Clinicopathological Profile of Medullary Thyroid Carcinoma—Could We Predict Aggressive Behavior?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Group
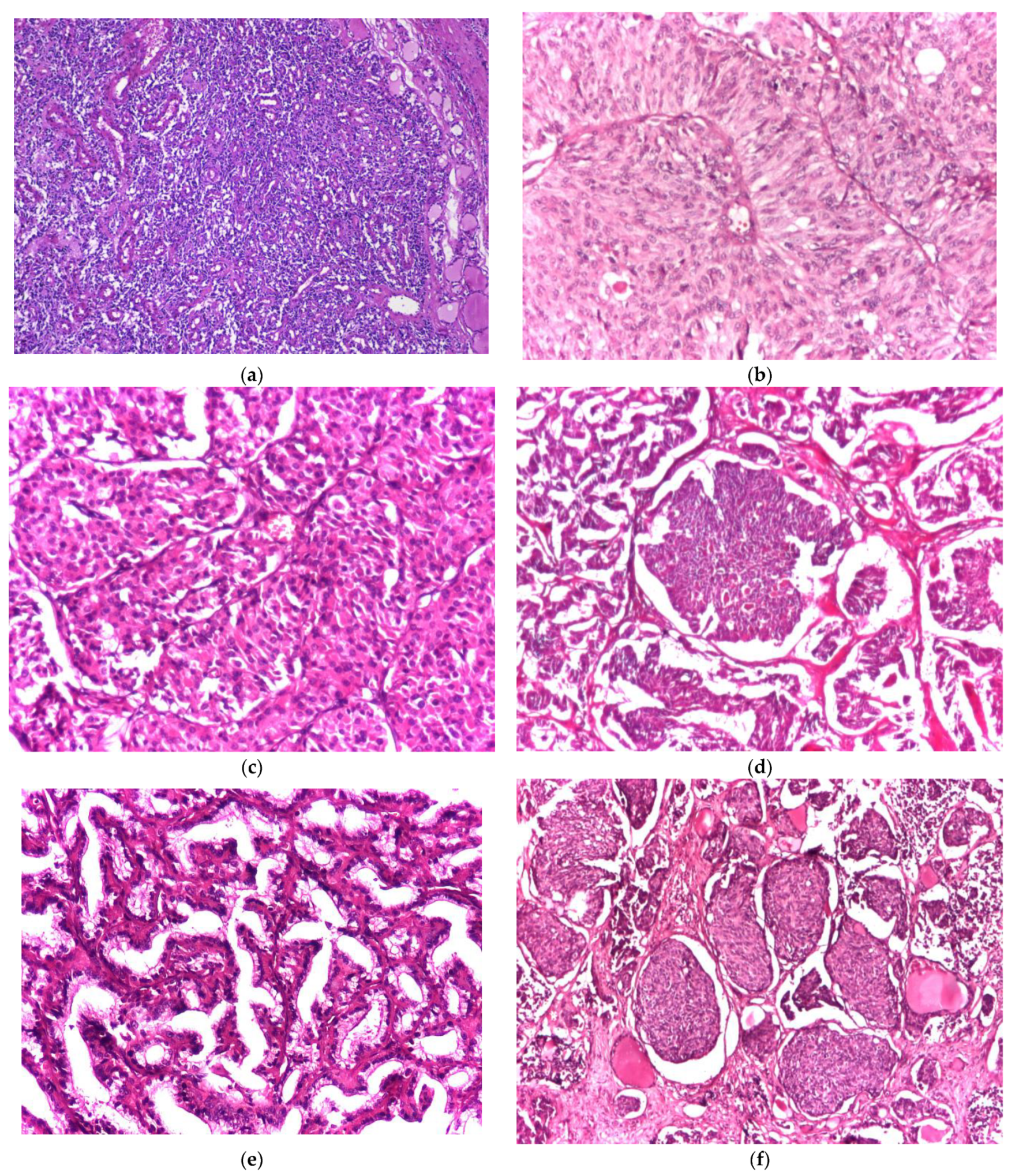
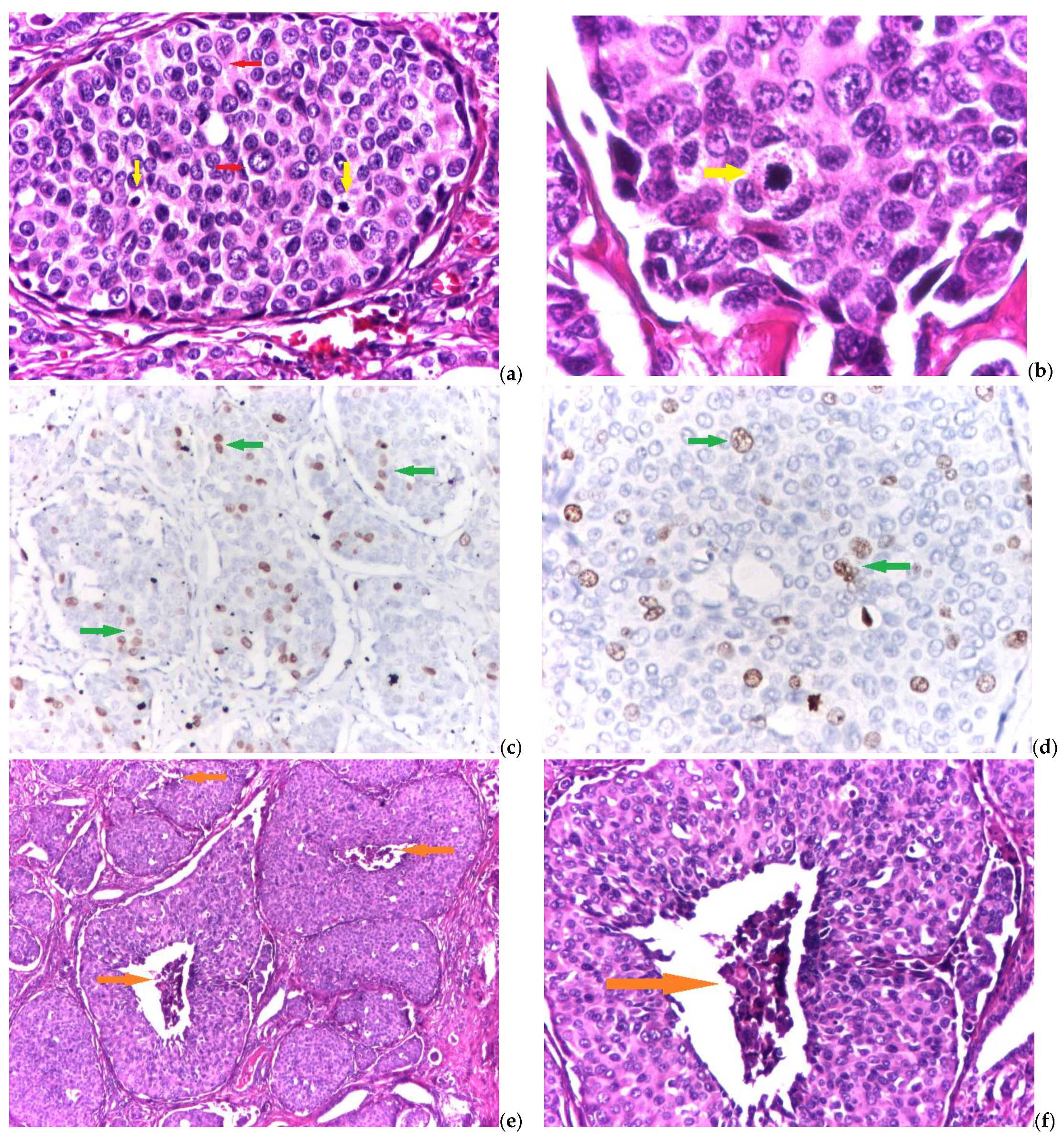
2.2. Histological and Immunohistochemical Evaluation
2.3. Grading System
2.4. Statistical Analysis
3. Results
3.1. General Clinicopathological Characteristics
3.2. Grading System Parameters
3.3. Relationships between Clinicopathological Prognostic Factors
3.3.1. Extrathyroidal Extension
3.3.2. Lymphovascular Invasion
3.3.3. Lymph Node Metastasis
4. Discussion
4.1. Significance of Classical Clinicopathological Parameters
4.2. Significance of the Grading System
4.3. Significance of Aggressiveness Parameters
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- DeLellis, R.A.; Ghuzlan, A.; Saavedra, A.J.; Baloch, Z.W.; Baloso, F.; Elisei, R.; Kaserer, K.; LiVolsi, V.; Matias-Guiu, X.; Mete, O.; et al. Medullary thyroid carcinoma. In World Health Organization (WHO) Classification of Tumours of Endocrine Organs, 4th ed.; Lloyd, R.V., Osamura, R.Y., Kloppel, G., Rosai, J., Eds.; WHO International Agency for Research on Cancer (IARC) Press: Lyon, France, 2017; pp. 108–116. Available online: https://www.iarc.who.int/news-events/who-classification-of-tumours-of-endocrine-organs/ (accessed on 15 September 2022).
- Hazard, J.B.; Hawk, W.A.; Crile, J.R.G. Medullary (solid) carcinoma of the thyroid; a clinicopathologic entity. J. Clin. Endocrinol. Metab. 1959, 19, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Hazard, J.B. The C cells (parafollicular cells) of the thyroid gland and medullary thyroid carcinoma. A review. Am. J. Pathol. 1977, 88, 213–250. [Google Scholar] [PubMed]
- Bhattacharyya, N. A population-based analysis of survival factors in differentiated and medullary thyroid carcinoma. Otolaryngol. Head Neck Surg. 2003, 128, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Wells, S.A., Jr.; Asa, S.L.; Dralle, H.; Elisei, R.; Evans, D.B.; Gagel, R.F.; Lee, N.; Machens, A.; Moley, J.F.; Pacini, F.; et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid 2015, 25, 567–610. [Google Scholar] [CrossRef] [PubMed]
- Gogna, S.; Goldberg, M.; Samson, D.; Gachabayov, M.; Felsenreich, D.M.; Azim, A.; Dong, E. Medullary thyroid cancer in patients older than 45-epidemiologic trends and predictors of survival. Cancers 2020, 12, 3124. [Google Scholar] [CrossRef] [PubMed]
- Băetu, M.; Olariu, C.A.; Stancu, C.; Caragheorgheopol, A.; Ioachim, D.; Moldoveanu, G.; Corneci, C.; Badiu, C. Thresholds of basal- and calcium-stimulated calcitonin for diagnosis of thyroid malignancy. Horm. Metab. Res. 2021, 53, 779–786. [Google Scholar] [CrossRef]
- Wells, S.A., Jr.; Pacini, F.; Robinson, B.G.; Santoro, M. Multiple endocrine neoplasia type 2 and familial medullary thyroid carcinoma: An update. J. Clin. Endocrinol. Metab. 2013, 98, 3149–3164. [Google Scholar] [CrossRef]
- Wells, S.A., Jr. Progress in endocrine neoplasia. Clin. Cancer Res. 2016, 22, 4981–4988. [Google Scholar] [CrossRef]
- Viola, D.; Elisei, R. Management of medullary thyroid cancer. Endocrinol. Metab. Clin. 2019, 48, 285–301. [Google Scholar] [CrossRef]
- Thyroid Cancer Survivors’ Association (ThyCa). Medullary Thyroid Cancer Handbook; ThyCa: Olney, MD, USA, 2014; Available online: https://www.thyca.org/download/document/630/MTChandbook.pdf (accessed on 15 September 2022).
- Xu, B.; Fuchs, T.L.; Ahmadi, S.; Alghamdi, M.; Alzumaili, B.; Bani, M.A.; Baudin, E.; Chou, A.; De Leo, A.; Fagin, J.A.; et al. International Medullary Thyroid Carcinoma Grading System: A validated grading system for medullary thyroid carcinoma. J. Clin. Oncol. 2022, 40, 96–104. [Google Scholar] [CrossRef]
- Găleşanu, M.R.; Rusu, E.; Bild, E.; Tarcău, L.; Zbranca, E.; Găleşanu, C. Cases of thyroid cancer irradiated at the Iaşi Radiology-Oncology Clinic (1984–1988). Rev. Med. Chir. Soc. Med. Nat. Iasi 1989, 93, 751–757. [Google Scholar] [PubMed]
- Mogoş, V.; Zbranca, E.; Strat, V.; Diaconescu, M.R.; Chifan, M.; Florea, N.; Costăchescu, G.; Găleşanu, M.R.; Rusu, V.; Georgescu, G. Thyroid cancers. Rev. Med. Chir. Soc. Med. Nat. Iasi 1995, 99, 72–81. [Google Scholar] [PubMed]
- Ivan, A.; Azoicăi, D. Epidemiological aspects and methodological difficulties in establishing the causal relationship of Chernobyl nuclear accident with cancer. Rev. Med. Chir. Soc. Med. Nat. Iasi 2002, 106, 659–664. [Google Scholar] [PubMed]
- Buzduga, C.; Mogoş, V.; Găleşanu, C.; Vulpoi, C.; Ungureanu, C.M.; Cristea, C.; Preda, C.; Ciobanu, D.; Ferariu, D.; Florea, N.; et al. Epidemiology and histology of malignant thyroid nodules in North East Region of Romania (Moldavia) before and after alimentary salt universal iodination. Rev. Med. Chir. Soc. Med. Nat. Iasi 2011, 115, 45–48. [Google Scholar] [PubMed]
- Teodoriu, L.; Ungureanu, M.C.; Leustean, L.; Preda, C.; Ciobanu, D.; Grierosu, I.; Matei, M.; Iacob, R.; Stefanescu, C. Updated incidence of thyroid cancer in the North East region of Romania after 35 years of Chernobyl fallout. Is there a link between? Diagnostics 2021, 11, 907. [Google Scholar] [CrossRef] [PubMed]
- Dal Maso, L.; Tavilla, A.; Pacini, F.; Serraino, D.; van Dijk, B.A.C.; Chirlaque, M.D.; Capocaccia, R.; Larrañaga, N.; Colonna, M.; Agius, D.; et al. Survival of 86,690 patients with thyroid cancer: A population-based study in 29 European countries from EUROCARE-5. Eur. J. Cancer 2017, 77, 140–152. [Google Scholar] [CrossRef]
- Lloyd, R.V.; Osamura, R.Y.; Kloppel, G.; Rosai, J. Tumours of the thyroid gland. In WHO Classification of the Tumours of Endocrine Organs, 4th ed.; Lloyd, R.V., Osamura, R.Y., Kloppel, G., Rosai, J., Eds.; WHO IARC Press: Lyon, France, 2017; pp. 65–142. Available online: https://www.iarc.who.int/news-events/who-classification-of-tumours-of-endocrine-organs/ (accessed on 15 September 2022).
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours, 8th ed.; Wiley-Blackwell: New York, NY, USA, 2017; Available online: https://www.uicc.org/resources/tnm-classification-malignant-tumours-8th-edition (accessed on 15 September 2022).
- Amin, M.B.; Edge, S.; Greene, F.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. AJCC Cancer Staging Manual, 8th ed.; Springer: New York, NY, USA, 2017. [Google Scholar]
- Alzumaili, B.; Xu, B.; Spanheimer, P.M.; Tuttle, R.M.; Sherman, E.; Katabi, N.; Dogan, S.; Ganly, I.; Untch, B.R.; Ghossein, R.A. Grading of medullary thyroid carcinoma on the basis of tumor necrosis and high mitotic rate is an independent predictor of poor outcome. Mod. Pathol. 2020, 33, 1690–1701. [Google Scholar] [CrossRef]
- Thomas, C.M.; Asa, S.L.; Ezzat, S.; Sawka, A.M.; Goldstein, D. Diagnosis and pathologic characteristics of medullary thyroid carcinoma—Review of current guidelines. Curr. Oncol. 2019, 26, 338–344. [Google Scholar] [CrossRef]
- Ahmed, S.R.; Ball, D.W. Clinical review: Incidentally discovered medullary thyroid cancer: Diagnostic strategies and treatment. J. Clin. Endocrinol. Metab. 2011, 96, 1237–1245. [Google Scholar] [CrossRef]
- Hadoux, J.; Pacini, F.; Tuttle, R.M.; Schlumberger, M. Management of advanced medullary thyroid cancer. Lancet Diab. Endocrinol. 2016, 4, 64–71. [Google Scholar] [CrossRef]
- Kebebew, E.; Ltuarte, P.H.; Siperstein, A.E. Medullar thyroid carcinoma: Clinical characteristics, treatment, prognostic factors, and a comparison of staging systems. Cancer 2000, 88, 39–48. [Google Scholar] [CrossRef]
- Huang, Y.; Min, Y.; Yang, G.; Wang, H.; Yin, G.; Zhang, L. Construction and validation of a prediction model for identifying clinical risk factors of lateral lymph node metastasis in medullary thyroid carcinoma. Int. J. Gen. Med. 2022, 15, 2301–2309. [Google Scholar] [CrossRef]
- Scerrino, G.; Cocorullo, G.; Orlando, G.; Rotolo, G.; Corigliano, A.; Amato, C.; Proclamà, M.P.; Vitale, I.; Melfa, G.; Paladino, N.C. Predictive factors for lymph node involvement in sporadic medullary thyroid microcarcinoma: A systematic review. Eur. Rev. Med. Pharm. Sci. 2022, 26, 1004–1016. [Google Scholar] [CrossRef]
- Fan, W.; Xiao, C.; Wu, F. Analysis of risk factors for cervical lymph node metastases in patients with sporadic medullary thyroid carcinoma. J. Int. Med. Res. 2018, 46, 1982–1989. [Google Scholar] [CrossRef]
- Noullet, S.; Trésallet, C.; Godiris-Petit, G.; Hoang, G.; Leenhardt, L.; Menegaux, F. Traitement chirurgical du cancer médullaire sporadique de la thyroïde. J. de Chir. Viscérale 2011, 148, 277–283. [Google Scholar] [CrossRef]
- Manjunath, P.R.; Vadayath, U.M.; Nair, V.; Pavithran, P.V.; Bhavani, N.; Kumar, H.; Abraham, N.; Menon, A.S.; Narayanan, P. Clinical profile of medullary thyroid carcinoma: Audit from a tertiary care center in South India. Indian J. Endocr. Metab. 2020, 24, 355–359. [Google Scholar] [CrossRef]
- Jayasinghe, R.; Basnayake, O.; Jayarajah, U.; Seneviratne, S. Management of medullary carcinoma of the thyroid: A review. J. Int. Med. Res. 2022, 50, 3000605221110698. [Google Scholar] [CrossRef]
- DeLellis, R.; Williams, E. Thyroid and parathyroid tumours. In Pathology and Genetics of Tumours of Endocrine Organs, WHO Classification of Tumours; De Lellis, R.A., Lloyd, R.V., Heitz, P.U., Eng, C., Eds.; IARC Press: Lyon, France, 2004; pp. 50–66. [Google Scholar]
- American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer; Cooper, D.S.; Doherty, G.M.; Haugen, B.R. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009, 19, 1167–1214. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Ralhan, R.; Cao, J.; Lim, T. EpCAM nuclear localization identifies aggressive thyroid cancer and is a marker for poor prognosis. BMC Cancer 2010, 10, 331. [Google Scholar] [CrossRef]
| Antibody | Manufacturer | Clone | Antigen Retrieval | Class | Dilution | Labeling | Cellular Localization |
|---|---|---|---|---|---|---|---|
| AntiCgA | Leica Biosystems, Deer Park, IL, USA | 5H7 | Citrate, pH 6 | Monoclonal mouse antihuman chromogranin A | 1:400 | Neuroendocrine cells | Cytoplasm |
| AntiSyn | Leica Biosystems, Deer Park, IL, USA | 27G12 | Citrate, pH 6 | Monoclonal mouse antisynaptophisin antibody | 1:50 | Neuroendocrine cells | Cytoplasm |
| Anticalcitonin | Leica Biosystems, Deer Park, IL, USA | CL 1948 | Citrate, pH 6 | Monoclonal mouse anticalcitonin antibody | 1:250 | C cells | Cytoplasm |
| AntiCD56 | Agilent Dako, Santa Clara, CA, USA | 123C3 | Citrate, pH 6 | Monoclonal mouse antiCD56 antibody | 1:75 | Neuroendocrine cells | Membrane |
| AntiTTF1 | Agilent Dako, Santa Clara, CA, USA | mAb 8G7G3/1 | pH 9 | Monoclonal mouse antiTTF1 antibody | 1:50 | Follicular thyroid cells | Nuclear |
| AntiKi67 | ThermoFisher Scientific, Waltham, MA, USA | SP6 | Citrate, pH 6 | Monoclonal rabbit antiKi67 antibody | 1:250 | Proliferating cells | Nuclear |
| Preoperative Calcitonin Value | Postoperative Calcitonin Value | ||||||
|---|---|---|---|---|---|---|---|
| pTN | Order of Tens (n = 7) | Order of Hundreds (n = 10) | Order of Thousands (n = 4) | Normal Values (≤11.5 pg/mL) (n = 12) | Order of Tens (n = 4) | Order of Hundreds (n = 4) | Order of Thousands (n = 1) |
| T1 | 3 (14.2%) | 5 (23.8%) | 1(4.7%) | 8 (38.0%) | 1 (4.7%) | 0 (0%) | 0 (0%) |
| T2 | 3 (14.2%) | 3 (14.2%) | 0 (0%) | 3 (14.2%) | 2 (9.5%) | 1 (4.7%) | 0 (0%) |
| T3 | 1 (4.7%) | 2 (9.5%) | 3 (14.2%) | 1 (4.7%) | 1 (4.7%) | 3 (14.2%) | 1 (4.7%) |
| N1 | 0 (0%) | 3 (14.2%) | 2 (9.5%) | 0 (0%) | 3 (14.2%) | 1 (4.7%) | 1 (4.7%) |
| N0 | 3 (14.2%) | 5 (23.8%) | 0 (0%) | 9 (42.8%) | 0 (0%) | 1 (4.7%) | 0 (0%) |
| Nx | 4 (19.0%) | 2 (9.5%) | 2 (9.5%) | 3 (14.2%) | 1 (4.7%) | 2 (9.5%) | 0 (0%) |
| Grading System Parameter | Total MTC Cases (n = 59) | |
|---|---|---|
| Low-Grade MTC n, % | High-Grade MTC n, % | |
| Mitotic index | ||
| 0 | 0 (0%) | |
| 1 | 8 (13.56%) | |
| 2 | 8 (13.56%) | |
| 3 | 10 (16.95%) | |
| 4 | 12 (20.33%) | |
| 5 | 11 (18.64%) | |
| 6 | 1 (1.70%) | |
| 7 | 8 * (13.56%) | |
| 8 | 0 (0%) | |
| 9 | 0 (0%) | |
| 10 | 1 # (1.70%) | |
| Ki67 proliferative index | ||
| 0–1.9% | 22 (37.28%) | |
| 2–2.9% | 22 (37.28%) | |
| 3–3.9% | 7 (11.86%) | |
| 4–4.9% | 2 (3.40) | |
| 5–5.9% | 1 ** (1.70%) | |
| 6–6.9% | 3 ** (5.08%) | |
| 7–7.9% | 0 | |
| 8–8.9% | 1 ^ (1.70%) | |
| 9–10% | 1 ** (1.70%) | |
| Tumor necrosis | ||
| Absent | 56 (94.91%) | |
| Present | 3 (5.09%) | |
| Clinicopathological Characteristics | Extrathyroidal Extension | Univariate Analysis | OR (95% CI) | |
|---|---|---|---|---|
| Present (n = 24) | Absent (n = 35) | |||
| Age at diagnosis | ||||
| <55 yo | 7 (29.1%) | 10 (28.5%) | 0.964 | 0.971 (0.308–3.054) |
| >55 yo | 17 (70.8%) | 25 (71.4%) | ||
| Tumor size (mm) | ||||
| <10 mm | 1 (4.1%) | 8 (22.8%) | 0.088 | 1.925 (0.516–7.177) |
| 10–40 mm | 16 (66.6%) | 22 (62.8%) | ||
| >40 mm | 7 (29.1%) | 5 (14.2%) | ||
| Histological variant | ||||
| Conventional | 12 (50%) | 23 (65.7%) | 0.227 | 1.916 (0.662–5.542) |
| Other variants | 12 (50%) | 12 (43.8%) | ||
| Focality of the tumor | ||||
| Unifocal | 18 (75%) | 32 (91.4%) | 0.084 | 3.555 (0.792–15.957) |
| Multifocal | 6 (25%) | 3 (8.5%) | ||
| Resection margins | ||||
| R0 | 20 (83.33%) | 33 (94.29%) | 0.190 | 3.300 (0.553–19.685) |
| R1 | 4 (16.67%) | 2 (5.71%) | ||
| Tumor recurrence | ||||
| present | 7 (29.1%) | 7 (20%) | 0.418 | 1.647 (0.492–5.516) |
| absent | 17 (70.9%) | 28 (80%) | ||
| Coexisting thyroid pathology | ||||
| Colloid goiter † | 11 (45.8%) | 13 (37.1%) | 0.700 | 0.545 (0.096–3.075) |
| Nodular goiter | 11 (45.8%) | 17 (48.5%) | ||
| Hashimoto thyroiditis | 2 (8.3%) | 5 (14.2%) | ||
| Mitotic index | ||||
| <5/2 mm2 (low-grade) | 14 (58.33%) | 24 (68.57%) | 0.421 | 1.558 (0.529–4.592) |
| >5/2 mm2 (high-grade) | 10 (41.67%) | 11 (31.43%) | ||
| Ki67 proliferative index | ||||
| <5% (low-grade) | 20 (83.33%) | 33 (94.29%) | 0.190 | 3.300 (0.553–19.685) |
| >5% (high grade) | 4 (16.67%) | 2 (5.71%) | ||
| Tumor necrosis | ||||
| absent | 23 (95.83%) | 33 (94.29%) | 0.790 | 0.717 (0.061–8.387) |
| present | 1 (4.17%) | 2 (5.71%) | ||
| Tumor grade | ||||
| high-grade | 10 (41.67%) | 11 (31.43%) | 0.421 | 1.558 (0.529–4.592) |
| low-grade | 14 (58.33%) | 24 (68.57%) | ||
| Clinicopathological Characteristics | Lympho-Vascular Invasion | Univariate Analysis | OR (95% CI) | |
|---|---|---|---|---|
| Present (n = 34) | Absent (n = 25) | |||
| Age at diagnosis | ||||
| <55 yo | 10 (29.4%) | 7 (28%) | 0.905 | 0.933 (0.297–2.927) |
| >55 yo | 24 (70.5%) | 18 (72%) | ||
| Tumor size (mm) | ||||
| <10 mm | 0 (0%) | 9 (36%) | 0.0001 * | 13.695 (1.662–112.846) |
| 10–40 mm | 23 (67.6%) | 15 (60%) | ||
| >40 mm | 11 (32.4%) | 1 (4%) | ||
| Histological variant | ||||
| Conventional | 19 (55.8%) | 16 (64%) | 0.398 | 0.664 (0.257–1.717) |
| Other variants | 15 (44.1%) | 19 (76%) | ||
| Focality of the tumor | ||||
| Unifocal | 27 (79.4%) | 23 (92%) | 0.183 | 2.981 (0.562–15.790) |
| Multifocal | 7 (20.5%) | 2 (8%) | ||
| Resection margins | ||||
| R0 | 29 (85.3%) | 24 (96%) | 0.179 | 4.138 (0.452–37.875) |
| R1 | 5 (14.7%) | 1 (4%) | ||
| Tumor recurrence | ||||
| Present | 10 (29.4%) | 4 (16%) | 0.238 | 2.187 (0.597–8.019) |
| Absent | 24 (70.6%) | 21 (84%) | ||
| Coexisting thyroid pathology | ||||
| Colloid goiter † | 14 (41.1%) | 10 (40%) | 0.2307 | 0.250 (0.0442–1.4136) |
| Nodular goiter | 18 (52.9%) | 10 (40%) | ||
| Hashimoto thyroiditis | 2 (5.8%) | 5 (20%) | ||
| Mitotic index | ||||
| <5/2 mm2 (low-grade) | 19 (55.9%) | 19 (76%) | 0.111 | 2.500 (0.799–7.821) |
| >5/2 mm2 (high-grade) | 15 (44.1%) | 6 (24%) | ||
| Ki67 proliferative index | ||||
| <5% (low-grade) | 29 (85.3%) | 24 (96%) | 0.179 | 4.138 (0.452–37.875) |
| >5% (high grade) | 5 (14.7%) | 1 (4%) | ||
| Tumor necrosis | ||||
| absent | 31 (91.2%) | 25 (100%) | 0.127 | 0.554 (0.438–0.700) |
| present | 3 (8.8%) | 0 (0%) | ||
| Tumor grade | ||||
| high-grade | 15 (44.1%) | 6 (24%) | 0.111 | 2.500 (0.799–7.821) |
| low-grade | 19 (55.9%) | 19 (76%) | ||
| Clinicopathological Characteristics | Lymph Node Metastasis | Univariate Analysis | OR (95% CI) | |
|---|---|---|---|---|
| Present (n = 20) | Absent (n = 23) | |||
| Age at diagnosis | ||||
| <55 yo | 8 (40%) | 5 (21.7%) | 0.193 | 0.416 (0.109–1.583) |
| >55 yo | 12 (60%) | 18 (78.2%) | ||
| Tumor size (mm) | ||||
| <10 mm | 0 (0%) | 3 (13%) | 0.022 * | 6 (1.081–33.275) |
| 10–40 mm | 12 (60%) | 18 (78.2%) | ||
| >40 mm | 8 (40%) | 2 (8.6%) | ||
| Histopathologic MTC type | ||||
| Conventional | 12 (60%) | 12 (52,1%) | 0.606 | 0.727 (0.216–2.444) |
| Other variants | 8 (40%) | 11 (47.8%) | ||
| Focality of the tumor | ||||
| Unifocal | 14 (70%) | 22 (95.6%) | 0.023 * | 9.428 (1.023–86.860) |
| Multifocal | 6 (30%) | 1 (4.4%) | ||
| Resection margins | ||||
| R0 | 16 (80%) | 22 (95.6%) | 0.110 | 5.500 (0.560–53.986) |
| R1 | 4 (20%) | 1 (4.3%) | ||
| Tumor recurrence | ||||
| present | 6 (30%) | 5 (21.7%) | 0.536 | 1.543 (0.389–6.115) |
| absent | 14 (70%) | 18 (78.3%) | ||
| Coexisting thyroid pathology | ||||
| Colloid goiter † | 8 (40%) | 10 (43.4%) | 0.967 | 1.125 (0.308–4.104) |
| Nodular goiter | 9 (45%) | 10 (43.4%) | ||
| Hashimoto thyroiditis | 3 (15%) | 3 (13%) | ||
| Mitotic index | ||||
| <5/2 mm2 (low-grade) | 11 (55%) | 16 (69.6%) | 0.324 | 1.870 (0.535–7.776) |
| >5/2 mm2 (high-grade) | 9 (45%) | 7 (30.4%) | ||
| Ki67 proliferative index | ||||
| <5% (low-grade) | 16 (80%) | 22 (95.6%) | 0.110 | 5.500 (0.560–53.986) |
| >5% (high grade) | 4 (20%) | 1 (4.4%) | ||
| Tumor necrosis | ||||
| Absent | 19 (95%) | 22 (95.6%) | 0.919 | 1.158 (0.068–19.798) |
| Present | 1 (5%) | 1 (4.4%) | ||
| Tumor grade | ||||
| High-grade | 9 (45%) | 7 (30.4%) | 0.324 | 1.870 (0.535–6.534) |
| Low-grade | 11 (55%) | 16 (69.6%) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giusca, S.E.; Andriescu, E.C.; Caruntu, I.D.; Ciobanu, D. Clinicopathological Profile of Medullary Thyroid Carcinoma—Could We Predict Aggressive Behavior? Biomedicines 2023, 11, 116. https://doi.org/10.3390/biomedicines11010116
Giusca SE, Andriescu EC, Caruntu ID, Ciobanu D. Clinicopathological Profile of Medullary Thyroid Carcinoma—Could We Predict Aggressive Behavior? Biomedicines. 2023; 11(1):116. https://doi.org/10.3390/biomedicines11010116
Chicago/Turabian StyleGiusca, Simona Eliza, Elena Corina Andriescu, Irina Draga Caruntu, and Delia Ciobanu. 2023. "Clinicopathological Profile of Medullary Thyroid Carcinoma—Could We Predict Aggressive Behavior?" Biomedicines 11, no. 1: 116. https://doi.org/10.3390/biomedicines11010116
APA StyleGiusca, S. E., Andriescu, E. C., Caruntu, I. D., & Ciobanu, D. (2023). Clinicopathological Profile of Medullary Thyroid Carcinoma—Could We Predict Aggressive Behavior? Biomedicines, 11(1), 116. https://doi.org/10.3390/biomedicines11010116






