CrossRef]
- Hung, K.-Y.; Chen, Y.-M.; Wang, C.-C.; Wang, Y.-H.; Lin, C.-Y.; Chang, Y.-T.; Huang, K.-T.; Lin, M.-C.; Fang, W.-F. Insufficient Nutrition and Mortality Risk in Septic Patients Admitted to ICU with a Focus on Immune Dysfunction. Nutrients 2019, 11, 367. [Google Scholar] [CrossRef]
- Fang, W.-F.; Huang, C.-H.; Chen, Y.-M.; Hung, K.-Y.; Chang, Y.-C.; Lin, C.-Y.; Fang, Y.-T.; Chen, H.-C.; Huang, K.-T.; Chang, H.-C.; et al. Application of dynamic pulse pressure and vasopressor tools for predicting outcomes in patients with sepsis in intensive care units. J. Crit. Care 2019, 52, 156–162. [Google Scholar] [CrossRef]
- Fang, W.-F.; Chen, Y.-M.; Wang, Y.-H.; Huang, C.-H.; Hung, K.-Y.; Fang, Y.-T.; Chang, Y.-C.; Lin, C.-Y.; Chang, Y.-T.; Chen, H.-C.; et al. Incorporation of dynamic segmented neutrophil-to-monocyte ratio with leukocyte count for sepsis risk stratification. Sci. Rep. 2019, 9, 19756. [Google Scholar] [CrossRef]
- Tsai, Y.-H.; Lin, C.-Y.; Chen, Y.-M.; Chang, Y.-P.; Hung, K.-Y.; Chang, Y.-C.; Chen, H.-C.; Huang, K.-T.; Chen, Y.-C.; Wang, Y.-H.; et al. Impact of Body Mass Index on the Survival of Patients with Sepsis with Different Modified NUTRIC Scores. Nutrients 2021, 13, 1873. [Google Scholar]
- Hsu, C.-Y.; Tsai, Y.-H.; Lin, C.-Y.; Chang, Y.-C.; Chen, H.-C.; Chang, Y.-P.; Chen, Y.-M.; Huang, K.-T.; Wang, Y.-H.; Wang, C.-C.; et al. Application of a 72 h National Early Warning Score and Incorporation with Sequential Organ Failure Assessment for Predicting Sepsis Outcomes and Risk Stratification in an Intensive Care Unit: A Derivation and Validation Cohort Study. J. Pers. Med. 2021, 11, 910. [Google Scholar] [CrossRef]
- Hung, K.-Y.; Tsai, Y.-H.; Lin, C.-Y.; Chang, Y.-C.; Wang, Y.-H.; Lin, M.-C.; Fang, W.-F. Application of Peak Glucose Range and Diabetes Status in Mortality Risk Stratification in Critically Ill Patients with Sepsis. Diagnostics 2021, 11, 1798. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Wang, Y.-H.; Chen, Y.-M.; Hung, K.-Y.; Chang, Y.-C.; Fang, Y.-T.; Chen, H.-C.; Huang, K.-T.; Chang, H.-C.; Chen, Y.-C.; et al. Dynamic monitoring of kidney injury status over 3 days in the intensive care unit as a sepsis phenotype associated with hospital mortality and hyperinflammation. Biomed. J. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-C.; Fang, Y.-T.; Chen, H.-C.; Lin, C.-Y.; Chang, Y.-P.; Tsai, Y.-H.; Chen, Y.-M.; Huang, K.-T.; Chang, H.-C.; Wang, C.-C.; et al. The Survival of Septic Patients with Compensated Liver Cirrhosis Is Not Inferior to That of Septic Patients without Liver Cirrhosis: A Propensity Score Matching Analysis. J. Clin. Med. 2022, 11, 1629. [Google Scholar] [CrossRef]
- Fang, W.-F.; Fang, Y.-T.; Huang, C.-H.; Chen, Y.-M.; Chang, Y.-C.; Lin, C.-Y.; Hung, K.-Y.; Chen, H.-C.; Huang, K.-T.; Chang, H.-C.; et al. Risk factors and associated outcomes of ventilator-associated events developed in 28 days among sepsis patients admitted to intensive care unit. Sci. Rep. 2020, 10, 12702. [Google Scholar] [CrossRef]
- Levy, M.M.; Fink, M.P.; Marshall, J.C.; Abraham, E.; Angus, D.; Cook, D.; Cohen, J.; Opal, S.M.; Vincent, J.-L.; Ramsay, G. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit. Care Med. 2003, 31, 1250–1256. [Google Scholar] [CrossRef]
- Shankar-Hari, M.; Phillips, G.S.; Levy, M.L.; Seymour, C.W.; Liu, V.X.; Deutschman, C.S.; Angus, D.C.; Rubenfeld, G.D.; Singer, M.; for the Sepsis Definitions Task Force. Developing a New Definition and Assessing New Clinical Criteria for Septic Shock: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 775–787. [Google Scholar] [CrossRef]
- Bratusch-Marrain, P.R.; DeFronzo, R.A. Impairment of insulin-mediated glucose metabolism by hyperosmolality in man. Diabetes 1983, 32, 1028–1034. [Google Scholar] [CrossRef]
- Druml, W.; Kleinberger, G.; Lenz, K.; Laggner, A.; Schneeweiss, B. Fructose-induced hyperlactemia in hyperosmolar syndromes. Klin. Wochenschr. 1986, 64, 615–618. [Google Scholar] [CrossRef]
- Kozeny, G.; Murdock, D.; Euler, D.; Hano, J.; Scanlon, P.; Bansal, V.; Vertuno, L. In vivo effects of acute changes in osmolality and sodium concentration on myocardial contractility. Am. Heart J. 1985, 109, 290–296. [Google Scholar] [CrossRef]
- Adrogue, H.J.; Madias, N.E. Hypernatremia. N. Engl. J. Med. 2000, 342, 1493–1499. [Google Scholar] [CrossRef]
- Funk, G.-C.; Lindner, G.; Druml, W.; Metnitz, B.; Schwarz, C.; Bauer, P.; Metnitz, P.G.H. Incidence and prognosis of dysnatremias present on ICU admission. Intensive Care Med. 2010, 36, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Polderman, K.H.; Schreuder, W.O.; van Schijndel, R.J.S.; Thijs, L.G. Hypernatremia in the intensive care unit: An indicator of quality of care? Crit. Care Med. 1999, 27, 1105–1108. [Google Scholar] [CrossRef] [PubMed]
- van Ijzendoorn, M.C.O.; Buter, H.; Kingma, W.P.; Navis, G.J.; Boerma, E.C. The Development of Intensive Care Unit Acquired Hypernatremia Is Not Explained by Sodium Overload or Water Deficit: A Retrospective Cohort Study on Water Balance and Sodium Handling. Crit. Care Res. Pract. 2016, 2016, 9571583. [Google Scholar] [CrossRef] [PubMed]
- Chao, W.-C.; Tseng, C.-H.; Chien, Y.-C.; Sheu, C.-C.; Tsai, M.-J.; Fang, W.-F.; Chen, Y.-M.; Kao, K.-C.; Hu, H.-C.; Perng, W.-C.; et al. Association of day 4 cumulative fluid balance with mortality in critically ill patients with influenza: A multicenter retrospective cohort study in Taiwan. PLoS ONE 2018, 13, e0190952. [Google Scholar]
- Bendall, L.J.; Bradstock, K.F. G-CSF: From granulopoietic stimulant to bone marrow stem cell mobilizing agent. Cytokine Growth Factor Rev. 2014, 25, 355–367. [Google Scholar] [CrossRef]
- Jantsch, J.; Schatz, V.; Friedrich, D.; Schröder, A.; Kopp, C.; Siegert, I.; Maronna, A.; Wendelborn, D.; Linz, P.; Binger, K.J.; et al. Cutaneous Na+ storage strengthens the antimicrobial barrier function of the skin and boosts macrophage-driven host defense. Cell Metab. 2015, 21, 493–501. [Google Scholar] [CrossRef]
- Brownlie, R.; Zamoyska, R. T cell receptor signalling networks: Branched, diversified and bounded. Nat. Rev. Immunol. 2013, 13, 257–269. [Google Scholar] [CrossRef]
- Junger, W.G.; Liu, F.C.; Loomis, W.H.; Hoyt, D.B. Hypertonic saline enhances cellular immune function. Circ. Shock 1994, 42, 190–196. [Google Scholar]
- Wu, C.; Yosef, N.; Thalhamer, T.; Zhu, C.; Xiao, S.; Kishi, Y.; Regev, A.; Kuchroo, V.K. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature 2013, 496, 513–517. [Google Scholar] [CrossRef]
- Kleinewietfeld, M.; Manzel, A.; Titze, J.; Kvakan, H.; Yosef, N.; Linker, R.A.; Muller, D.N.; Hafler, D.A. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature 2013, 496, 518–522. [Google Scholar] [CrossRef]
- Cvetkovic, L.; Perisic, S.; Titze, J.; Jäck, H.-M.; Schuh, W. The Impact of Hyperosmolality on Activation and Differentiation of B Lymphoid Cells. Front. Immunol. 2019, 10, 828. [Google Scholar] [CrossRef] [PubMed]
- Fahlman, C.; Jacobsen, F.W.; Veiby, O.P.; Mcniece, I.K.; Blomhoff, H.K.; Jacobsen, S.E. Tumor necrosis factor-alpha (TNF-alpha) potently enhances in vitro macrophage production from primitive murine hematopoietic progenitor cells in combination with stem cell factor and interleukin-7: Novel stimulatory role of p55 TNF receptors. Blood 1994, 84, 1528–1533. [Google Scholar] [CrossRef] [PubMed]
- Witsell, A.L.; Schook, L.B. Tumor necrosis factor alpha is an autocrine growth regulator during macrophage differentiation. Proc. Natl. Acad. Sci. USA 1992, 89, 4754–4758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otto, N.M.; Schindler, R.; Lun, A.; Boenisch, O.; Frei, U.; Oppert, M. Hyperosmotic stress enhances cytokine production and decreases phagocytosis in vitro. Crit. Care 2008, 12, R107. [Google Scholar] [CrossRef]
- Schwartz, L.; Abolhassani, M.; Pooya, M.; Steyaert, J.-M.; Wertz, X.; Israël, M.; Guais, A.; Chaumet-Riffaud, P. Hyperosmotic stress contributes to mouse colonic inflammation through the methylation of protein phosphatase 2A. Am. J. Physiol. Gastrointest Liver Physiol. 2008, 295, G934–G941. [Google Scholar] [CrossRef]
- Wenstedt, E.F.; Verberk, S.G.; Kroon, J.; Neele, A.E.; Baardman, J.; Claessen, N.; Pasaoglu, T.; Rademaker, E.; Schrooten, E.M.; Wouda, R.D.; et al. Salt increases monocyte CCR2 expression and inflammatory responses in humans. JCI Insight 2019, 4, e130508. [Google Scholar] [CrossRef]
- Rochwerg, B.; Alhazzani, W.; Sindi, A.; Heels-Ansdell, D.; Thabane, L.; Fox-Robichaud, A.; Mbuagbaw, L.; Szczeklik, W.; Alshamsi, F.; Altayyar, S. Fluid resuscitation in sepsis: A systematic review and network meta-analysis. Ann. Intern. Med. 2014, 161, 347–355. [Google Scholar] [CrossRef]
- Alshayeb, H.M.; Showkat, A.; Babar, F.; Mangold, T.; Wall, B.M. Severe hypernatremia correction rate and mortality in hospitalized patients. Am. J. Med. Sci. 2011, 341, 356–360. [Google Scholar] [CrossRef]
- Quinn, J.W.; Sewell, K.; Simmons, D.E. Recommendations for active correction of hypernatremia in volume-resuscitated shock or sepsis patients should be taken with a grain of salt: A systematic review. SAGE Open Med. 2018, 6, 2050312118762043. [Google Scholar] [CrossRef]
- Chi, W.C.; Patel, S.; Cheung, N.W. Admission sodium levels and hospital outcomes. Intern. Med. J. 2021, 51, 93–98. [Google Scholar] [CrossRef]
- Akirov, A.; Diker-Cohen, T.; Steinmetz, T.; Amitai, O.; Shimon, I. Sodium levels on admission are associated with mortality risk in hospitalized patients. Eur. J. Intern. Med. 2017, 46, 25–29. [Google Scholar] [CrossRef] [PubMed]

| Clinical Parameters | All (n = 520) | Hyponatremia (n = 164) | Eunatremia (n = 305) | Hypernatremia (n = 51) | p-Value |
|---|---|---|---|---|---|
| Age, mean (SD) | 66.6 (15.3) | 66.2 (14.9) | 65.9 (15.7) | 71.8 (12.9) | 0.036 * |
| BMI, mean (SD) | 22.8 (5.0) | 22.8 (4.8) | 22.8 (5.1) | 22.4 (4.5) | 0.912 |
| Gender (male), n (%) | 212 (40.8) | 77 (47.0) | 112 (36.7) | 23 (45.1) | 0.080 |
| Diabetes mellitus, n (%) | 232 (44.6) | 81 (49.4) | 128 (42.0) | 23 (45.1) | 0.304 |
| Hypertension, n (%) | 276 (53.1) | 87 (53.0) | 160 (52.5) | 29 (56.9) | 0.844 |
| CAD, n (%) | 130 (25.0) | 38 (23.2) | 78 (25.6) | 14 (27.5) | 0.775 |
| COPD, n (%) | 62 (11.9) | 14 (8.5) | 39 (12.8) | 9 (17.6) | 0.165 |
| Cirrhosis, n (%) | 40 (7.7) | 8 (4.9) | 28 (9.2) | 4 (7.8) | 0.249 |
| Stroke, n (%) | 109 (21.0) | 31 (18.9) | 64 (21.0) | 14 (27.5) | 0.424 |
| CKD, n (%) | 138 (26.5) | 41 (25.0) | 85 (27.9) | 12 (23.5) | 0.700 |
| APCHE II score, mean (SD) | 25.0 (8.8) | 24.3 (8.6) | 24.8 (8.8) | 27.6 (9.1) | 0.088 |
| All (n = 520) | Hyponatremia (n = 164) | Eunatremia (n = 305) | Hypernatremia (n = 51) | p-Value | ||
|---|---|---|---|---|---|---|
| 7 day mortality, n (%) | 77 (14.8) | 22 (13.4) | 41 (13.4) | 14 (27.5) | 0.030 * | |
| 14 day mortality, n (%) | 115 (22.1) | 34 (20.7) | 61 (20.0) | 20 (39.2) | 0.009 * | |
| 28 day mortality, n (%) | 153 (29.4) | 49 (29.9) | 80 (26.2) | 24 (47.1) | 0.010 * | |
| Hospital mortality, n (%) | 225 (43.3) | 74 (45.1) | 121 (39.7) | 30 (58.8) | 0.033 * | |
| All (n = 249) | Sustained Eunatremia (n = 197) | Acquired Hypernatremia (n = 52) | p-Value | |||
| 7 day mortality, n (%) | 25 (10.0) | 15 (7.6) | 10 (19.2) | 0.019 * | ||
| 14 day mortality, n (%) | 43 (17.3) | 29 (14.7) | 14 (26.9) | 0.042 * | ||
| 28 day mortality, n (%) | 60 (24.1) | 41 (20.8) | 19 (36.5) | 0.028 * | ||
| Hospital mortality, n (%) | 99 (39.8) | 72 (36.5) | 27 (51.9) | 0.056 | ||
| Parameter | Odds Ratio | 95% Confidence Interval | p-Value |
|---|---|---|---|
| Age | 1.001 | 0.986–1.016 | 0.882 |
| BMI | 0.957 | 0.916–1.000 | 0.048 * |
| Gender (male) | 0.956 | 0.634–1.441 | 0.830 |
| Diabetes mellitus | 0.814 | 0.533–1.242 | 0.340 |
| Hypertension | 0.823 | 0.524–1.293 | 0.399 |
| CAD | 1.186 | 0.725–1.941 | 0.496 |
| COPD | 0.996 | 0.543–1.828 | 0.991 |
| Cirrhosis | 1.441 | 0.680–3.053 | 0.340 |
| Stroke | 0.686 | 0.405–1.162 | 0.161 |
| CKD | 1.227 | 0.771–1.954 | 0.388 |
| APCHE II score | 1.037 | 1.014–1.060 | 0.002 * |
| Hypernatremia | 2.300 | 1.179–4.487 | 0.015 * |
| All | Hyponatremia | Eunatremia | Hypernatremia | p-Value | ||
|---|---|---|---|---|---|---|
| Day 1 | (n = 128) | (n = 42) | (n = 75) | (n = 11) | ||
| G-CSF | 2.4 (4.8) | 2.4 (5.6) | 2.6 (4.7) | 1.0 (2.6) | 0.020 * | |
| TNF-α | 2.6 (6.0) | 2.1 (4.8) | 2.8 (28.2) | 1.9 (3.1) | 0.010 * | |
| IL-6 | 3.4 (18.8) | 2.8 (6.6) | 3.9 (62.4) | 2.8 (2.6) | 0.071 | |
| IL-10 | 1.7 (1.0) | 1.7 (1.1) | 1.6 (1.2) | 1.4 (0.9) | 0.758 | |
| HLA-DR expression, % | 92.2 (15.0) | 95.5 (8.2) | 90.0 (18.9) | 87.3 (35.4) | 0.025 * | |
| Day 3 | (n = 115) | (n = 39) | (n = 67) | (n = 9) | ||
| G-CSF | 2.4 (4.4) | 2.3 (4.9) | 2.6 (3.9) | 1.0 (3.4) | 0.899 | |
| TNF-α | 2.3 (4.5) | 2.0 (4.5) | 2.7 (28.2) | 1.9 (2.4) | 0.724 | |
| IL-6 | 3.2 (12.5) | 2.8 (5.8) | 3.6 (62.4) | 2.8 (2.4) | 0.729 | |
| IL-10 | 1.7 (0.9) | 1.7 (1.0) | 1.6 (1.0) | 1.4 (0.8) | 0.910 | |
| HLA-DR expression, % | 92.4 (16.0) | 95.6 (7.9) | 90.0 (19.4) | 87.3 (35.8) | 0.336 | |
| All | Sustained Eunatremia | Acquired Hypernatremia | p-Value | |||
| Day 1 | (n = 60) | (n = 49) | (n = 11) | |||
| G-CSF | 2.6 (6.0) | 2.4 (3.8) | 4.7 (21.6) | 0.184 | ||
| TNF-α | 2.8 (51.5) | 2.6 (28.4) | 16.8 (95.4) | 0.248 | ||
| IL-6 | 4.3 (83.8) | 3.7 (81.3) | 59.2 (103.4) | 0.344 | ||
| IL-10 | 1.6 (1.2) | 1.6 (0.9) | 2.2 (5.9) | 0.240 | ||
| HLA-DR expression, % | 89.9 (20.9) | 90.5 (20.2) | 81.5 (20.3) | 0.136 | ||
| Day 3 | (n = 58) | (n = 47) | (n = 11) | |||
| G-CSF | 2.6 (4.8) | 2.3 (3.8) | 4.7 (21.6) | 0.049 * | ||
| TNF-α | 2.7 (4.4) | 2.6 (28.4) | 16.8 (95.4) | 0.009 * | ||
| IL-6 | 3.8 (78.7) | 3.6 (76.3) | 59.2 (103.4) | 0.077 | ||
| IL-10 | 1.6 (1.1) | 1.5 (0.8) | 2.2 (5.9) | 0.293 | ||
| HLA-DR expression, % | 90.0 (21.3) | 90.8 (20.8) | 81.5 (20.3) | 0.413 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-Y.; Chen, Y.-M.; Tsai, Y.-H.; Hung, K.-Y.; Fang, Y.-T.; Chang, Y.-P.; Tsai, M.-Y.; Wu, H.-F.; Lin, M.-C.; Fang, W.-F. Association of Hypernatremia with Immune Profiles and Clinical Outcomes in Adult Intensive Care Unit Patients with Sepsis. Biomedicines 2022, 10, 2285. https://doi.org/10.3390/biomedicines10092285
Lin C-Y, Chen Y-M, Tsai Y-H, Hung K-Y, Fang Y-T, Chang Y-P, Tsai M-Y, Wu H-F, Lin M-C, Fang W-F. Association of Hypernatremia with Immune Profiles and Clinical Outcomes in Adult Intensive Care Unit Patients with Sepsis. Biomedicines. 2022; 10(9):2285. https://doi.org/10.3390/biomedicines10092285
Chicago/Turabian StyleLin, Chiung-Yu, Yu-Mu Chen, Yi-Hsuan Tsai, Kai-Yin Hung, Ying-Tang Fang, Yu-Ping Chang, Meng-Yun Tsai, Hsuan-Feng Wu, Meng-Chih Lin, and Wen-Feng Fang. 2022. "Association of Hypernatremia with Immune Profiles and Clinical Outcomes in Adult Intensive Care Unit Patients with Sepsis" Biomedicines 10, no. 9: 2285. https://doi.org/10.3390/biomedicines10092285
APA StyleLin, C.-Y., Chen, Y.-M., Tsai, Y.-H., Hung, K.-Y., Fang, Y.-T., Chang, Y.-P., Tsai, M.-Y., Wu, H.-F., Lin, M.-C., & Fang, W.-F. (2022). Association of Hypernatremia with Immune Profiles and Clinical Outcomes in Adult Intensive Care Unit Patients with Sepsis. Biomedicines, 10(9), 2285. https://doi.org/10.3390/biomedicines10092285







