Stem Cell Therapy for Alzheimer’s Disease: A Scoping Review for 2017–2022
Abstract
:1. Introduction
1.1. Alzheimer’s Disease and Its Treatments
1.2. Types of Stem Cell for AD Therapy
1.2.1. Neural Stem Cell (NSC)
1.2.2. Mesenchymal Stem Cell (MSC)
1.2.3. Embryonic Stem Cell (ESC)
1.2.4. Induced Pluripotent Stem Cell (iPSC)
1.3. Overview of Study and Objective
‘How did the different types of stem cells used for Alzheimer’s disease in studies on the cellular level, animal model, and clinical level imply their effectiveness?’
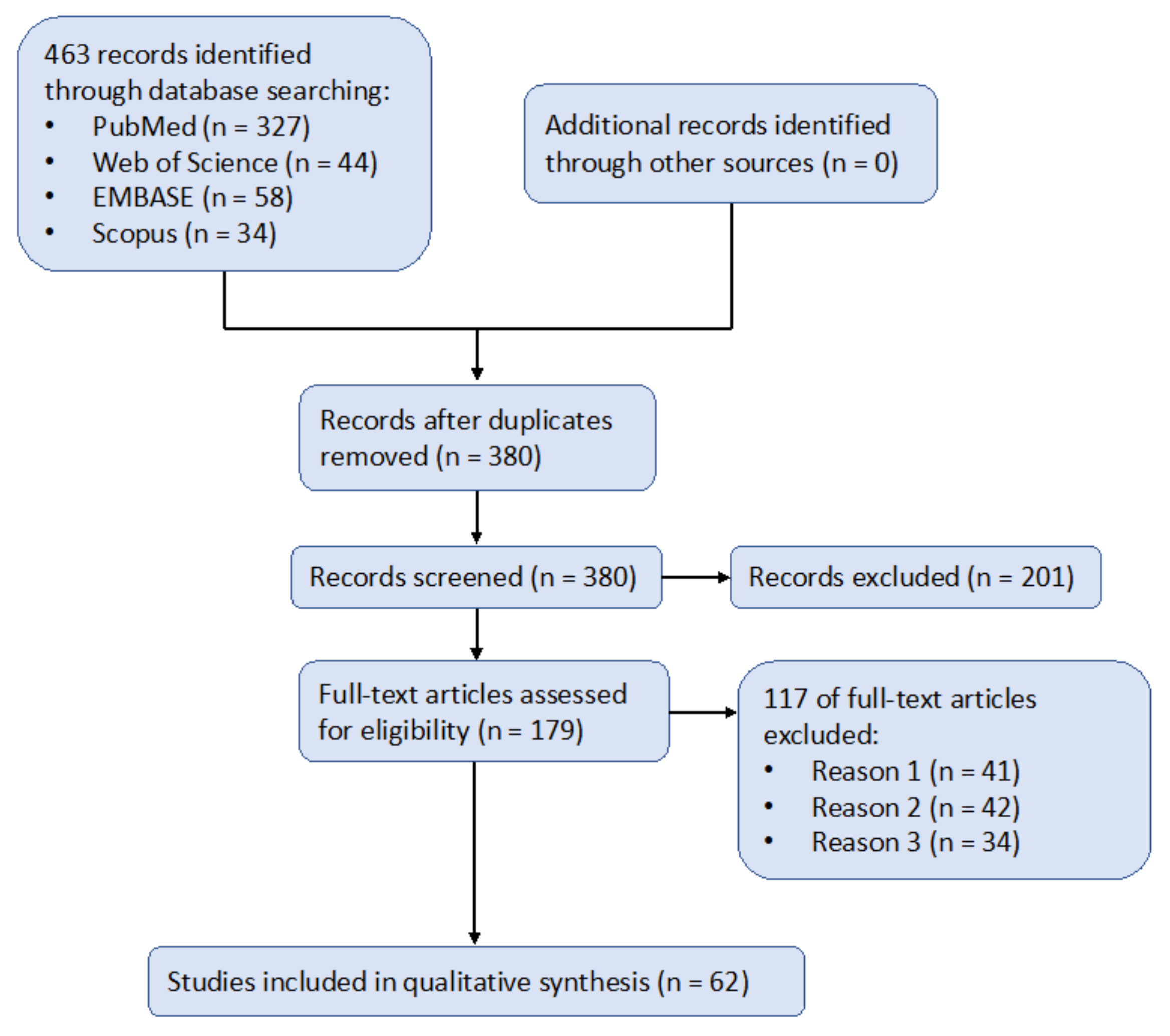
2. Methodology
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
- Reason 1 (n = 41): “stem cell” was mentioned in the article, not as a means of therapy but as a medium for other medical treatments or preclinical research. For instance, Kim et al. [43] mentioned induced pluripotent stem cells (iPSCs) only as a model for AD to aid their research on estrogen’s mitigating effect on AD. Even though this kind of research was related to stem cells, it did not have a significant relationship with stem cell therapy and thus was excluded from our records.
- Reason 2 (n = 42): the article contained brief mentioning of AD, but was not targeted toward AD. For instance, Rajan et al. [44] mentioned a collection of neurodegenerative diseases, with AD just as a sub-section of the entire article. Even though this article explained well the mechanisms of stem cell therapy, it did not include enough information for the treatment of AD and thus could not be included in our records.
- Reason 3 (n = 34): the exposure group (i.e., treated with stem cell therapy) could not be qualified as eligible to be included in the synthesis. For instance, Campos et al. [45] compared two types of stem cell therapies (neural and mesenchymal), instead of one with a control group. Therefore, it is difficult to qualify and quantify the effects of their research.
3. Analysis of the Included Studies
3.1. Cellular Level
3.2. Animal Model
3.3. Clinical Level
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alipour, M.; Nabavi, S.M.; Arab, L.; Vosough, M.; Pakdaman, H.; Ehsani, E.; Shahpasand, K. Stem cell therapy in Alzheimer’s disease: Possible benefits and limiting drawbacks. Mol. Biol. Rep. 2019, 46, 1425–1446. [Google Scholar] [CrossRef]
- Bagheri-Mohammadi, S. Stem cell-based therapy as a promising approach in Alzheimer’s disease: Current perspectives on novel treatment. Cell Tissue Bank. 2021, 22, 339–353. [Google Scholar] [CrossRef] [PubMed]
- Bali, P.; Lahiri, D.K.; Banik, A.; Nehru, B.; Anand, A. Potential for Stem Cells Therapy in Alzheimer’s Disease: Do Neurotrophic Factors Play Critical Role? Curr. Alzheimer Res. 2017, 14, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Agatonovic-Kustrin, S.; Kettle, C.; Morton, D.W. A molecular approach in drug development for Alzheimer’s disease. Biomed. Pharmacother. 2018, 106, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.; Lee, G.; Ritter, A.; Sabbagh, M.; Zhong, K. Alzheimer’s disease drug development pipeline: 2020. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2020, 6, e12050. [Google Scholar] [CrossRef]
- Kabir, M.T.; Uddin, M.S.; Mamun, A.A.; Jeandet, P.; Aleya, L.; Mansouri, R.A.; Ashraf, G.M.; Mathew, B.; Bin-Jumah, M.N.; Abdel-Daim, M.M. Combination drug therapy for the management of Alzheimer’s disease. Int. J. Mol. Sci. 2020, 21, 3272. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, Y.; Sterling, K.; Song, W. Brain-derived neurotrophic factor in Alzheimer’s disease and its pharmaceutical potential. Transl. Neurodegener. 2022, 11, 4. [Google Scholar] [CrossRef]
- Craft, S.; Raman, R.; Chow, T.W.; Rafii, M.S.; Sun, C.K.; Rissman, R.A.; Donohue, M.C.; Brewer, J.B.; Jenkins, C.; Harless, K.; et al. Safety, Efficacy, and Feasibility of Intranasal Insulin for the Treatment of Mild Cognitive Impairment and Alzheimer Disease Dementia: A Randomized Clinical Trial. JAMA Neurol. 2020, 77, 1099–1109. [Google Scholar] [CrossRef]
- De la Torre, J.C. Treating cognitive impairment with transcranial low level laser therapy. J. Photochem. Photobiol. B 2017, 168, 149–155. [Google Scholar] [CrossRef]
- Dos Santos-Neto, L.L.; de Vilhena Toledo, M.A.; Medeiros-Souza, P.; de Souza, G.A. The use of herbal medicine in Alzheimer’s disease-a systematic review. Evid.-Based Complement. Altern. Med. 2006, 3, 441–445. [Google Scholar] [CrossRef]
- Wu, A.J.; Tong, B.C.; Huang, A.S.; Li, M.; Cheung, K.H. Mitochondrial Calcium Signaling as a Therapeutic Target for Alzheimer’s Disease. Curr. Alzheimer Res. 2020, 17, 329–343. [Google Scholar] [CrossRef]
- Jadiya, P.; Kolmetzky, D.W.; Tomar, D.; Di Meco, A.; Lombardi, A.A.; Lambert, J.P.; Luongo, T.S.; Ludtmann, M.H.; Praticò, D.; Elrod, J.W. Impaired mitochondrial calcium efflux contributes to disease progression in models of Alzheimer’s disease. Nat. Commun. 2019, 10, 3885. [Google Scholar] [CrossRef] [Green Version]
- Godoy, J.A.; Rios, J.A.; Zolezzi, J.M.; Braidy, N.; Inestrosa, N.C. Signaling pathway cross talk in Alzheimer’s disease. Cell Commun. Signal. 2014, 12, 23. [Google Scholar] [CrossRef] [Green Version]
- Dhana, K.; Evans, D.A.; Rajan, K.B.; Bennett, D.A.; Morris, M.C. Healthy lifestyle and the risk of Alzheimer dementia: Findings from 2 longitudinal studies. Neurology 2020, 95, e374–e383. [Google Scholar] [CrossRef]
- Chakari-Khiavi, F.; Dolati, S.; Chakari-Khiavi, A.; Abbaszadeh, H.; Aghebati-Maleki, L.; Pourlak, T.; Mehdizadeh, A.; Yousefi, M. Prospects for the application of mesenchymal stem cells in Alzheimer’s disease treatment. Life Sci. 2019, 231, 116564. [Google Scholar] [CrossRef]
- Duncan, T.; Valenzuela, M. Alzheimer’s disease, dementia, and stem cell therapy. Stem Cell Res. Ther. 2017, 8, 111. [Google Scholar] [CrossRef]
- Lucke-Wold, B.P.; Logsdon, A.F.; Manoranjan, B.; Turner, R.C.; McConnell, E.; Vates, G.E.; Huber, J.D.; Rosen, C.L.; Simard, J.M. Aneurysmal Subarachnoid Hemorrhage and Neuroinflammation: A Comprehensive Review. Int. J. Mol. Sci. 2016, 17, 497. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Lee, Y.; Lee, S.; Kim, K.; Song, M.; Lee, J. Mesenchymal Stem Cell Therapy and Alzheimer’s Disease: Current Status and Future Perspectives. J. Alzheimer’s Dis. 2020, 77, 1–14. [Google Scholar] [CrossRef]
- Boese, A.C.; Hamblin, M.H.; Lee, J.P. Neural stem cell therapy for neurovascular injury in Alzheimer’s disease. Exp. Neurol. 2020, 324, 113112. [Google Scholar] [CrossRef]
- Guo, M.; Yin, Z.; Chen, F.; Lei, P. Mesenchymal stem cell-derived exosome: A promising alternative in the therapy of Alzheimer’s disease. Alzheimer’s Res. Ther. 2020, 12, 109. [Google Scholar] [CrossRef]
- Hosseini, S.A.; Mohammadi, R.; Noruzi, S.; Mohamadi, Y.; Azizian, M.; Mousavy, S.M.; Ghasemi, F.; Hesari, A.; Sahebkar, A.; Salarinia, R.; et al. Stem cell- and gene-based therapies as potential candidates in Alzheimer’s therapy. J. Cell. Biochem. 2018, 119, 8723–8736. [Google Scholar] [CrossRef] [PubMed]
- Cosacak, M.I.; Bhattarai, P.; Kizil, C. Alzheimer’s disease, neural stem cells and neurogenesis: Cellular phase at single-cell level. Neural Regen. Res. 2020, 15, 824–827. [Google Scholar] [CrossRef] [PubMed]
- McGinley, L.M.; Kashlan, O.N.; Bruno, E.S.; Chen, K.S.; Hayes, J.M.; Kashlan, S.R.; Raykin, J.; Johe, K.; Murphy, G.G.; Feldman, E.L. Human neural stem cell transplantation improves cognition in a murine model of Alzheimer’s disease. Sci. Rep. 2018, 8, 14776. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Lin, H.T.; Lee, C.C.; Tsai, K.J. Effects of neural stem cell transplantation in Alzheimer’s disease models. J. Biomed. Sci. 2020, 27, 29. [Google Scholar] [CrossRef] [Green Version]
- Apodaca, L.A.; Baddour, A.A.D.; Garcia, C., Jr.; Alikhani, L.; Giedzinski, E.; Ru, N.; Agrawal, A.; Acharya, M.M.; Baulch, J.E. Human neural stem cell-derived extracellular vesicles mitigate hallmarks of Alzheimer’s disease. Alzheimer’s Res. Ther. 2021, 13, 57. [Google Scholar] [CrossRef] [PubMed]
- Revuelta, M.; Urrutia, J.; Villarroel, A.; Casis, O. Microglia-Mediated Inflammation and Neural Stem Cell Differentiation in Alzheimer’s Disease: Possible Therapeutic Role of K(V)1.3 Channel Blockade. Front. Cell. Neurosci. 2022, 16, 868842. [Google Scholar] [CrossRef]
- Reza-Zaldivar, E.E.; Hernández-Sapiéns, M.A.; Gutiérrez-Mercado, Y.K.; Sandoval-Ávila, S.; Gomez-Pinedo, U.; Márquez-Aguirre, A.L.; Vázquez-Méndez, E.; Padilla-Camberos, E.; Canales-Aguirre, A.A. Mesenchymal stem cell-derived exosomes promote neurogenesis and cognitive function recovery in a mouse model of Alzheimer’s disease. Neural Regen. Res. 2019, 14, 1626–1634. [Google Scholar] [CrossRef]
- Nakano, M.; Kubota, K.; Kobayashi, E.; Chikenji, T.S.; Saito, Y.; Konari, N.; Fujimiya, M. Bone marrow-derived mesenchymal stem cells improve cognitive impairment in an Alzheimer’s disease model by increasing the expression of microRNA-146a in hippocampus. Sci. Rep. 2020, 10, 10772. [Google Scholar] [CrossRef]
- Wei, H.; Xu, Y.; Chen, Q.; Chen, H.; Zhu, X.; Li, Y. Mesenchymal stem cell-derived exosomal miR-223 regulates neuronal cell apoptosis. Cell Death Dis. 2020, 11, 290. [Google Scholar] [CrossRef]
- Kim, H.J.; Cho, K.R.; Jang, H.; Lee, N.K.; Jung, Y.H.; Kim, J.P.; Lee, J.I.; Chang, J.W.; Park, S.; Kim, S.T.; et al. Intracerebroventricular injection of human umbilical cord blood mesenchymal stem cells in patients with Alzheimer’s disease dementia: A phase I clinical trial. Alzheimer’s Res. Ther. 2021, 13, 154. [Google Scholar] [CrossRef]
- Ubina, T.; Magallanes, M.; Srivastava, S.; Warden, C.D.; Yee, J.K.; Salvaterra, P.M. A Human Embryonic Stem Cell Model of Aβ-Dependent Chronic Progressive Neurodegeneration. Front. Neurosci. 2019, 13, 1007. [Google Scholar] [CrossRef] [Green Version]
- Fan, W.; Tang, S.; Fan, X.; Fang, Y.; Xu, X.; Li, L.; Xu, J.; Li, J.L.; Wang, Z.; Li, X. SIRT1 regulates sphingolipid metabolism and neural differentiation of mouse embryonic stem cells through c-Myc-SMPDL3B. eLife 2021, 10, e67452. [Google Scholar] [CrossRef]
- Kim, D.Y.; Choi, S.H.; Lee, J.S.; Kim, H.J.; Kim, H.N.; Lee, J.E.; Shin, J.Y.; Lee, P.H. Feasibility and Efficacy of Intra-Arterial Administration of Embryonic Stem Cell Derived-Mesenchymal Stem Cells in Animal Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2020, 76, 1281–1296. [Google Scholar] [CrossRef]
- Atkinson-Dell, R.; Mohamet, L. Induced Pluripotent Stem Cell-Derived Astroglia: A New Tool for Research towards the Treatment of Alzheimer’s Disease. Adv. Exp. Med. Biol. 2019, 1175, 383–405. [Google Scholar] [CrossRef]
- Butler Iii, R.R.; Kozlova, A.; Zhang, H.; Zhang, S.; Streit, M.; Sanders, A.R.; Laudanski, K.; Pang, Z.P.; Gejman, P.V.; Duan, J. The Genetic Relevance of Human Induced Pluripotent Stem Cell-Derived Microglia to Alzheimer’s Disease and Major Neuropsychiatric Disorders. Mol. Neuropsychiatry 2020, 5, 85–96. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, M.; Ren, Q.; Liu, G.; Meng, S.; Xiahou, K.; Zhang, Y.; Jiang, N.; Zhou, W. Human Induced Pluripotent Stem Cell-Derived Neural Cells from Alzheimer’s Disease Patients Exhibited Different Susceptibility to Oxidative Stress. Stem Cells Dev. 2020, 29, 1444–1456. [Google Scholar] [CrossRef]
- Armijo, E.; Edwards, G.; Flores, A.; Vera, J.; Shahnawaz, M.; Moda, F.; Gonzalez, C.; Sanhueza, M.; Soto, C. Induced Pluripotent Stem Cell-Derived Neural Precursors Improve Memory, Synaptic and Pathological Abnormalities in a Mouse Model of Alzheimer’s Disease. Cells 2021, 10, 1802. [Google Scholar] [CrossRef]
- Aboul-Soud, M.A.M.; Alzahrani, A.J.; Mahmoud, A. Induced Pluripotent Stem Cells (iPSCs)-Roles in Regenerative Therapies, Disease Modelling and Drug Screening. Cells 2021, 10, 2319. [Google Scholar] [CrossRef]
- Kolagar, T.A.; Farzaneh, M.; Nikkar, N.; Khoshnam, S.E. Human Pluripotent Stem Cells in Neurodegenerative Diseases: Potentials, Advances and Limitations. Curr. Stem Cell Res. Ther. 2020, 15, 102–110. [Google Scholar] [CrossRef]
- Chang, C.Y.; Ting, H.C.; Liu, C.A.; Su, H.L.; Chiou, T.W.; Lin, S.Z.; Harn, H.J.; Ho, T.J. Induced Pluripotent Stem Cell (iPSC)-Based Neurodegenerative Disease Models for Phenotype Recapitulation and Drug Screening. Molecules 2020, 25, 2000. [Google Scholar] [CrossRef]
- Kwak, K.A.; Lee, S.P.; Yang, J.Y.; Park, Y.S. Current Perspectives regarding Stem Cell-Based Therapy for Alzheimer’s Disease. Stem Cells Int. 2018, 2018, 6392986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yepes-Nuñez, J.; Urrutia, G.; Romero-Garcia, M.; Alonso-Fernandez, S. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Rev. Esp. Cardiol. (Engl. Ed.) 2021, 74, 790–799. [Google Scholar]
- Kim, J.Y.; Mo, H.; Kim, J.; Kim, J.W.; Nam, Y.; Rim, Y.A.; Ju, J.H. Mitigating Effect of Estrogen in Alzheimer’s Disease-Mimicking Cerebral Organoid. Front. Neurosci. 2022, 16, 816174. [Google Scholar] [CrossRef] [PubMed]
- Rajan, T.S.; Scionti, D.; Diomede, F.; Grassi, G.; Pollastro, F.; Piattelli, A.; Cocco, L.; Bramanti, P.; Mazzon, E.; Trubiani, O. Gingival Stromal Cells as an In Vitro Model: Cannabidiol Modulates Genes Linked with Amyotrophic Lateral Sclerosis. J. Cell. Biochem. 2017, 118, 819–828. [Google Scholar] [CrossRef]
- Campos, H.C.; Ribeiro, D.E.; Hashiguchi, D.; Hukuda, D.Y.; Gimenes, C.; Romariz, S.A.A.; Ye, Q.; Tang, Y.; Ulrich, H.; Longo, B.M. Distinct Effects of the Hippocampal Transplantation of Neural and Mesenchymal Stem Cells in a Transgenic Model of Alzheimer’s Disease. Stem Cell Rev. Rep. 2022, 18, 781–791. [Google Scholar] [CrossRef]
- Lin, Y.T.; Seo, J.; Gao, F.; Feldman, H.M.; Wen, H.L.; Penney, J.; Cam, H.P.; Gjoneska, E.; Raja, W.K.; Cheng, J.; et al. APOE4 Causes Widespread Molecular and Cellular Alterations Associated with Alzheimer’s Disease Phenotypes in Human iPSC-Derived Brain Cell Types. Neuron 2018, 98, 1141–1154.e7. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Fu, Y.; Yamazaki, Y.; Ren, Y.; Davis, M.D.; Liu, C.C.; Lu, W.; Wang, X.; Chen, K.; Cherukuri, Y.; et al. APOE4 exacerbates synapse loss and neurodegeneration in Alzheimer’s disease patient iPSC-derived cerebral organoids. Nat. Commun. 2020, 11, 5540. [Google Scholar] [CrossRef]
- Farahzadi, R.; Fathi, E.; Vietor, I. Mesenchymal Stem Cells Could Be Considered as a Candidate for Further Studies in Cell-Based Therapy of Alzheimer’s Disease via Targeting the Signaling Pathways. ACS Chem. Neurosci. 2020, 11, 1424–1435. [Google Scholar] [CrossRef]
- Xu, Z.; Nan, W.; Zhang, X.; Sun, Y.; Yang, J.; Lu, K.; Liu, Y.; Gao, Y.; Yang, F.; Mao, W.; et al. Umbilical Cord Mesenchymal Stem Cells Conditioned Medium Promotes Aβ25-35 phagocytosis by Modulating Autophagy and Aβ-Degrading Enzymes in BV2 Cells. J. Mol. Neurosci. 2018, 65, 222–233. [Google Scholar] [CrossRef]
- Chang, K.H.; Lee-Chen, G.J.; Huang, C.C.; Lin, J.L.; Chen, Y.J.; Wei, P.C.; Lo, Y.S.; Yao, C.F.; Kuo, M.W.; Chen, C.M. Modeling Alzheimer’s Disease by Induced Pluripotent Stem Cells Carrying APP D678H Mutation. Mol. Neurobiol. 2019, 56, 3972–3983. [Google Scholar] [CrossRef] [Green Version]
- Coronel, R.; Lachgar, M.; Bernabeu-Zornoza, A.; Palmer, C.; Domínguez-Alvaro, M.; Revilla, A.; Ocaña, I.; Fernández, A.; Martínez-Serrano, A.; Cano, E.; et al. Neuronal and Glial Differentiation of Human Neural Stem Cells Is Regulated by Amyloid Precursor Protein (APP) Levels. Mol. Neurobiol. 2019, 56, 1248–1261. [Google Scholar] [CrossRef]
- Lo Giudice, M.; Mihalik, B.; Turi, Z.; Dinnyés, A.; Kobolák, J. Calcilytic NPS 2143 Reduces Amyloid Secretion and Increases sAβPPα Release from PSEN1 Mutant iPSC-Derived Neurons. J. Alzheimer’s Dis. 2019, 72, 885–899. [Google Scholar] [CrossRef] [Green Version]
- Marzano, M.; Bejoy, J.; Cheerathodi, M.R.; Sun, L.; York, S.B.; Zhao, J.; Kanekiyo, T.; Bu, G.; Meckes, D.G., Jr.; Li, Y. Differential Effects of Extracellular Vesicles of Lineage-Specific Human Pluripotent Stem Cells on the Cellular Behaviors of Isogenic Cortical Spheroids. Cells 2019, 8, 993. [Google Scholar] [CrossRef] [Green Version]
- Meyer, K.; Feldman, H.M.; Lu, T.; Drake, D.; Lim, E.T.; Ling, K.H.; Bishop, N.A.; Pan, Y.; Seo, J.; Lin, Y.T.; et al. REST and Neural Gene Network Dysregulation in iPSC Models of Alzheimer’s Disease. Cell Rep. 2019, 26, 1112–1127.e9. [Google Scholar] [CrossRef] [Green Version]
- Van der Kant, R.; Langness, V.F.; Herrera, C.M.; Williams, D.A.; Fong, L.K.; Leestemaker, Y.; Steenvoorden, E.; Rynearson, K.D.; Brouwers, J.F.; Helms, J.B.; et al. Cholesterol Metabolism Is a Druggable Axis that Independently Regulates Tau and Amyloid-β in iPSC-Derived Alzheimer’s Disease Neurons. Cell Stem Cell 2019, 24, 363–375.e9. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Sheng, H.; Liao, L.; Xu, C.; Zhang, A.; Yang, Y.; Zhao, L.; Duan, L.; Chen, H.; Zhang, B. Mesenchymal Stem Cell-Conditioned Medium Improves Mitochondrial Dysfunction and Suppresses Apoptosis in Okadaic Acid-Treated SH-SY5Y Cells by Extracellular Vesicle Mitochondrial Transfer. J. Alzheimer’s Dis. 2020, 78, 1161–1176. [Google Scholar] [CrossRef]
- Chen, Y.A.; Lu, C.H.; Ke, C.C.; Chiu, S.J.; Jeng, F.S.; Chang, C.W.; Yang, B.H.; Liu, R.S. Mesenchymal Stem Cell-Derived Exosomes Ameliorate Alzheimer’s Disease Pathology and Improve Cognitive Deficits. Biomedicines 2021, 9, 594. [Google Scholar] [CrossRef]
- Boutajangout, A.; Noorwali, A.; Atta, H.; Wisniewski, T. Human Umbilical Cord Stem Cell Xenografts Improve Cognitive Decline and Reduce the Amyloid Burden in a Mouse Model of Alzheimer’s Disease. Curr. Alzheimer Res. 2017, 14, 104–111. [Google Scholar] [CrossRef]
- Cui, Y.; Ma, S.; Zhang, C.; Cao, W.; Liu, M.; Li, D.; Lv, P.; Xing, Q.; Qu, R.; Yao, N.; et al. Human umbilical cord mesenchymal stem cells transplantation improves cognitive function in Alzheimer’s disease mice by decreasing oxidative stress and promoting hippocampal neurogenesis. Behav. Brain Res. 2017, 320, 291–301. [Google Scholar] [CrossRef]
- Harach, T.; Jammes, F.; Muller, C.; Duthilleul, N.; Cheatham, V.; Zufferey, V.; Cheatham, D.; Lukasheva, Y.A.; Lasser, T.; Bolmont, T. Administrations of human adult ischemia-tolerant mesenchymal stem cells and factors reduce amyloid beta pathology in a mouse model of Alzheimer’s disease. Neurobiol. Aging 2017, 51, 83–96. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, N.K.; Yoo, D.; Lee, J.; Choi, S.J.; Oh, W.; Chang, J.W.; Na, D.L. Lowering the concentration affects the migration and viability of intracerebroventricular-delivered human mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2017, 493, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Zhou, Y.; Zhang, R.; Wu, K.; Lu, Y.; Li, Y.; Duan, R.; Yao, Y.; Zhu, D.; Jia, Y. MicroRNA Let-7f-5p Promotes Bone Marrow Mesenchymal Stem Cells Survival by Targeting Caspase-3 in Alzheimer Disease Model. Front. Neurosci. 2018, 12, 333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, S.; Son, M.; Choi, J.; Lee, S.; Byun, K. sRAGE prolonged stem cell survival and suppressed RAGE-related inflammatory cell and T lymphocyte accumulations in an Alzheimer’s disease model. Biochem. Biophys. Res. Commun. 2018, 495, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Jia, J.; Wang, Z. Mesenchymal Stem Cell-Derived Extracellular Vesicles Suppresses iNOS Expression and Ameliorates Neural Impairment in Alzheimer’s Disease Mice. J. Alzheimer’s Dis. 2018, 61, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ma, S.; Yang, B.; Huang, T.; Meng, N.; Xu, L.; Xing, Q.; Zhang, Y.; Zhang, K.; Li, Q.; et al. Resveratrol promotes hUC-MSCs engraftment and neural repair in a mouse model of Alzheimer’s disease. Behav. Brain Res. 2018, 339, 297–304. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Xie, Z.; Bi, J.; Zhu, Z. Anti-inflammatory effects of bone marrow mesenchymal stem cells on mice with Alzheimer’s disease. Exp. Ther. Med. 2018, 16, 5015–5020. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.; Hei, Y.; Liu, W. Upregulation of seladin-1 and nestin expression in bone marrow mesenchymal stem cell transplantation via the ERK1/2 and PI3K/Akt signaling pathways in an Alzheimer’s disease model. Oncol. Lett. 2018, 15, 7443–7449. [Google Scholar] [CrossRef]
- Esmaeilzade, B.; Artimani, T.; Amiri, I.; Najafi, R.; Shahidi, S.; Sabec, M.; Farzadinia, P.; Zare, M.; Zahiri, M.; Soleimani Asl, S. Dimethyloxalylglycine preconditioning enhances protective effects of bone marrow-derived mesenchymal stem cells in Aβ-induced Alzheimer disease. Physiol. Behav. 2019, 199, 265–272. [Google Scholar] [CrossRef]
- Hu, W.; Feng, Z.; Xu, J.; Jiang, Z.; Feng, M. Brain-derived neurotrophic factor modified human umbilical cord mesenchymal stem cells-derived cholinergic-like neurons improve spatial learning and memory ability in Alzheimer’s disease rats. Brain Res. 2019, 1710, 61–73. [Google Scholar] [CrossRef]
- Nasiri, E.; Alizadeh, A.; Roushandeh, A.M.; Gazor, R.; Hashemi-Firouzi, N.; Golipoor, Z. Melatonin-pretreated adipose-derived mesenchymal stem cells efficeintly improved learning, memory, and cognition in an animal model of Alzheimer’s disease. Metab. Brain Dis. 2019, 34, 1131–1143. [Google Scholar] [CrossRef]
- Eftekharzadeh, M.; Simorgh, S.; Doshmanziari, M.; Hassanzadeh, L.; Shariatpanahi, M. Human adipose-derived stem cells reduce receptor-interacting protein 1, receptor-interacting protein 3, and mixed lineage kinase domain-like pseudokinase as necroptotic markers in rat model of Alzheimer’s disease. Indian J. Pharmacol. 2020, 52, 392–401. [Google Scholar] [CrossRef]
- Guo, X.; Liu, Y.; Morgan, D.; Zhao, L.R. Reparative Effects of Stem Cell Factor and Granulocyte Colony-Stimulating Factor in Aged APP/PS1 Mice. Aging Dis. 2020, 11, 1423–1443. [Google Scholar] [CrossRef]
- Liu, Y.; Huber, C.C.; Wang, H. Disrupted blood-brain barrier in 5×FAD mouse model of Alzheimer’s disease can be mimicked and repaired in vitro with neural stem cell-derived exosomes. Biochem. Biophys. Res. Commun. 2020, 525, 192–196. [Google Scholar] [CrossRef]
- Mehrabadi, S.; Motevaseli, E.; Sadr, S.S.; Moradbeygi, K. Hypoxic-conditioned medium from adipose tissue mesenchymal stem cells improved neuroinflammation through alternation of toll like receptor (TLR) 2 and TLR4 expression in model of Alzheimer’s disease rats. Behav. Brain Res. 2020, 379, 112362. [Google Scholar] [CrossRef]
- Park, B.N.; Kim, J.H.; Lim, T.S.; Park, S.H.; Kim, T.G.; Yoon, B.S.; Son, K.S.; Yoon, J.K.; An, Y.S. Therapeutic effect of mesenchymal stem cells in an animal model of Alzheimer’s disease evaluated by β-amyloid positron emission tomography imaging. Aust. N. Z. J. Psychiatry 2020, 54, 883–891. [Google Scholar] [CrossRef]
- Park, D.; Choi, E.K.; Cho, T.H.; Joo, S.S.; Kim, Y.B. Human Neural Stem Cells Encoding ChAT Gene Restore Cognitive Function via Acetylcholine Synthesis, Aβ Elimination, and Neuroregeneration in APPswe/PS1dE9 Mice. Int. J. Mol. Sci. 2020, 21, 3958. [Google Scholar] [CrossRef]
- Ramezani, M.; Komaki, A.; Hashemi-Firouzi, N.; Mortezaee, K.; Faraji, N.; Golipoor, Z. Therapeutic effects of melatonin-treated bone marrow mesenchymal stem cells (BMSC) in a rat model of Alzheimer’s disease. J. Chem. Neuroanat. 2020, 108, 101804. [Google Scholar] [CrossRef]
- Zhao, J.; Su, M.; Lin, Y.; Liu, H.; He, Z.; Lai, L. Administration of Amyloid Precursor Protein Gene Deleted Mouse ESC-Derived Thymic Epithelial Progenitors Attenuates Alzheimer’s Pathology. Front. Immunol. 2020, 11, 1781. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhang, N.; Hu, N.; Jiang, R.; Lu, H.; Xuan, A.; Long, D.; Chen, Y. Neural stem cell transplantation improves learning and memory by protecting cholinergic neurons and restoring synaptic impairment in an amyloid precursor protein/presenilin 1 transgenic mouse model of Alzheimer’s disease. Mol. Med. Rep. 2020, 21, 1172–1180. [Google Scholar] [CrossRef]
- Cone, A.S.; Yuan, X.; Sun, L.; Duke, L.C.; Vreones, M.P.; Carrier, A.N.; Kenyon, S.M.; Carver, S.R.; Benthem, S.D.; Stimmell, A.C.; et al. Mesenchymal stem cell-derived extracellular vesicles ameliorate Alzheimer’s disease-like phenotypes in a preclinical mouse model. Theranostics 2021, 11, 8129–8142. [Google Scholar] [CrossRef]
- Huang, D.; Cao, Y.; Yang, X.; Liu, Y.; Zhang, Y.; Li, C.; Chen, G.; Wang, Q. A Nanoformulation-Mediated Multifunctional Stem Cell Therapy with Improved β-Amyloid Clearance and Neural Regeneration for Alzheimer’s Disease. Adv. Mater. 2021, 33, 2006357. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Kim, O.J.; Oh, S.H.; Lee, S.; Reum Lee, H.A.; Lee, K.O.; Lee, B.Y.; Kim, N.K. Extracellular Vesicles Released from Neprilysin Gene-Modified Human Umbilical Cord-Derived Mesenchymal Stem Cell Enhance Therapeutic Effects in an Alzheimer’s Disease Animal Model. Stem Cells Int. 2021, 2021, 5548630. [Google Scholar] [CrossRef] [PubMed]
- Kuo, S.C.; Chio, C.C.; Yeh, C.H.; Ma, J.T.; Liu, W.P.; Lin, M.T.; Lin, K.C.; Chang, C.P. Mesenchymal stem cell-conditioned medium attenuates the retinal pathology in amyloid-β-induced rat model of Alzheimer’s disease: Underlying mechanisms. Aging Cell 2021, 20, e13340. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.Y.; Park, S.I.; Park, S.A.; Jeon, J.H.; Jung, H.Y.; Yon, J.M.; Jeun, S.S.; Lim, H.K.; Kim, S.W. Potential application of human neural crest-derived nasal turbinate stem cells for the treatment of neuropathology and impaired cognition in models of Alzheimer’s disease. Stem Cell Res. Ther. 2021, 12, 402. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.H.; Ji, W.L.; Chen, H.; Sun, Y.Y.; Zhao, X.Y.; Wang, F.; Shi, Y.; Hu, Y.N.; Liu, B.X.; Wu, J.W.; et al. Intranasal Transplantation of Human Neural Stem Cells Ameliorates Alzheimer’s Disease-Like Pathology in a Mouse Model. Front. Aging Neurosci. 2021, 13, 650103. [Google Scholar] [CrossRef]
- Neves, A.F.; Camargo, C.; Premer, C.; Hare, J.M.; Baumel, B.S.; Pinto, M. Intravenous administration of mesenchymal stem cells reduces Tau phosphorylation and inflammation in the 3xTg-AD mouse model of Alzheimer’s disease. Exp. Neurol. 2021, 341, 113706. [Google Scholar] [CrossRef]
- Santamaria, G.; Brandi, E.; Vitola, P.; Grandi, F.; Ferrara, G.; Pischiutta, F.; Vegliante, G.; Zanier, E.R.; Re, F.; Uccelli, A.; et al. Intranasal delivery of mesenchymal stem cell secretome repairs the brain of Alzheimer’s mice. Cell Death Differ. 2021, 28, 203–218. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Li, J.; Wang, T.; Hei, Y.; Li, H.; Wang, X.; Wang, L.; Zhao, R.; Liu, W.; et al. Tail-vein injection of MSC-derived small extracellular vesicles facilitates the restoration of hippocampal neuronal morphology and function in APP / PS1 mice. Cell Death Discov. 2021, 7, 230. [Google Scholar] [CrossRef]
- Zhang, X.M.; Ouyang, Y.J.; Yu, B.Q.; Li, W.; Yu, M.Y.; Li, J.Y.; Jiao, Z.M.; Yang, D.; Li, N.; Shi, Y.; et al. Therapeutic potential of dental pulp stem cell transplantation in a rat model of Alzheimer’s disease. Neural Regen. Res. 2021, 16, 893–898. [Google Scholar] [CrossRef]
- Choi, J.M.; Park, H.S.; He, M.T.; Kim, Y.S.; Kim, H.Y.; Lee, A.Y.; Cho, E.J. Membrane-Free Stem Cells and Pyridoxal 5’-Phosphate Synergistically Enhance Cognitive Function in Alzheimer’s Disease Mouse Model. Antioxidants 2022, 11, 601. [Google Scholar] [CrossRef]
- Guo, W.; Zeng, Z.; Xing, C.; Zhang, J.; Bi, W.; Yang, J.; Shah, R.; Wang, D.; Li, Y.; Zhang, X.; et al. Stem cells from human exfoliated deciduous teeth affect mitochondria and reverse cognitive decline in a senescence-accelerated mouse prone 8 model. Cytotherapy 2022, 24, 59–71. [Google Scholar] [CrossRef]
- Liu, S.; Fan, M.; Xu, J.X.; Yang, L.J.; Qi, C.C.; Xia, Q.R.; Ge, J.F. Exosomes derived from bone-marrow mesenchymal stem cells alleviate cognitive decline in AD-like mice by improving BDNF-related neuropathology. J. Neuroinflam. 2022, 19, 35. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, J.; Fu, X.; Zhang, J.; Song, J.; Wang, Y.; Duan, L.; Shao, P.; Xu, X.; Zeng, L.; et al. Fe3O4@polydopamine nanoparticle-loaded human umbilical cord mesenchymal stem cells improve the cognitive function in Alzheimer’s disease mice by promoting hippocampal neurogenesis. Nanomedicine 2022, 40, 102507. [Google Scholar] [CrossRef]
- Zhang, H.A.; Yuan, C.X.; Liu, K.F.; Yang, Q.F.; Zhao, J.; Li, H.; Yang, Q.H.; Song, D.; Quan, Z.Z.; Qing, H. Neural stem cell transplantation alleviates functional cognitive deficits in a mouse model of tauopathy. Neural Regen Res. 2022, 17, 152–162. [Google Scholar] [CrossRef]
- Zhang, X.; Lei, T.; Wang, D.; Cai, S.; Hang, Z.; Yang, Y.; Bi, W.; Xiao, Z.; Du, H. Stem cells from human exfoliated deciduous teeth relieves Alzheimer’s disease symptoms in SAMP8 mice by up-regulating the PPARγ pathway. Biomed. Pharmacother. 2022, 152, 113169. [Google Scholar] [CrossRef]
- Wang, Y.; Jing, N.; Su, L.; Shi, C.; Zhang, P.; Wang, Z.; Sun, H.; Yang, J.; Liu, Y.; Wen, X.; et al. Establishment of induced pluripotent stem cell line (ZZUi009-A) from an Alzheimer’s disease patient carrying a PSEN1 gene mutation. Stem Cell Res. 2018, 27, 30–33. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, H.; Yang, J.; Shi, C.; Liu, Y.; Xu, Y.; Zhang, J. Generation of induced pluripotent stem cell line (ZZUi0013-A) from a 65-year-old patient with a novel MEOX2 gene mutation in Alzheimer’s disease. Stem Cell Res. 2019, 34, 101366. [Google Scholar] [CrossRef]
- Dai, Q.; Zhang, W.; Guo, J.; Di, W.; Zhao, J.; Zhang, B.; Wang, Y. Generation of an induced pluripotent stem cell line (SIAISi003-A) from a 79-year-old patient with Alzheimer’s disease having APOE3/4 genetic background. Stem Cell Res. 2020, 48, 101949. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Du, X.; Ma, Z.; Liu, B.; Guo, R.; Feng, B.; Ma, J.; Cui, H. Induced pluripotent stem cells derived from one 70-years-old male donor with the APOE-ε4/ε4 alleles. Stem Cell Res. 2021, 53, 102395. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, H.; Zhang, Q.; Yang, J.; Liu, Y.; Liu, H.; Shi, C.; Wang, Y.; Xu, Y. Generation of induced pluripotent stem cell line (ZZUi0024-A) from a 51-year-old patient with APP gene mutation in Alzheimer’ s disease. Stem Cell Res. 2021, 53, 102267. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, M.; Lee, M.; So, S.; Kang, S.S.; Choi, J.; Kim, D.; Heo, H.; Lee, S.S.; Park, H.R.; et al. Mitochondrial genome mutations and neuronal dysfunction of induced pluripotent stem cells derived from patients with Alzheimer’s disease. Cell Prolif. 2022, 55, e13274. [Google Scholar] [CrossRef] [PubMed]
- Majd, S.; Power, J.; Majd, Z. Alzheimer’s Disease and Cancer: When Two Monsters Cannot Be Together. Front. Neurosci. 2019, 13, 155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takagi, Y. History of Neural Stem Cell Research and Its Clinical Application. Neurol. Med.-Chir. 2016, 56, 110–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stopa, E.G.; Gonzalez, A.M.; Chorsky, R.; Corona, R.J.; Alvarez, J.; Bird, E.D.; Baird, A. Basic fibroblast growth factor in Alzheimer’s disease. Biochem. Biophys. Res. Commun. 1990, 171, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, S.; He, X.B.; Cheng, C.; Le, W. Induced pluripotent stem cells in Alzheimer’s disease: Applications for disease modeling and cell-replacement therapy. Mol. Neurodegener. 2016, 11, 39. [Google Scholar] [CrossRef] [Green Version]
- Qin, C.; Lu, Y.; Wang, K.; Bai, L.; Shi, G.; Huang, Y.; Li, Y. Transplantation of bone marrow mesenchymal stem cells improves cognitive deficits and alleviates neuropathology in animal models of Alzheimer’s disease: A meta-analytic review on potential mechanisms. Transl. Neurodegener. 2020, 9, 20. [Google Scholar] [CrossRef]
- Volarevic, V.; Markovic, B.S.; Gazdic, M.; Volarevic, A.; Jovicic, N.; Arsenijevic, N.; Armstrong, L.; Djonov, V.; Lako, M.; Stojkovic, M. Ethical and Safety Issues of Stem Cell-Based Therapy. Int. J. Med. Sci. 2018, 15, 36–45. [Google Scholar] [CrossRef] [Green Version]
- Gorecka, J.; Kostiuk, V.; Fereydooni, A.; Gonzalez, L.; Luo, J.; Dash, B.; Isaji, T.; Ono, S.; Liu, S.; Lee, S.R.; et al. The potential and limitations of induced pluripotent stem cells to achieve wound healing. Stem Cell Res. Ther. 2019, 10, 87. [Google Scholar] [CrossRef] [Green Version]
- Wei, W.L.; Tsai, S.T.; Chiou, T.W.; Harn, H.J. Human-Induced Pluripotent Stem Cells and Herbal Small-Molecule Drugs for Treatment of Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 1327. [Google Scholar] [CrossRef] [Green Version]
- Patwardhan, A.G.; Belemkar, S. An update on Alzheimer’s disease: Immunotherapeutic agents, stem cell therapy and gene editing. Life Sci. 2021, 282, 119790. [Google Scholar] [CrossRef]
- Zhao, X.R.; Li, D.D.; Zhang, L.; Niu, Y.H.; Wang, W.Z.; Niu, B. Mesenchymal stem cell therapies for Alzheimer’s disease: Preclinical studies. Metab. Brain Dis. 2021, 36, 1687–1695. [Google Scholar] [CrossRef]
- Salwa; Kumar, L. Engrafted stem cell therapy for Alzheimer’s disease: A promising treatment strategy with clinical outcome. J. Control. Release 2021, 338, 837–857. [Google Scholar] [CrossRef]


| Author | Year | Type of Stem Cell | Intervention | Control Group | Measured Outcomes of Interest | Reference | |
|---|---|---|---|---|---|---|---|
| Animal-induced stem cells | Farahzadi et al. | 2020 | BM-MSC | Injection of BM-MSCs with Aβ-treated neural cells | Aβ-treated neural cells without BM-MSC injection | Significant increase in telomerase activity, which indicates mitigation of AD | [48] |
| Human-oriented stem cells | Xu et al. | 2018 | ucMSC | Culture ucMSCs to BV2 cell line with Aβ25–35 oligomers | BV2 cell line without culturing ucMSCs | MSCs inhibited proliferation of BV2 cells, mediated microglial autophagy, and enhanced clearance of Aβ, hence mitigating AD | [49] |
| Lin et al. | 2018 | iPSC | Using CRISPR/Cas9 to generate isogenic iPSCs harboring homozygous APOE4 alleles from unaffected parental APOE3 cells | Parental APOE3 cells without injection of isogenic iPSC lines | APOE4 iPSC-derived neurons recapitulate phenotypes of AD at multiple levels, including increased synapse number and elevated Aβ42 secretion | [46] | |
| Chang et al. | 2019 | iPSC | Injection of a potential Aβ aggregation reducer indole compound NC009–1 with iPSC on live human neurons from AD patients | AD neurons without injection of iPSC | Improved neurite outgrowth in AD-iPSC-derived neurons | [50] | |
| Coronel et al. | 2019 | hNSC | Transiently increase APP level in hNSCs | hNSCs without increasing APP level | Promoted gliogenesis and inhibited neurogenesis in hNSCs | [51] | |
| Giudice et al. | 2019 | iPSC | Injection of γ-secretase inhibitor to iPSC-derived neurons from AD patients | AD neurons without injection of iPSC-derived neurons | Increased expression of CaSR protein and modulated PSEN1 expression in plasma membrane of AD neurons | [52] | |
| Marzano et al. | 2019 | hiPSC | Injection of hiPSC-derived extracellular vesicles (EVs) to AD-associated SY-UBH cell lines | SY-UBH cells without hiPSC injection | hiPSC-derived EVs exhibited neural protective abilities in Aβ42 oligomer-treated cultures, enhancing cell viability and reducing oxidative stress | [53] | |
| Meyer et al. | 2019 | iPSC | iPSCs generated from sporadic AD (SAD) patients | iPSCs generated from age-matched controls of familial AD (FAD) patients | SAD iPSC lines showed a significant level of increase in neural differentiation-related gene expression, leading to premature neuronal differentiation, and reduced neural progenitor cell renewal | [54] | |
| van der Kant et al. | 2019 | iPSC | iPSC-derived neurons from SAD patients | iPSC-derived neurons from FAD patients | Allosteric activation of CYP46A1 lowered cholesteryl esters (CE) in iPSC-derived neurons, indicating a druggable CYP46A1-CE-Tau axis in AD. | [55] | |
| Zhang et al. | 2020 | hucMSC | Treatment of hucMSC conditioned medium to AD cellular model established by okadaic acid-treated SH-SY5Y cells | SH-SY5Y cells without treatment of hucMSC | hucMSCs significantly decreased tau phosphorylation at Thur181 level, and alleviated intracellular and mitochondrial oxidative stress in AD cells | [56] | |
| Zhao et al. | 2020 | iPSC | Cerebral organoid model using iPSCs with APOE3 or APOE4 genotype from individuals with AD dementia | Cerebral organoid model using iPSCs with APOE3 or APOE4 genotype from individuals with normal cognition | Cerebral organoids model using iPSCs from AD patients carrying APOE4 show greater apoptosis and decreased synaptic integrity | [47] | |
| Chen et al. | 2021 | MSC | Culture MSC-exosomes into human neural cell with AD mutations | Human neural cells without culturing MSC-exosomes | Improvement in brain glucose metabolism and cognitive functioning | [57] |
| Author | Year | Type of Stem Cell | Intervention | Control Group | Measured Outcomes of Interest | Reference |
|---|---|---|---|---|---|---|
| Boutajangout et al. | 2017 | hucMSC | Direct injection of hucMSCs into carotid artery of APP/PS1 Tg AD model mice | AD mice without injection of hucMSCs | Reduction of amyloid beta burden in cortex and hippocampus which correlated with a reduction of cognitive loss | [58] |
| Cui et al. | 2017 | ucMSC | Intravenous transplantation of ucMSCs to Tg2576 mice, which express AD-like APP pathological forms | Tg2576 mice without transplantation of ucMSCs | Reduced oxidative stress in hippocampus of AD mice due to decrease of malondialdehyde (MDA) and hence up-regulated neuronal synaptic plasticity | [59] |
| Harach et al. | 2017 | MSC | Administer MSC intravenously to APP/PS1 transgenic mice that developed cerebral Aβ | APPPS1 transgenic mice without administering MSCs | Reduced soluble cerebral Aβ levels and increased Aβ-degrading enzymes to modulate cerebral cytokines | [60] |
| Kim et al. | 2017 | hUCB-MSC | Injection of hUCB-MSCs in C3H/C57 wild-type mice | C3H/C57 mice without injection of hUCB-MSC | Increased cell viability and rate of cell migration in the brain intracerebroventricular route, hence mitigating AD. | [61] |
| Han et al. | 2018 | BM-MSC | Exposure to MicroRNA let-7f-5p modified MSCs in vitro in C57BL/6 mice | C57BL/6 mice without MSC transplantation | Increased caspase-3 expression and hence decreased cytotoxicity for AD models | [62] |
| McGinley et al. | 2018 | hNSC | Transplantation of hNSC targeted to fimbria fornix of APP/PS1 murine | APP/PS1 mice without transplantation of hNSCs | Reduced amyloid plaque load and increased recruitment of activated microglia, indicating functional and pathological improvements in AD mice | [23] |
| Oh et al. | 2018 | sRAGE-MSC | Injection of sRAGE-MSCs with Aβ1–42 to entorhinal cortices of male Sprague Dawley rats | Sprague Daley rats without injection of sRAGE-MSCs | Longer survival time for mice with sRAGE-MSC injection due to prevented apoptosis, and decrease in neuron numbers | [63] |
| Wang et al. | 2018 | MSC | Intracerebroventricular injection of MSC-derived extracellular vesicles to APP/PS1 mice | APP/PS1 mice without injection of MSCs | Alleviated exogenous Aβ-induced iNOS mRNA and protein expression, therefore improved cognitive behavior and rescued impairment of CA1 synaptic transition and long-term potentiation in AD mice | [64] |
| Wang et al. | 2018 | ucMSC | Injection of ucMSCs with resveratrol to hippocampus of AD mice | AD mice without ucMSC injection | Improved learning and memory, enhanced neurogenesis, and alleviated neural apoptosis in AD mice | [65] |
| Wei et al. | 2018 | BM-MSC | Injection of BM-MSCs on APP/PS1 mice | APP/PS1 mice without BM-MSC injection | Reduced Aβ1–42 content and BACE1 gene expression; ameliorated symptoms of AD | [66] |
| Yu et al. | 2018 | BM-MSC | Transplantation of BM-MSCs to murine models with AD | AD murine model without BM-MSC transplantation | Increased protein expression of seladin-1 and nestin, and hence reduced neurodegeneration | [67] |
| Esmaeilzade et al. | 2019 | MSC | Incubation of MSCs with dimethyloxalylglycine (DMOG) in Aβ-injected rats | Aβ-injected rats without MSC incubation | Increased cell viability, migration, and expression of CXCR4, CCR2, Nrf2, and HIF-1α, which enhanced antioxidant capacity in the hippocampus | [68] |
| Hu et al. | 2019 | ucMSC | Transplantation of ucMSCs with brain-derived neurotrophic factor (BDNF) to mice with Aβ1–42 | AD mice without transplantation of ucMSCs | Significantly improved spatial learning and memory abilities in the AD rats; increased release of acetylcholine and ChAT expression in the hippocampus | [69] |
| Nasiri et al. | 2019 | AD-MSC | Injection of melatonin-pretreated AD-MSCs to male Wistar rats | Male Wistar rats without injection of AD-MSCs | Cleared Aβ deposition and reduced microglial cells | [70] |
| Reza-Zaldivar et al. | 2019 | MSC | Administration of MSC-derived exosomes to amyloid 1–42 treated AD mice | AD mice without injection of MSC-derived exosomes | Alleviated beta amyloid 1–42-induced cognitive impairment | [27] |
| Eftekharzadeh et al. | 2020 | hADSC | Intravenous injection of hADSCs to murine model of AD | Murine model without administration of hADSCs | hADSCs significantly decreased the expression of necroptotic markers and reduced necroptosis and declined death of neuronal cells in the hippocampus of AD rats | [71] |
| Guo et al. | 2020 | SCF | Subcutaneously injection of SCF to APP/PS1 transgenic mice with C57BL/6J genetic background | APP/PS1 transgenic mice without injection of SCF | Increased association of TREM2+/Iba1+ cells with Aβ plaques and enhanced cerebral expression that ameliorated AD pathology at late stage | [72] |
| Kim et al. | 2020 | ES-MSC | Intra-arterial administration of ES-MSCs in AD rat model | AD rats without administration of ES-MSCs | Significantly inhibited Aβ-induced cell death in hippocampus and promoted autophagolysosomal clearance of Aβ | [33] |
| Liu et al. | 2020 | NSC | Injection of NSC-derived exosomes to 5 × FAD mice | 5 × FAD mice without injection of NSC-derived exosomes | Reversed AD-caused blood–brain barrier deficiency | [73] |
| Mehrabadi et al. | 2020 | ATSCs | Injection of hypoxic-conditioned medium from ATSCs intravenously to AD mice | AD mice without injection of ATSCs | Decreased beta amyloid plaques, TLR2 and TLR4 expression and enhanced neuronal survival | [74] |
| Park et al. | 2020 | BM-MSC | Intravenous injection of BM-MSCs to 3xTg AD mice | AD mice without injection of BM-MSCs | Enhanced memory function and less β-amyloid-immunopositive plaques | [75] |
| Park et al. | 2020 | NSC | Transplantation of NSCs into APPswe/PS1dE9 AD model mice | AD mice without NSC transplantation | Increased ACh (acetylcholinesterase) level and improved learning and memory function | [76] |
| Ramezani et al. | 2020 | BM-MSC | Transplantation of BM-MSCs to male Wistar rats | Wistar rats without BM-MSC transplantation | Enhanced learning, cognition and memory that mitigated neurodegeneration of AD | [77] |
| Zhao et al. | 2020 | ESC | Transplantation of mouse ESC-derived thymic epithelial progenitors into AD mice | Without ESC transplantation | Reduced cerebral Aβ plaque load and improved cognitive performance with increased T cell number | [78] |
| Zhu et al. | 2020 | NSC | Implantation of NSCs to hippocampus of APP/PS1 Tg (transgenic) AD mice | AD mice without NSC implantation | Protected cholinergic neurons, restored synaptic impairment in amyloid precursor and hence improved learning and memory function | [79] |
| Apodaca et al. | 2021 | hNSC | Intravenous injection of hNSC-derived extracellular vesicles on 5xFAD accelerated transgenic AD mice | AD mice without injection of hNSC-derived extracellular vesicles | Reduced dense core Aβ plaque accumulation and microglial activation, restoration of homeostatic levels of circulating pro-inflammatory cytokines in AD mice, with improved cognition and synaptic function | [25] |
| Armijo et al. | 2021 | iPSC | Stereotaxically injection of iPSC-derived neural precursors to hippocampus of 3xTg AD mice | AD mice without injection of iPSC-derived neural precursors | Improved memory, synaptic plasticity, and reduced brain pathology, including a reduction of amyloid and tangles deposits | [37] |
| Cone et al. | 2021 | MSC | Intranasally injection of NSC derived extracellular vesicles to non-transgenic 5xFAD mice | AD mice without NSC injection | Lowered Aβ plaque load in the hippocampus. Less colocalization between GFAP and Aβ plaques and hence better cognition functions | [80] |
| Huang et al. | 2021 | Nanoformulation-mediated NSC | Injection of nanoformulation-mediated NSC into APPswe/PS1dE9 double transgenic mouse model | Mouse model without NSC injection | Improved neural regeneration, and efficient and long-lasting Aβ degradation | [81] |
| Jeong et al. | 2021 | hucMSC | Intravenous transplantation of hucMSCs into Aβ injected AD animal model | AD animal model without hucMSC transplantation | Superior neurogenesis and anti-inflammation properties with increased NEP in hippocampus | [82] |
| Kuo et al. | 2021 | MSC | Intracerebroventricular administration of MSC-conditioned medium to Aβ-induced rat model | Rat model without administration of MSC | Decreased expression of tight junction proteins, SIRT1 and β-catenin, which attenuated retinal pathology of AD | [83] |
| Lim et al. | 2021 | hNTSC | Transplantation of hNTSC into 5xFAD transgenic AD mice | AD mice without hNTSC transplantation | Reduced Aβ42 levels and plaque formation in the brain, increased survival of hippocampal and cortex neurons | [84] |
| Lu et al. | 2021 | hNSC | Intranasal transplantation of hNSCs into APP/PS1 transgenic AD mice | AD mice without hNSC transplantation | Attenuated beta-amyloid accumulation by upregulating the expression of beta-amyloid degrading enzymes, insulin degrading enzymes and neprilysin, which ameliorated neuroinflammation, cholingergic dysfunction, and synaptic loss | [85] |
| Neves et al. | 2021 | BM-MSC | Administer of allogeneic BM-MSCs intravenously in 3xTg AD mice | AD mice without administering BM-MSCs | Reduced β-secretase cleavage of amyloid precursor protein and decreased tau phosphorylation | [86] |
| Santamaria et al. | 2021 | MSC | In vivo systematic administration of MSCs to APP/PS1 AD mice | AD mice without administration of MSCs | Induced persistent memory recovery and reduced plaques with β-amyloid oligomers | [87] |
| Wang et al. | 2021 | MSC | Tail-vein injection of MSC-derived small extracellular vesicles into APP/PS1 AD mice | AD mice without injection of MSCs | Restored hippocampal neuronal morphology, with improved cognitive impairments and reduced neuronal loss | [88] |
| Zhang et al. | 2021 | Dental pulp stem cell | Injection of 5 × 10 dental pulp stem cells into the hippocampus of AD mice | AD mice without injection of dental pulp stem cells | Increased expression of neuron-related doublecortin, NeuN, and neurofilament 200 in the hippocampus with decreased expression of Aβ, hence improving cognitive and behavioral abilities | [89] |
| Choi et al. | 2022 | MFSCE | Treatment of MFSCE on Aβ25–35-injected AD mice | AD mice without MFSCE treatment | Suppressed Bax and cleaved caspase-3 protein expression, downregulated amyloidogenic-pathway-related protein expressions and hence improved cognitive functioning | [90] |
| Guo et al. | 2022 | SHED | SHEDs cultured in vitro and injected into AD SAMP8 mice by caudal vein | AD mice without SHED injection | Improved cognitive ability and reversed memory loss through the recovery of dysfunctional mitochondria | [91] |
| Liu et al. | 2022 | BM-MSC | Lateral ventricle administration of BM-MSCs to adult C57BL/6 AD mice | AD mice without administration of BM-MSCs | Inhibited hyper activation of microglia and astrocytes in the hippocampus of AD mice | [92] |
| Wang et al. | 2022 | hucMSC | Injection of hucMSCs with Fe3O4 polydopamine nanoparticles into APP/PS1 transgenic mice | AD mice without hucMSC injection | Improved memory and cognitive ability of AD by increased expression of brain-derived neurotrophic factor | [93] |
| Zhang et al. | 2022 | NSC | Transplantation of NSCs into hippocampal CA1 region of rTg (tau P301L) 4510 mouse model | Mouse model without transplantation of NSCs | Reduced abnormal aggregation of tau, and hence improvements in short-term memory | [94] |
| Zhang et al. | 2022 | SHED | Injection of SHED into SAMP8 AD mice | AD mice without SHED injection | Relieved AD symptoms by up-regulating PPARγ pathway | [95] |
| Author | Year | Type of Stem Cell | Intervention | Control Group | Measured Outcomes of Interest | Reference |
|---|---|---|---|---|---|---|
| Wang et al. | 2018 | iPSC | Induction of iPSC with Sendai-virus delivery system | N/A | Obtain iPSC cell line (ZZUi009-A) from AD patient with PSEN1 gene mutation | [96] |
| Wang et al. | 2019 | iPSC | Induction of iPSC with novel MEOX2 mutation in a family with AD | N/A | Obtain iPSC cell line (ZZUi0013-A) with episomal plasmids expressing OCT3/4, SOX2, KLF4, LIN28, and L-MYC genes | [97] |
| Dai et al. | 2020 | iPSC | Generation of iPSC from peripheral blood mononuclear cells with AD patient with APOE3/4 genotype | N/A | With immunocytochemistry iPSC displayed potential to differentiate spontaneously into three germ layers in vitro | [98] |
| Kim et al. | 2021 | hUCB-MSC | Intracerebroventricular injection of hUCB-MSCs to AD patients | AD patients without hUCB-MSC injection | Phase I clinical trial showed significant effect in mitigating neurodegeneration despite few adverse events (fever, headache, vomit) | [30] |
| Wang et al. | 2021 | iPSC | Induction of iPSC from male AD patient with APOE-ε4/ε4 alleles | N/A | Obtain iPSCs with differentiation potential for treating neurological disorder and multiple sclerosis | [99] |
| Wang et al. | 2021 | iPSC | Induction of iPSC with APP gene mutation in a female patient with AD | N/A | Obtain iPSC cell line (ZZUi0024-A) with dermal fibroblasts expressing OCT3/4, SOX2, KLF4, LIN28, and L-MYC genes | [100] |
| Lee et al. | 2022 | iPSC | Induction of iPSC from AD patients with mtDNA mutations | N/A | mtDNA mutations induced growth advantage with higher viability and proliferation, lower mitochondrial respiration, and membrane potential | [101] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, Y.; Lyu, L.; Zhan, S. Stem Cell Therapy for Alzheimer’s Disease: A Scoping Review for 2017–2022. Biomedicines 2023, 11, 120. https://doi.org/10.3390/biomedicines11010120
Duan Y, Lyu L, Zhan S. Stem Cell Therapy for Alzheimer’s Disease: A Scoping Review for 2017–2022. Biomedicines. 2023; 11(1):120. https://doi.org/10.3390/biomedicines11010120
Chicago/Turabian StyleDuan, Yunxiao, Linshuoshuo Lyu, and Siyan Zhan. 2023. "Stem Cell Therapy for Alzheimer’s Disease: A Scoping Review for 2017–2022" Biomedicines 11, no. 1: 120. https://doi.org/10.3390/biomedicines11010120





