Laminin-5, Fibronectin, and Type IV Collagen as Potential Biomarkers of Brain Glioma Malignancy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Biological Material
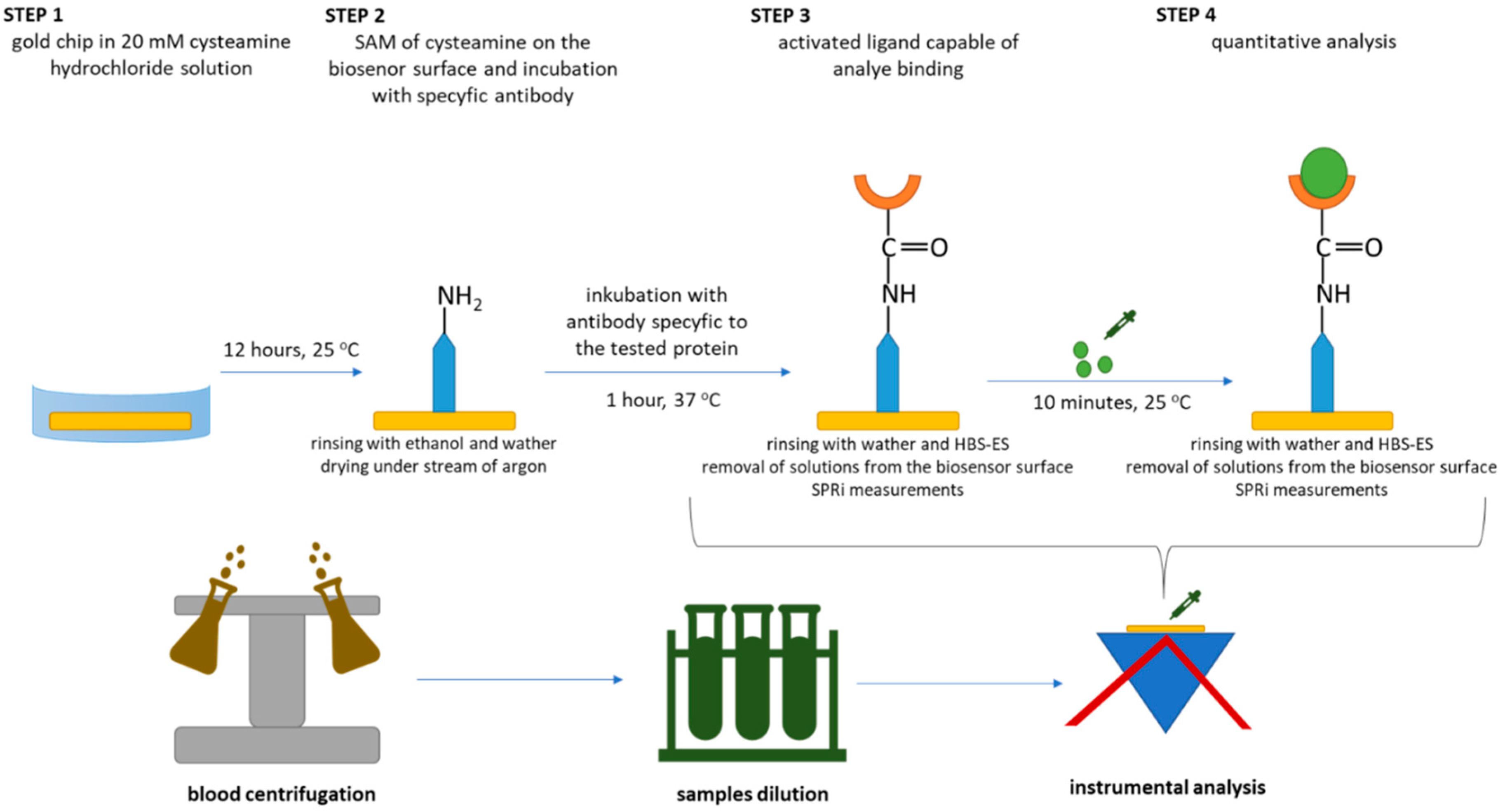
2.3. Procedure for Quantifying LN-5, FN, COL IV
2.4. SPRi Measurements
2.5. Methods Calibration and Quantitative Determinations
3. Results
3.1. Statistical Analysis
3.2. ROC Analysis
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

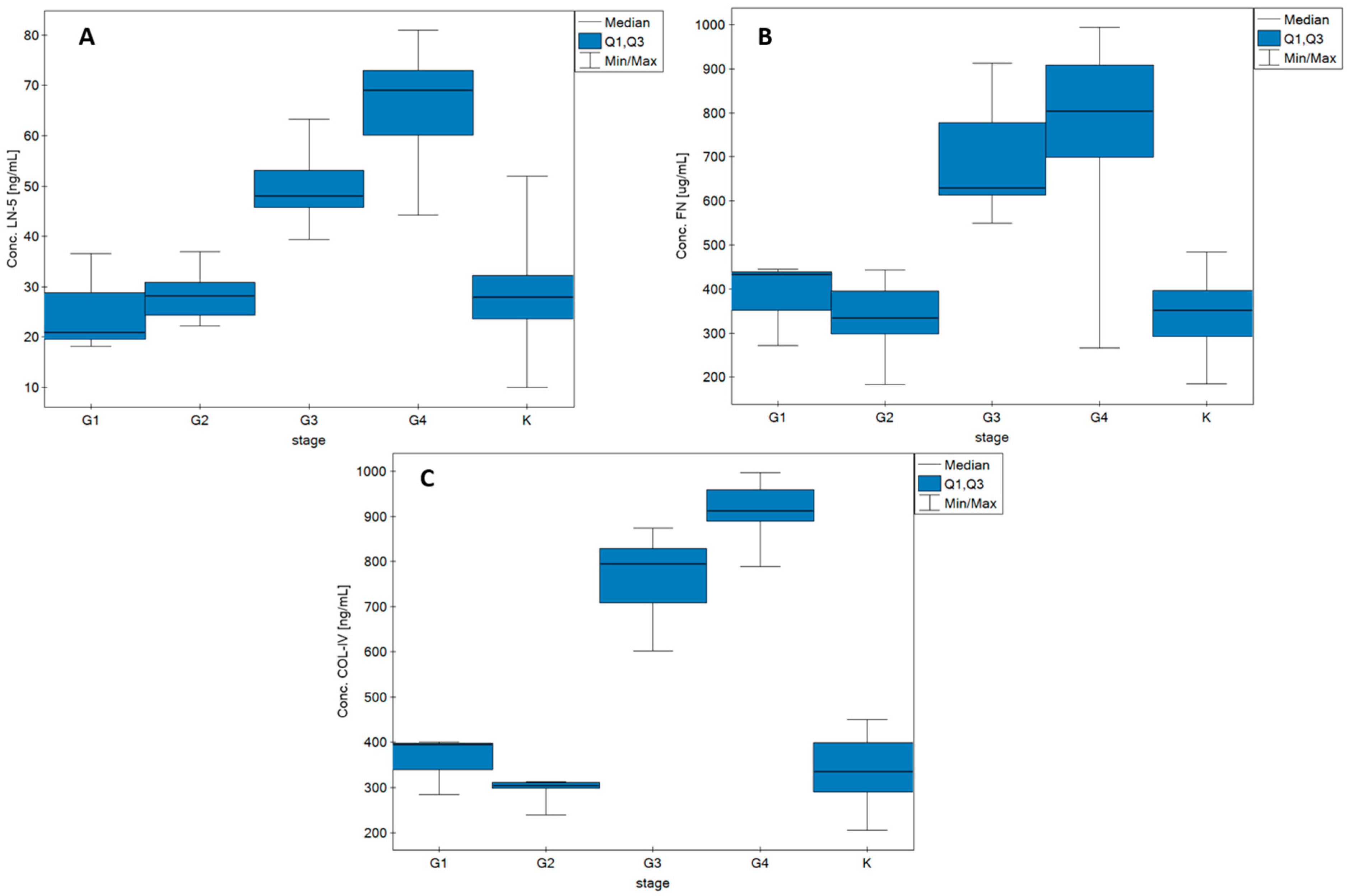
| Paramete/ Biomarker | LN-5 [ng/mL] | FN [µg/mL] | COL IV [ng/mL] | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K | G1 | G2 | G3 | G4 | K | G1 | G2 | G3 | G4 | K | G1 | G2 | G3 | G4 | |
| Median | 27.89 | 21.52 | 28.15 | 48.04 | 69.75 | 351.41 | 432.36 | 334.71 | 628.92 | 803.34 | 334.35 | 394.37 | 304.09 | 795.19 | 912.39 |
| Q1 | 32.25 | 28.79 | 30.91 | 53.19 | 73.00 | 396.47 | 438.62 | 395.04 | 777.51 | 908.45 | 398.82 | 397.09 | 310.09 | 829.04 | 958.91 |
| Q3 | 23.57 | 19.57 | 24.32 | 45.70 | 60.11 | 292.16 | 352.09 | 297.42 | 613.45 | 698.63 | 288.69 | 338.79 | 297.35 | 708.64 | 889.69 |
| MIN | 10.04 | 18.10 | 22.20 | 39.37 | 44.20 | 184.14 | 271.82 | 183.04 | 549.54 | 266.40 | 205.61 | 283.21 | 239.64 | 601.92 | 789.65 |
| MAX | 52.23 | 36.58 | 37.00 | 63.36 | 81.00 | 484.18 | 444.98 | 443.27 | 912.20 | 994.07 | 449.87 | 399.81 | 312.69 | 874.64 | 997.64 |
| Age—FN | |||||
|---|---|---|---|---|---|
| G1 | G2 | G3 | G4 | All samples | |
| RSpearman | −1 | 0.29 | −0.27 | 0.02 | 0.42 |
| p | - | >0.05 | >0.05 | >0.05 | <0.05 |
| Age—LN-5 | |||||
| G1 | G2 | G3 | G4 | All samples | |
| RSpearman | 1 | −0.13 | −0.17 | 0.01 | 0.49 |
| p | - | >0.05 | >0.05 | >0.05 | <0.05 |
| Age—COL IV | |||||
| G1 | G2 | G3 | G4 | All samples | |
| RSpearman | −0.5 | −0.48 | 0.45 | −0.05 | 0.47 |
| p | >0.05 | >0.05 | >0.05 | >0.05 | <0.05 |
| Tumour size—FN | |||||
| G1 | G2 | G3 | G4 | All samples | |
| RSpearman | - | −0.6 | 0 | −0.04 | −0.01 |
| p | - | >0.05 | >0.05 | >0.05 | >0.05 |
| Tumour size—LN-5 | |||||
| G1 | G2 | G3 | G4 | All samples | |
| RSpearman | - | −0.4 | −0.2 | 0.2 | 0.16 |
| p | - | >0.05 | >0.05 | >0.35 | >0.05 |
| Tumour size–COL IV | |||||
| G1 | G2 | G3 | G4 | All samples | |
| RSpearman | - | −0.4 | 0.8 | −0.46 | −0.19 |
| p | - | >0.05 | >0.05 | <0.05 | >0.05 |
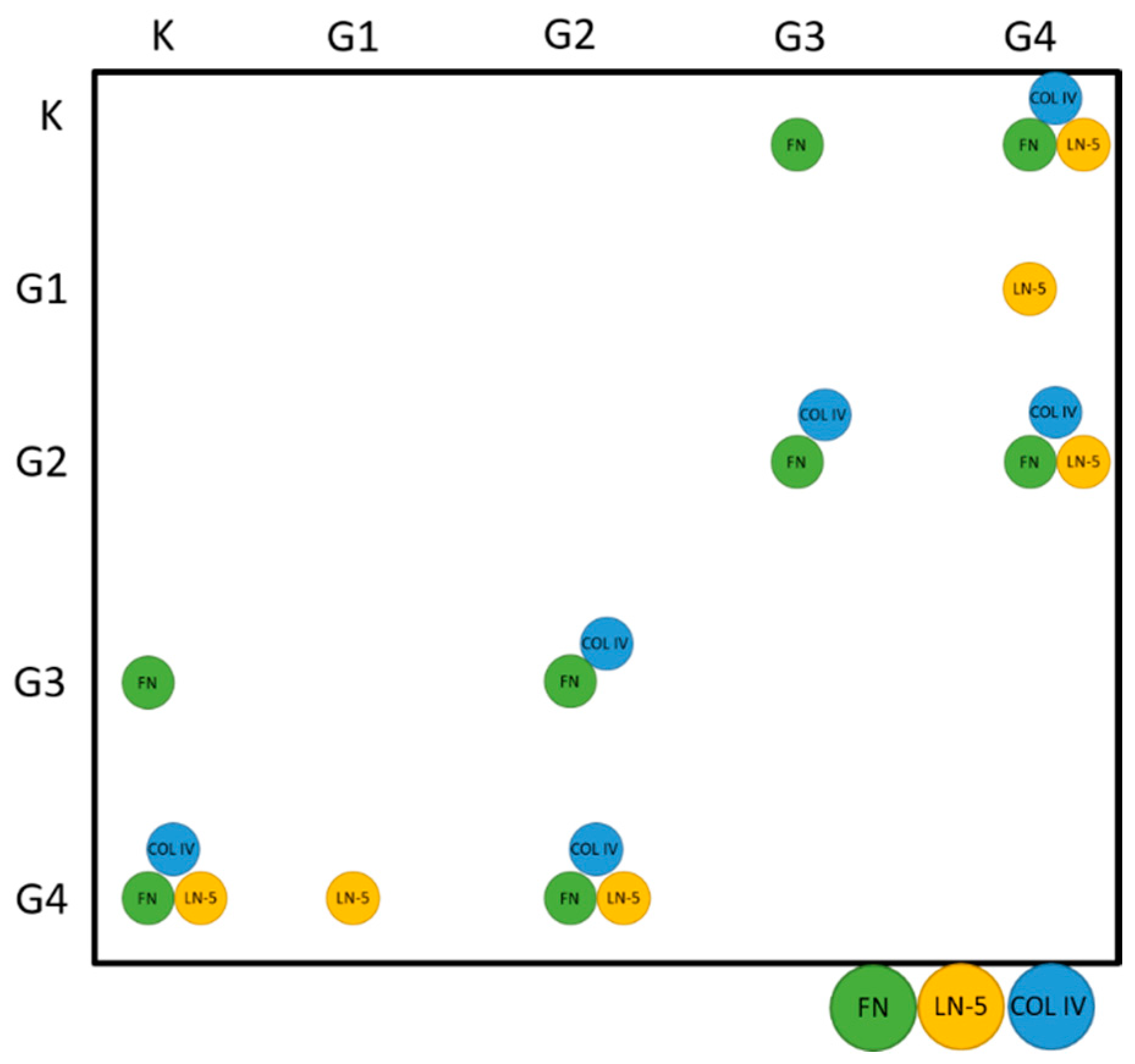
| G1 | |||
|---|---|---|---|
| FN—LN5 | FN—COL4 | LN-5 COL4 | |
| RSpearman | −1 | 0.5 | −0.5 |
| p | >0.05 | >0.05 | >0.05 |
| G2 | |||
| FN—LN5 | FN—COL4 | LN-5 COL4 | |
| RSpearman | −0.16 | −0.37 | 0.018 |
| p | >0.05 | >0.05 | >0.05 |
| G3 | |||
| FN—LN5 | FN—COL4 | LN-5 COL4 | |
| RSpearman | −0.50 | 0.57 | −0.72 |
| p | >0.05 | >0.05 | >0.05 |
| G4 | |||
| FN—LN5 | FN—COL4 | LN-5 COL4 | |
| RSpearman | 0.03 | 0.16 | −0.02 |
| p | >0.05 | >0.05 | >0.05 |
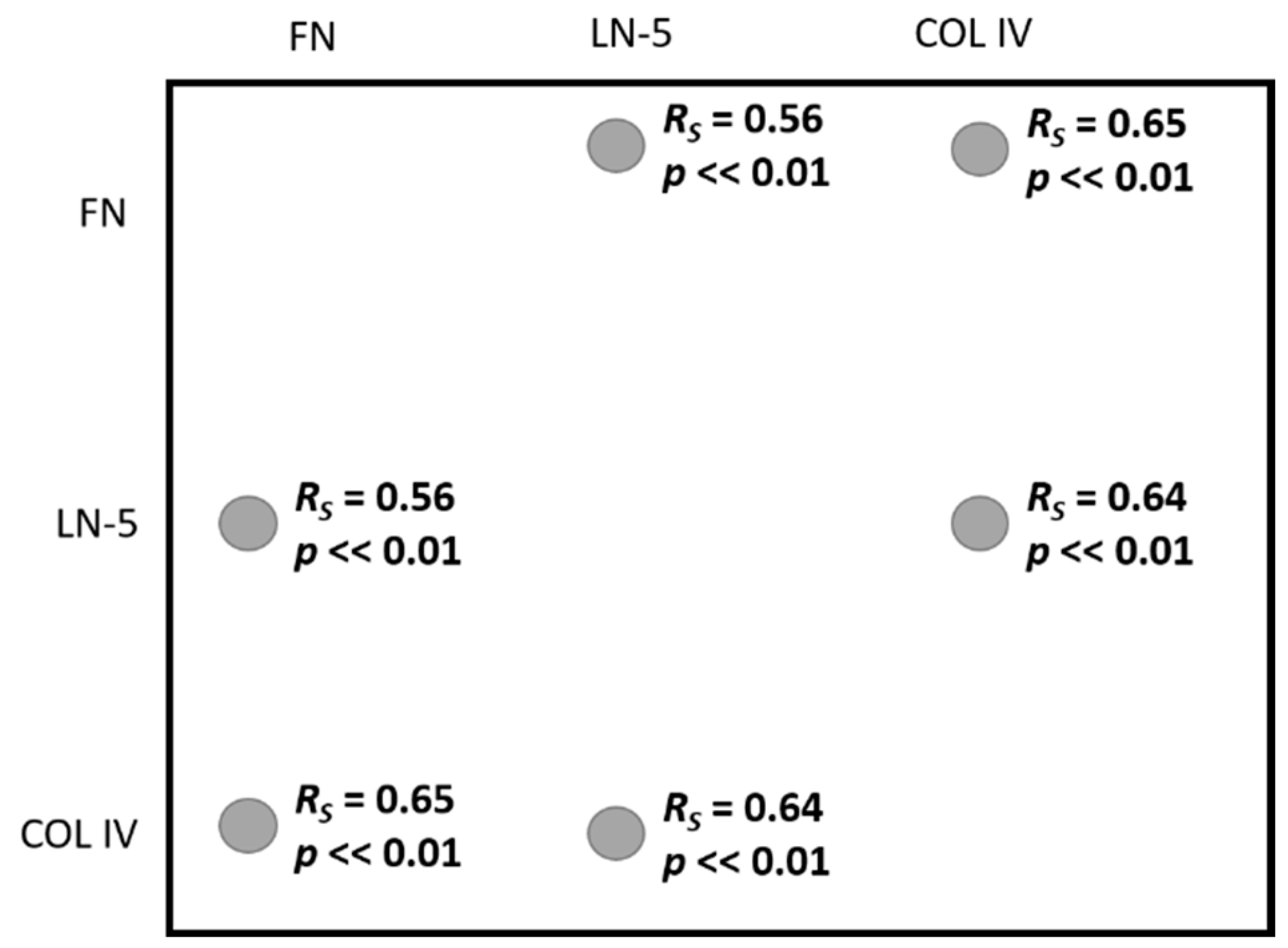
| All samples | |||
| FN—LN5 | FN—COL4 | LN-5 COL4 | |
| RSpearman | 0.56 | 0.65 | 0.64 |
| p | <<0.01 | <<0.01 | <<0.01 |
References
- Lohi, J. Laminin-5 in the Progression of Carcinomas. Int. J. Cancer 2001, 94, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Kong, L.L.; Matthews, R.T.; Viapiano, M.S. The Proteoglycan Brevican Binds to Fibronectin after Proteolytic Cleavage and Promotes Glioma Cell Motility. J. Biol. Chem. 2008, 283, 24848–24859. [Google Scholar] [CrossRef] [PubMed]
- Quo, P.; Imanishi, Y.; Cackowski, F.C.; Jarzynka, M.J.; Tao, H.Q.; Nishikawa, R.; Hirose, T.; Hu, B.; Cheng, S.Y. Up-Regulation of Angiopoietin-2, Matrix Metalloprotease-2, Membrane Type 1 Metalloprotease, and Laminin 5 γ 2 Correlates with the Invasiveness of Human Glioma. Am. J. Pathol. 2005, 166, 877–890. [Google Scholar]
- Kawataki, T.; Yamane, T.; Naganuma, H.; Rousselle, P.; Andurén, I.; Tryggvason, K.; Patarroyo, M. Laminin Isoforms and Their Integrin Receptors in Glioma Cell Migration and Invasiveness: Evidence for a Role of A5-Laminin(s) and A3β1 Integrin. Exp. Cell Res. 2007, 313, 3819–3831. [Google Scholar] [CrossRef]
- Martin, K.J.; Kwan, C.P.; Nagasaki, K.; Zhang, X.; O’Hare, M.J.; Kaelin, C.M.; Burgeson, R.E.; Pardee, A.B.; Sager, R. Down-Regulation of Laminin-5 in Breast Carcinoma Cells. Mol. Med. 1998, 4, 602–613. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.C.; Yang, C.H.; Cheng, L.H.; Chang, W.T.; Lin, Y.R.; Cheng, H.C. Fibronectin in Cancer: Friend or Foe. Cells 2019, 9, 27. [Google Scholar] [CrossRef]
- Chintala, S.K.; Sawaya, R.; Gokaslan, Z.L.; Fuller, G.; Rao, J.S. Immimohistochemical Localization of Extracellular Matrix Proteins in Human Glioma, Both in Vivo and in Vitro. Cancer Lett. 1996, 101, 107–114. [Google Scholar] [CrossRef]
- Higuchi, M.; Ohnishi, T.; Arita, N.; Hiraga, S.; Iwasaki, H.; Mori, S.; Hayakawa, T. Immunohistochemical Localization of Fibronectin, Laminin and Fibronectin-Receptor in Human Malignant Gliomas. In Relation to Tumour Invasion. Brain Nerve 1991, 43, 17–23. [Google Scholar]
- Yu, Q.; Xue, Y.; Liu, J.; Xi, Z.; Li, Z.; Liu, Y. Fibronectin Promotes the Malignancy of Glioma Stem-like Cells via Modulation of Cell Adhesion, Differentiation, Proliferation and Chemoresistance. Front. Mol. Neurosci. 2018, 11, 130. [Google Scholar] [CrossRef]
- Wang, J.; Yan, S.; Chen, X.; Wang, A.; Han, Z.; Liu, B.; Shen, H. Identification of Prognostic Biomarkers for Glioblastoma Based on Transcriptome and Proteome Association Analysis. Technol. Cancer Res. Treat. 2022, 21, 1–10. [Google Scholar] [CrossRef]
- Payne, L.S.; Huang, P.H. The Pathobiology of Collagens in Glioma. Mol. Cancer Res. 2013, 11, 1129–1140. [Google Scholar] [CrossRef] [PubMed]
- Noreen, R.; Chien, C.C.; Chen, H.H.; Bobroff, V.; Moenner, M.; Javerzat, S.; Hwu, Y.; Petibois, C. FTIR Spectro-Imaging of Collagen Scaffold Formation during Glioma Tumour Development. Anal. Bioanal. Chem. 2013, 405, 8729–8736. [Google Scholar] [CrossRef]
- Bellail, A.C.; Hunter, S.B.; Brat, D.J.; Tan, C.; van Meir, E.G. Microregional Extracellular Matrix Heterogeneity in Brain Modulates Glioma Cell Invasion. Int. J. Biochem. Cell B 2004, 36, 1046–1069. [Google Scholar] [CrossRef] [PubMed]
- Tso, C.L.; Shintaku, P.; Chen, J.; Liu, Q.; Liu, J.; Chen, Z.; Yoshimoto, K.; Mischel, P.S.; Cloughesy, T.F.; Liau, L.M.; et al. Primary Glioblastomas Express Mesenchymal Stem-like Properties. Mol. Cancer Res. 2006, 4, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Samuel, M.S.; Lopez, J.I.; McGhee, E.J.; Croft, D.R.; Strachan, D.; Timpson, P.; Munro, J.; Schröder, E.; Zhou, J.; Brunton, V.G.; et al. Actomyosin-Mediated Cellular Tension Drives Increased Tissue Stiffness and β-Catenin Activation to Induce Epidermal Hyperplasia and Tumour Growth. Cancer Cell 2011, 19, 776–791. [Google Scholar] [CrossRef] [PubMed]
- Godard, S.; Getz, G.; Delorenzi, M.; Farmer, P.; Kobayashi, H.; Desbaillets, I.; Nozaki, M.; Diserens, A.C.; Hamou, M.F.; Dietrich, P.Y.; et al. Classification of Human Astrocytic Gliomas on the Basis of Gene Expression: A Correlated Group of Genes with Angiogenic Activity Emerges As a Strong Predictor of Subtypes. Cancer Res. 2003, 63, 6613–6625. [Google Scholar]
- Falkowski, P.; Mrozek, P.; Lukaszewski, Z.; Oldak, L.; Gorodkiewicz, E. An Immunosensor for the Determination of Cathepsin s in Blood Plasma by Array Spri—A Comparison of Analytical Properties of Silver–Gold and Pure Gold Chips. Biosensors 2021, 11, 298. [Google Scholar] [CrossRef]
- Sankiewicz, A.; Romanowicz, L.; Laudanski, P.; Zelazowska-Rutkowska, B.; Puzan, B.; Cylwik, B.; Gorodkiewicz, E. SPR Imaging Biosensor for Determination of Laminin-5 as a Potential Cancer Marker in Biological Material. Anal. Bioanal. Chem. 2016, 408, 5269–5276. [Google Scholar] [CrossRef]
- Qu, J.H.; Ordutowski, H.; van Tricht, C.; Verbruggen, R.; Barcenas Gallardo, A.; Bulcaen, M.; Ciwinska, M.; Gutierrez Cisneros, C.; Devriese, C.; Guluzade, S.; et al. Point-of-Care Therapeutic Drug Monitoring of Adalimumab by Integrating a FO-SPR Biosensor in a Self-Powered Microfluidic Cartridge. Biosens. Bioelectron. 2022, 206, 114125. [Google Scholar] [CrossRef]
- Singh, G.P.; Nigam, R.; Tomar, G.S.; Monisha, M.; Bhoi, S.K.; Arulselvi, S.; Sengar, K.; Akula, D.; Panta, P.; Anindya, R. Early and Rapid Detection of UCHL1 in the Serum of Brain-Trauma Patients: A Novel Gold Nanoparticle-Based Method for Diagnosing the Severity of Brain Injury. Analyst 2018, 143, 3366–3373. [Google Scholar] [CrossRef]
- Tian, Y.; Chen, Y.; Song, D.; Liu, X.; Bi, S.; Zhou, X.; Cao, Y.; Zhang, H. Acousto-Optic Tunable Filter-Surface Plasmon Resonance Immunosensor for Fibronectin. Anal. Chim. Acta 2005, 551, 98–104. [Google Scholar] [CrossRef]
- Dutra, R.F.; Kubota, L.T. An SPR Immunosensor for Human Cardiac Troponin T Using Specific Binding Avidin to Biotin at Carboxymethyldextran-Modified Gold Chip. Clin. Chim. Acta 2007, 376, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.H.; Kong, D.H.; Park, J.H.; Lee, S.T.; Hyun, J.; Kim, Y.M.; Ha, K.S. Rapid Analysis of Matrix Metalloproteinase-3 Activity by Gelatin Arrays Using a Spectral Surface Plasmon Resonance Biosensor. Analyst 2010, 135, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- Shoji, A.; Kabeya, M.; Sugawara, M. Real-Time Monitoring of Matrix Metalloproteinase-9 Collagenolytic Activity with a Surface Plasmon Resonance Biosensor. Anal. Biochem. 2011, 419, 53–60. [Google Scholar] [CrossRef]
- Lin, S.; Shih-Yuan Lee, A.; Lin, C.-C.; Lee, C.-K. Determination of Binding Constant and Stoichiometry for Antibody-Antigen Interaction with Surface Plasmon Resonance. Curr. Proteom. 2007, 3, 271–282. [Google Scholar] [CrossRef]
- Sankiewicz, A.; Romanowicz, L.; Pyc, M.; Hermanowicz, A.; Gorodkiewicz, E. SPR Imaging Biosensor for the Quantitation of Fibronectin Concentration in Blood Samples. J. Pharm. Biomed. Anal. 2018, 150, 1–8. [Google Scholar] [CrossRef]
- Sankiewicz, A.; Lukaszewski, Z.; Trojanowska, K.; Gorodkiewicz, E. Determination of Collagen Type IV by Surface Plasmon Resonance Imaging Using a Specific Biosensor. Anal. Biochem. 2016, 515, 40–46. [Google Scholar] [CrossRef]
- Hlubina, P.; Ciprian, D. Spectral Phase Shift of Surface Plasmon Resonance in the Kretschmann Configuration: Theory and Experiment. Plasmonics 2017, 12, 1071–1078. [Google Scholar] [CrossRef]
- Baeten, K.M.; Akassoglou, K. Extracellular Matrix and Matrix Receptors in Blood–Brain Barrier Formation and Stroke. Dev. Neurobiol. 2011, 71, 1018–1039. [Google Scholar] [CrossRef]
- Scanlon, C.S.; van Tubergen, E.A.; Inglehart, R.C.; D’Silva, N.J. Biomarkers of Epithelial-Mesenchymal Transition in Squamous Cell Carcinoma. J. Dent. Res. 2013, 92, 114–121. [Google Scholar] [CrossRef]
- Hindermann, W.; Berndt, A.; Haas, K.M.; Wunderlich, H.; Katenkamp, D.; Kosmehl, H. Immunohistochemical Demonstration of the Γ2 Chain of Laminin-5 in Urinary Bladder Urothelial Carcinoma Impact for Diagnosis and Prognosis. Cancer Detect. Prev. 2003, 27, 109–115. [Google Scholar] [CrossRef]
- Kiyoshima, K.; Oda, Y.; Kinukawa, N.; Naito, S.; Tsuneyoshi, M. Overexpression of Laminin-5 Γ2 Chain and Its Prognostic Significance in Urothelial Carcinoma of Urinary Bladder: Association with Expression of Cyclooxygenase 2, Epidermal Growth Factor, and Human Epidermal Growth Factor 2. Hum. Pathol. 2005, 36, 522–530. [Google Scholar] [CrossRef]
- Farha, K.M.M.A.; Janknegt, R.A.; Kester, A.D.M.; Arends, J.W. Value of Immunohistochemical Laminin Staining in Transitional Cell Carcinoma of Human Bladder. Urol. Int. 1993, 50, 133–140. [Google Scholar] [CrossRef]
- Schapers, R.F.; Pauwels, R.P.; Havenith, M.G.; Smeets, A.W.; van den Brandt, P.A.; Bosman, F.T. Prognostic Significance of Type IV Collagen and Laminin Immunoreactivity in Urothelial Carcinomas of the Bladder. Cancer 1990, 66, 2583–2588. [Google Scholar] [CrossRef]
- Daher, N.; Abourachid, H.; Bove, N.; Petit, J.; Burtin, P. Collagen IV Staining Pattern in Bladder Carcinomas: Relationship to Prognosis. Br. J. Cancer 1987, 55, 665–671. [Google Scholar] [CrossRef]
- Brunner, A.; Tzankov, A. The Role of Structural Extracellular Matrix Proteins in Urothelial Bladder Cancer (Review). Biomark. Insights 2007, 2, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Menéndez, V.; Fernández-Suárez, A.; Galán, J.A.; Pérez, M.; García-López, F. Diagnosis of Bladder Cancer by Analysis of Urinary Fibronectin. Urology 2005, 65, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Ioachim, E.; Michael, M.; Stavropoulos, N.E.; Kitsiou, E.; Salmas, M.; Malamou-Mitsi, V. A Clinicopathological Study of the Expression of Extracellular Matrix Components in Urothelial Carcinoma. BJU Int. 2005, 95, 655–659. [Google Scholar] [CrossRef]
- Malmstrom, P.U.; Larsson, A.; Johansson, S. Urinary Fibronectin in Diagnosis and Follow-up of Patients with Urinary Bladder Cancer. Br. J. Urol. 1993, 72, 307–310. [Google Scholar] [CrossRef]
- Hegele, A.; Heidenreich, A.; Varga, Z.; von Knobloch, R.; Olbert, P.; Kropf, J.; Hofmann, R. Cellular Fibronectin in Patients with Transitional Cell Carcinoma of the Bladder. Urol. Res. 2003, 30, 363–366. [Google Scholar] [CrossRef]
| Variable | Range | Number of Patients |
|---|---|---|
| Age [years] | <50 | 16 |
| >50 | 40 | |
| Gender | male | 34 |
| female | 22 | |
| Tumour grade | G1 | 3 |
| G2 | 10 | |
| G3 | 7 | |
| G4 | 36 | |
| Tumour size [cm2] | <15 | 15 |
| >15 | 15 | |
| The presence of other neoplasms in the immediate family | yes | 26 |
| no | 30 | |
| Concomitant non-cancerous diseases | yes | 28 |
| no | 28 |
| Analytical Characteristics of the Methods Used | Characteristics of the Quantification Procedure Used | |||||
|---|---|---|---|---|---|---|
| Biomarker | Method | Analytical Characteristic | References | Biological Material | Dilution | pH |
| LN-5 | SPRi biosensor [non fluidic] | Cantibody = 5 ng/mL LOD = 4 pg/mL LOQ = 14 pg/mL pH = 7.40 LR: 0.01–0.075 ng/mL | [18] | Blood plasma (blood centrifuged at 3000 rpm for 15 min then stored at −80 °C) | K G1 x1000 G2 G3 x1200 G4 | 7.40 |
| FN | SPRi biosensor [non fluidic] | Cantibody = 4 µg/mL LOD = 1.5 ng/mL LOQ = 5 ng/mL pH = 7.40 LR: 5–100 µg/mL | [26] | K G1 x5 G2 G3 x10 G4 | ||
| COL IV | SPRi biosensor [non fluidic] | Cantibody = 6 µg/mL LOD = 2.4 ng/mL LOQ = 8 ng/mL pH = 7.40 LR: 10–150 ng/mL | [27] | K G1 x5 G2 G3 x10 G4 | ||
| Parameter | LN-5 concentration [ng/mL] | FN concentration [µg/mL] | COL IV concentration [ng/mL] | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Range | Median | p-Value | Range | Median | p-Value | Range | Median | p-Value | |
| Tumour grade (G1–G2 vs. G3–G4) | |||||||||
| G1 (3) | 18.13–36.58 | 21.08 | <0.01 | 271.82–444.88 | 432.36 | <0.01 | 283.21–399.81 | 394.37 | <0.01 |
| G2 (10) | 22.26–37.00 | 28.15 | 183.04–443.27 | 334.70 | 239.64–312.69 | 304.10 | |||
| G3 (7) | 39.37–63.36 | 48.51 | 549.54–912.20 | 628.92 | 601.92–874.64 | 795.19 | |||
| G4 (36) | 44.20–81.02 | 69.23 | 266.40–994.07 | 803.34 | 789.65–997.64 | 912.39 | |||
| Tumour size [cm2] | |||||||||
| <15 (15) | 21.05–73.40 | 60.11 | 0.37 (NS) | 329.36–994.07 | 651.40 | 0.74 (NS) | 239.64–994.64 | 894.97 | 0.26 (NS) |
| >15 (15) | 18.13–79.52 | 64.25 | 183.04–964.44 | 771.84 | 297.44–973.23 | 829.64 | |||
| The presence of other neoplasms in the family | |||||||||
| YES (26) | 18.13–81.37 | 58.80 | 0.76 (NS) | 266.40–994.07 | 747.56 | 0.77 (NS) | 269.36–997.64 | 890.40 | 0.98 (NS) |
| NO (30) | 22.26–81.42 | 61.04 | 183.04–978.62 | 651.40 | 239.64–973.23 | 879.48 | |||
| Concomitant non-cancerous diseases | |||||||||
| YES (28) | 22.26–80.02 | 64.86 | 0.31 (NS) | 271.82–946.79 | 763.37 | 0.43 (NS) | 239.64–997.64 | 892.27 | 0.20 (NS) |
| NO (28) | 18.13–81.42 | 59.71 | 183.04–994.07 | 719.45 | 269.36–994.64 | 871.97 | |||
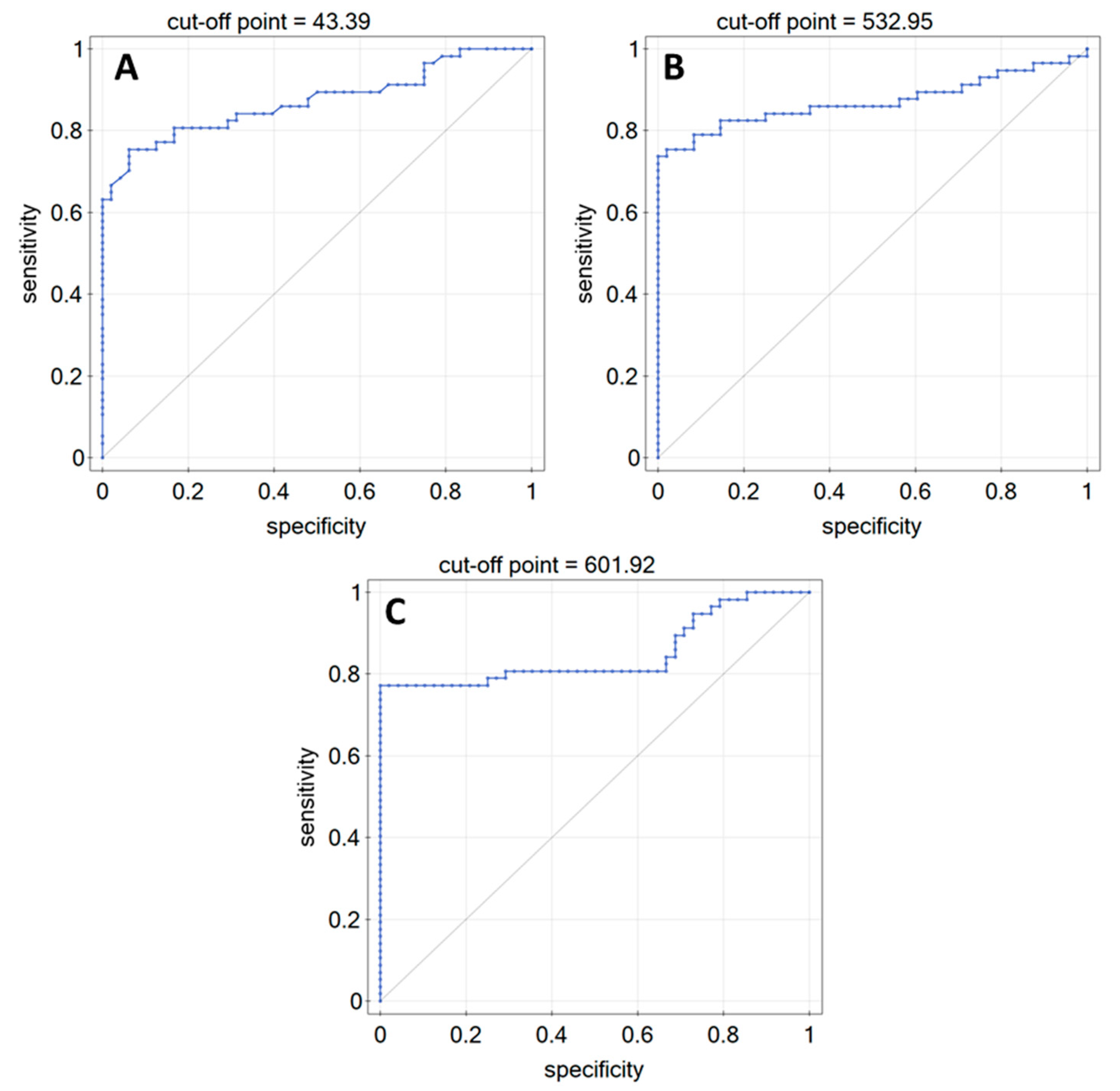
| AUC | p-Value | PPV [%] | NPV [%] | Sensitivity [%] | Specificity [%] | Cut-Off Point | |
|---|---|---|---|---|---|---|---|
| LN-5 | 0.87 | <<0.01 | 93.5 | 76.3 | 75.4 | 93.8 | 43.39 |
| FN | 0.87 | <<0.01 | 100 | 76.2 | 73.7 | 100 | 532.95 |
| COL IV | 0.85 | <<0.01 | 100 | 78.7 | 77.2 | 100 | 601.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oldak, L.; Chludzinska-Kasperuk, S.; Milewska, P.; Grubczak, K.; Reszec, J.; Gorodkiewicz, E. Laminin-5, Fibronectin, and Type IV Collagen as Potential Biomarkers of Brain Glioma Malignancy. Biomedicines 2022, 10, 2290. https://doi.org/10.3390/biomedicines10092290
Oldak L, Chludzinska-Kasperuk S, Milewska P, Grubczak K, Reszec J, Gorodkiewicz E. Laminin-5, Fibronectin, and Type IV Collagen as Potential Biomarkers of Brain Glioma Malignancy. Biomedicines. 2022; 10(9):2290. https://doi.org/10.3390/biomedicines10092290
Chicago/Turabian StyleOldak, Lukasz, Sylwia Chludzinska-Kasperuk, Patrycja Milewska, Kamil Grubczak, Joanna Reszec, and Ewa Gorodkiewicz. 2022. "Laminin-5, Fibronectin, and Type IV Collagen as Potential Biomarkers of Brain Glioma Malignancy" Biomedicines 10, no. 9: 2290. https://doi.org/10.3390/biomedicines10092290
APA StyleOldak, L., Chludzinska-Kasperuk, S., Milewska, P., Grubczak, K., Reszec, J., & Gorodkiewicz, E. (2022). Laminin-5, Fibronectin, and Type IV Collagen as Potential Biomarkers of Brain Glioma Malignancy. Biomedicines, 10(9), 2290. https://doi.org/10.3390/biomedicines10092290







