Insights into Pathogenesis, Nutritional and Drug Approach in Sarcopenia: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Focal Question
2.2. Databases
2.3. Eligible Criteria
2.4. Study Selection
2.5. Extraction of Data
2.6. Evaluation of the Risk of Bias in the Included Studies
3. Results
| Reference | Population | Intervention/Comparison | Outcomes |
|---|---|---|---|
| Leucine | |||
| [20] | Randomized, single-blind, placebo-controlled study (Spain) with 41 post-hospitalized older adults (82.1 ± 5.89 y) randomly divided into leucine + RTI (82.9 ± 5.67 y, 19♂ and 22♀) and placebo + RTI (81.2 ± 6.14 y, 10♂ and 10♀) groups | 20 g of whey protein isolated +3 g of leucine/2 non-consecutive days per week | ↑Physical performance, ↓frailty, and ↑myostatin (↑appendicular skeletal muscle mass) but not significantly between intervention and placebo |
| [21] | Randomized, double-blind, placebo-controlled study (Spain) with 50 elder (78.9 ± 7.9 y) living in nursing homes and able to walk 6 m were randomly divided into leucine (with MHS of 16.3 ± 8.5 kg) and placebo (with MHS of 19.2 ± 8.6 kg) groups | 6 g/day of leucine/13 weeks | ↑Functional performance, ↑walking time, ↑lean mass index, and ↑maximum static expiratory force |
| BCAA | |||
| [22] | Randomized, double-blind, placebo-controlled study (India) with 60 sarcopenic viral and alcohol-related cirrhosis patients (41.6 ± 9.9 y) randomly divided into BCAA (42.26 ± 10.07 y, 19♂ and 11♀) and placebo (40.83 ± 9.80 y, 21♂ and 9♀) groups | 12 g/day of BCAA/6 months | ↑Muscle mass, ↑MHS, ↑6-min walk distance, and ↑6-m gait speed but not significantly between intervention and placebo |
| [23] | Retrospective observational study (Japan) with 29 stroke patients divided into LEBDs (77–92 y, 6♂ and 9♀) and SBDs (77.8–86.3 y, 5♂ and 9♀) groups | LEBDs (2.07 g of BCAA, 1.44 g of leucine, 0.36 g of valine and 0.27 g of isoleucine) or SBDs (1.58 g of BCAA, 0.72 g of leucine, 0.48 g of valine and 0.38 g of isoleucine)/Patients received the intervention twice a day on the fifth and seventh days of hospitalization | Improvements in transthyretin and CRP were observed, but not significantly between the groups |
| [24] | Randomized, single-blind study (Japan) with 66 stroke patients randomly divided into breakfast (65.5 ± 13.1 y, 14♂ and 9♀) and post-exercise (67.5 ± 5 y, 14♂ and 9♀) groups | 3.5 g of amino acids and 6.5 g of protein + 40 IU of VD/day/2 months | ↑Leg press strength, ↑physical performance, ↓body fat mass, and improvement in Berg balance scale but without significant timing influence |
| Omega 3 | |||
| [25] | Randomized, double-blind, placebo-controlled study (Belgium) with 23 older adults (65–83 y, 15♂ and 8♀) randomly divided into PUFA (intervention) or PLAC (placebo) groups | 1100 mg ω-3 soft gels (1020 mg ω-3 + 410 mg DHA + 540 mg EPA + 4 mg vitamin E) 3× daily/14 weeks | ↑Knee-extensor strength and synergism with resistance training in improving muscle inflammatory and catabolic markers (FOXO1 and LC3b) |
| [26] | Population-based cross-sectional study (Iran) with 300 elderly adults (150♂ and 150♀, ≥65 y) were studied due to their eating habits | Anti-inflammatory (omega 3 + other nutrients) or other diets | Patients with anti-inflammatory diet presented lower odds of sarcopenia |
| Calcium | |||
| [27] | Randomized, double-blind, placebo-controlled study (Lebanon) with 248 overweight adults (55%♀, 71 ± 4.6 y, 30.2 ± 4.5 Kg/m2 of BMI) with baseline VD of 10–30 ng/mL randomly divided into VD and placebo groups | 3750 IU/day of VD/12 months | There were no improvements in the indices of sarcopenia or adiposity between the groups |
| [28] | Randomized, double-blind, placebo-controlled study (Belgium) with 15 adult patients with thermal burns dating from 2 to 5 years randomly divided into calcium + VD (22–58 y, 7♂ and 1♀) and placebo (29–64 y, 4♂ and 3♀) groups | Quarterly IM injection of 200,000 IU of VD + daily oral calcium/12 months | ↑Quadriceps strength when tested at high velocity significantly but without significant improvements in bone health |
| [29] | Randomized, double-blind, placebo-controlled study (United States). [Phase 1] NEPLA (14♂ and 11♀, 72 ± 1 y) and NEHMB (13♂ and 12♀, 73 ± 1 y) older adults groups; [Phase 2] REPLA (11♂ and 13♀, 73 ± 1 y) and REHMB (11♂ and 13♀, 73 ± 1 y) older adults groups. | [Phase 1] 3 g of CaHMB twice daily and [Phase 2] 3 g of CaHMB twice daily + RTI/24 weeks [Phase 1] and 24 weeks [Phase 2] | CaHMB significantly improved muscle strength and MQ independently of RTI |
| [30] | Prospective cohort study (Australia) with 740 non-institutionalized older adults (50%♀ and with mean age pf 62 ± 7 y) randomly sampled | Patients were analyzed for their nutrient intake at baseline and follow-up (2.6 ± 0.4 y later) | Significant positive associations were found between calcium intake and aLM |
| Reference | Population | Intervention/Comparison | Outcomes |
|---|---|---|---|
| SGLT2 inhibitors | |||
| [31] | Prospective cohort study (Japan) with 43 moderately obese Japanese patients (53.5 ± 8.04 y, 27♂ and 10♀) with T2DM treated with luseogliflozin | Personalized doses of luseogliflozin */52 weeks * | ↓Body fat and ↑skeletal muscle mass significantly |
| GH analogs | |||
| [32] | Secondary analyses of two previously completed randomized clinical trials (United States) with 341 individuals living with HIV and with abdominal obesity divided into tesamorelin (47.8 ± 7.3 y, 89.1%♂) and placebo (48 ± 7.6 y, 83.8%♂) groups | Personalized doses of tesamorelin/26 weeks * | Tesamorelin treatment significantly increased skeletal muscle mass, area, and density in those patients with significant decreases in visceral adipose tissue |
| GLP-1A | |||
| [33] | Prospective cohort study (Italy) with 6♂ (68.50 ± 4.23 y, 135.83 ± 31.38 mmol/l of FBG) and 3♀ (67.66 ± 3.78 y, 173.00 ± 49.24 mmol/l of FBG) | Liraglutide 3 mg/day/24 weeks | ↓Body fat mass and ↑muscle tropism protecting against sarcopenia |
| Metformin | |||
| [34] | Cross-sectional observational study (China) with 1427 (535♂ and 892♀) individuals with (504♂ and 775♀) and without (31♂ and 117♀) sarcopenia | Personalized doses of metformin * | Metformin was effective in protecting against muscle mass loss among T2DM individuals |
| [35] | Randomized, double-blind, placebo-controlled clinical study (Indonesia) with 91 non-diabetic elderly individuals randomly divided into metformin (67.77 ± 5.14 y, 19♂ and 24♀) and placebo (70.04 ± 5.34 y, 15♂ and 33♀) groups | 500 mg thrice daily/16 weeks | ↑Usual gait speed significantly but did not improve handgrip strength or myostatin levels |
| 3-Hydroxy-3 methylglutaryl coenzyme a inhibitors (statins) | |||
| [36] | Prospective cohort study (Finland) with 216 abdominal aortic aneurysms patients that underwent EVAR (77.7 ± 7.4 y, 188♂ and 28♀) divided into statin users (77.4 ± 7.5 y, 113♂ and 16♀) or nonusers (78.2 ± 7.3 y, 75♂ and 12♀) | 10 to 80 mg/day of atorvastatin, rosuvastatin, simvastatin orfluvastatin/At least a 4-month period of statin pre-treatment before EVAR | Statin treatment decreased long-term mortality among patients that underwent EVAR without pre-disposing to increased risk for sarcopenia |
| [37] | Population-based nationwide retrospective cohort study (Taiwan) with 67,001 clinically confirmed cases of CKD (2407 with sarcopenia) divided into statins users (547 with sarcopenia) and nonusers (1860 with sarcopenia) | Personalized doses of pravastatin, fluvastatin, atorvastatin, lovastatin, simvastatin or rosuvastatin * | Patients with CKD could receive statins treatment to reduce individual’s risk of developing newly diagnosed sarcopenia |
| [38] | Prospective cohort study (United Kingdom) with 639 older adults (321♂ and 318♀ with 64.1 ± 2.5 y and 65.9 ± 2.7 y, respectively) that were undergoing statins, thiazides or ACE inhibitors treatments | Personalized doses of statins, thiazides or ACE inhibitors/Mean follow-up time was about 4.4 y * | Any treatment was associated significantly with protection against handgrip strength decline |
| DPP-4 inhibitors | |||
| [39] | Retrospective cohort study (Turkey) with 90 T2DM geriatric patients (72.57 ± 7.089 y, 60%♀) divided into DPP4 users (n = 48, 72.88 ± 7.13 y) and nonusers (n = 42, 72.21 ± 7.10 y) | Personalized doses of DPP-4 inhibitors/6 months * | DPP-4 inhibitors therapy was effective in improving muscle strength among geriatric T2DM patients |
| [40] | Retrospective observational study (Japan) with 105 T2DM patients (62 ± 12 y, 39%♀) divided into DPP-4 inhibitors users (64 ± 13 y, 49%♂) and nonusers (60 ± 12y, 68%♂) | Personalized doses of statins, thiazides or DPP-4 inhibitors * | Among DPP-4 inhibitors users, the skeletal muscle index was significantly higher in comparison with nonusers |
| [41] | Cross-sectional cohort study (Italy) with 80 elderly diabetic patients (76.2 ± 5.4 y, 38♂ and 42♀) treated with DPP-4 (74.9 ± 4.8y, 17♂ and 20♀) or sulfonylureas (77.1 ± 5.3 y, 21♂ and 22♀) for at least 24 months | Personalized doses of statins, thiazides or DPP-4 inhibitors * | DPP-4 users had significant improvements in sarcopenia parameters such as fat-free mass decrease, skeletal muscle mass increase, and increases in muscle strength and gait speed |
| Reference | Question Focus | Appropriate Randomization | Allocation Blinding | Double-Blind | Losses (˂20%) | Porgnostics or Demographic Carachteristics | Outcomes | Intestion to Treat Analyses | Sample Calculation | Adequate Follow-Up |
|---|---|---|---|---|---|---|---|---|---|---|
| Leucine | ||||||||||
| [20] | Yes | No | Yes | No | Yes | Yes | Yes | No | Yes | Yes |
| [21] | Yes | NR | Yes | Yes | Yes | No | Yes | No | Yes | Yes |
| BCAA | ||||||||||
| [22] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| [23] | Yes | No | No | No | No | Yes | Yes | No | NR | No |
| [24] | Yes | Yes | Yes | No | Yes | No | Yes | Yes | Yes | Yes |
| Omega 3 | ||||||||||
| [25] | Yes | No | Yes | Yes | NR | No | Yes | NR | NR | Yes |
| [26] | Yes | No | No | No | Yes | Yes | Yes | No | Yes | No |
| Calcium | ||||||||||
| [27] | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes |
| [28] | Yes | Yes | Yes | No | Yes | Yes | Yes | No | No | Yes |
| [29] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | NR | Yes |
| [30] | Yes | No | No | No | No | Yes | Yes | No | NR | Yes |
| Reference | Question Focus | Appropriate Randomization | Allocation Blinding | Double-Blind | Losses (˂20%) | Porgnostics or Demographic Carachteristics | Outcomes | Intestion to Treat Analyses | Sample calculation | Adequate Follow-Up |
|---|---|---|---|---|---|---|---|---|---|---|
| SGLT2 inhibitors | ||||||||||
| [31] | Yes | No | No | No | Yes | Yes | Yes | No | NR | Yes |
| GH analogs | ||||||||||
| [32] | Yes | NR | Yes | No | NR | Yes | Yes | NR | NR | Yes |
| GLP-1A | ||||||||||
| [33] | Yes | No | No | No | Yes | Yes | Yes | Yes | NR | Yes |
| Metformin | ||||||||||
| [34] | Yes | No | No | No | Yes | Yes | Yes | Yes | NR | Yes |
| [35] | Yes | No | Yes | Yes | No | Yes | Yes | No | Yes | Yes |
| 3-Hydroxy-3 methylglutaryl coenzyme a inhibitors (statins) | ||||||||||
| [36] | Yes | No | No | No | NR | Yes | Yes | NR | No | Yes |
| [37] | Yes | No | No | No | Yes | Yes | Yes | No | NR | Yes |
| [38] | Yes | No | No | No | Yes | Yes | Yes | NR | NR | Yes |
| DPP-4 inhibitors | ||||||||||
| [39] | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes | Yes |
| [40] | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes | Yes |
| [41] | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes | Yes |
4. Discussion
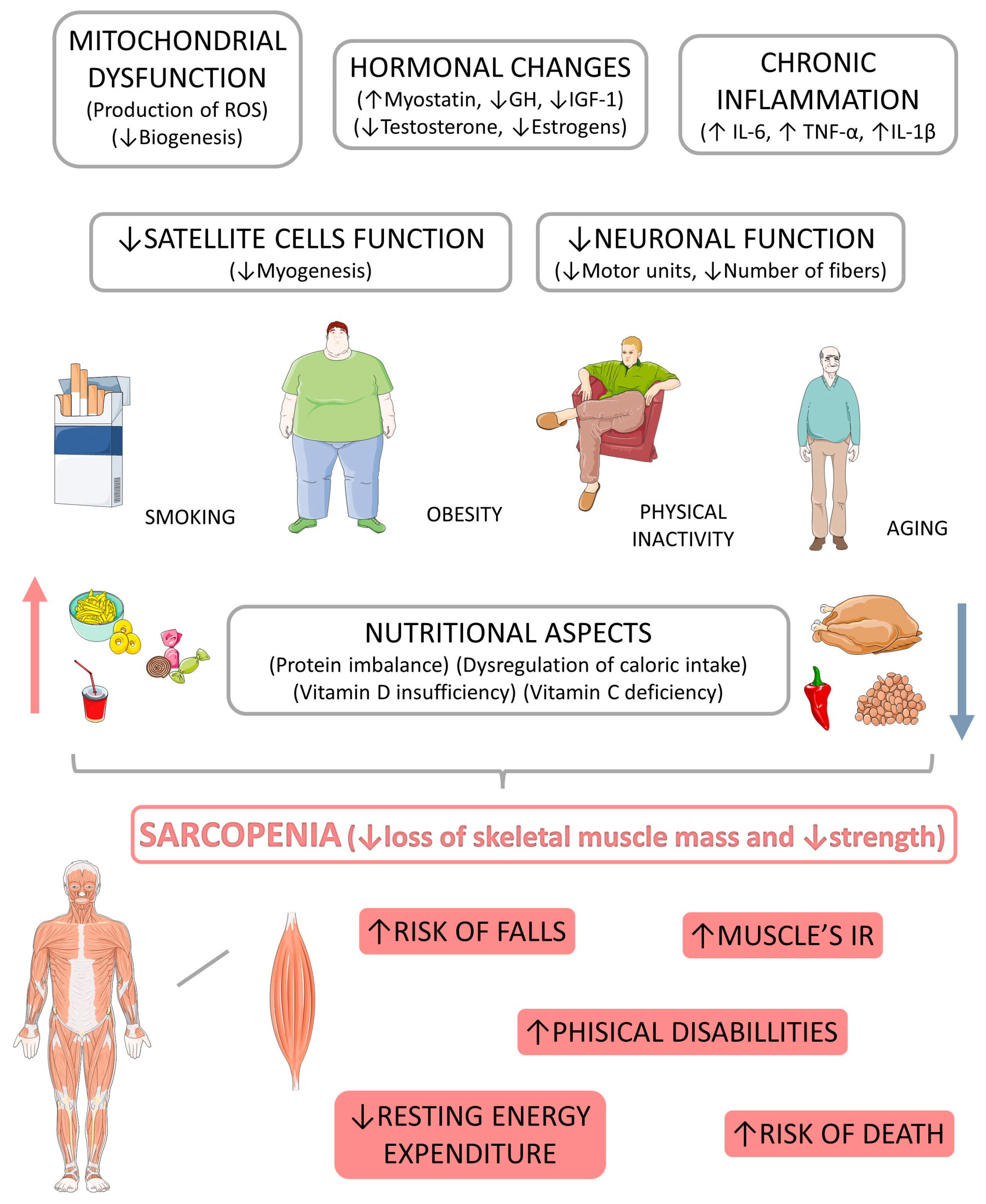
4.1. Sarcopenia: Pathophysiological Aspects
Diseases Related to Sarcopenia
4.2. Nutritional Approach to Sarcopenia: The Role of Amino Acids, Omega 3, Vitamin D, and Calcium
4.2.1. Amino Acids
Leucine
Branched-Chain Amino Acids (BCAA)
Creatine
4.2.2. Omega 3
4.2.3. Vitamin D
4.2.4. Calcium
4.3. Medication Approach to Sarcopenia
4.3.1. Sodium–Glucose Cotransporter 2 (SGLT2) Inhibitors
4.3.2. Growth Hormone (GH)
4.3.3. Glucagon-Like Peptide-1 Receptor Agonists (GLP-1A)
4.3.4. Metformin
4.3.5. 3-Hydroxy-3 Methylglutaryl Coenzyme A Inhibitors (Statins)
4.3.6. Losartan
4.3.7. Dipeptidyl Peptidase 4 (DPP-4) Inhibitors
5. Limitations of the Included Studies
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kirwan, R.; McCullough, D.; Butler, T.; Perez de Heredia, F.; Davies, I.G.; Stewart, C. Sarcopenia during COVID-19 lockdown restrictions: Long-term health effects of short-term muscle loss. Geroscience 2020, 42, 1547–1578. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Zanuso, B.; de Oliveira Dos Santos, A.R.; Miola, V.F.B.; Guissoni Campos, L.M.; Spilla, C.S.G.; Barbalho, S.M. Panax ginseng and aging related disorders: A systematic review. Exp. Gerontol. 2022, 161, 111731. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, M.; Obata, H.; Kamiya, K.; Matsunaga, A.; Hotta, K.; Izumi, T. Overlapping states of AWGS muscle dysfunction and inverse feasibility of ADL recovery by rehabilitation in older inpatients. Sci. Rep. 2022, 12, 22283. [Google Scholar] [CrossRef] [PubMed]
- Sayer, A.A.; Cruz-Jentoft, A. Sarcopenia definition, diagnosis and treatment: Consensus is growing. Age Ageing 2022, 51, afac220. [Google Scholar] [CrossRef]
- Pár, A.; Hegyi, J.P.; Váncsa, S.; Pár, G. Sarcopenia—2021: Pathophysiology, diagnosis, therapy. Orv. Hetil. 2021, 162, 3–12. [Google Scholar] [CrossRef]
- Guo, M.; Yao, J.; Li, J.; Zhang, J.; Wang, D.; Zuo, H.; Zhang, Y.; Xu, B.; Zhong, Y.; Shen, F.; et al. Irisin ameliorates age-associated sarcopenia and metabolic dysfunction. J. Cachexia Sarcopenia Muscle 2022. [Google Scholar] [CrossRef]
- Banack, H.R.; LaMonte, M.J.; Manson, J.E.; Zhu, K.; Evans, W.J.; Shankaran, M.; Wactawski-Wende, J. Association of muscle mass measured by D3-Creatine (D3Cr), sarcopenic obesity, and insulin-glucose homeostasis in postmenopausal women. PloS ONE 2022, 17, e0278723. [Google Scholar] [CrossRef]
- Wang, P.C.; Yeh, W.C.; Tsai, Y.W.; Chen, J.Y. Calf circumference has a positive correlation with physical performance among community-dwelling middle-aged, older women. Front. Public Health 2022, 10, 1038491. [Google Scholar] [CrossRef]
- Priego, T.; Martín, A.I.; González-Hedström, D.; Granado, M.; López-Calderón, A. Role of hormones in sarcopenia. Vitam. Horm. 2021, 115, 535–570. [Google Scholar] [CrossRef]
- Van Long, N.; Chien, P.N.; Tung, T.X.; Van Anh, L.T.; Giang, N.N.; Nga, P.T.; Linh, L.T.T.; Nam, S.Y.; Heo, C.Y. Complementary combination of biomarkers for diagnosis of sarcopenia in C57BL/6J mice. Life Sci. 2023, 312, 121213. [Google Scholar] [CrossRef]
- Yin, M.; Zhang, H.; Liu, Q.; Ding, F.; Hou, L.; Deng, Y.; Cui, T.; Han, Y.; Chen, Y.; Huang, C.; et al. Determination of skeletal muscle mass by aspartate aminotransferase/alanine aminotransferase ratio, insulin and FSH in Chinese women with sarcopenia. BMC Geriatr. 2022, 22, 893. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.R.; Lee, S.; Song, S.K. A Review of Sarcopenia Pathophysiology, Diagnosis, Treatment and Future Direction. J. Korean Med. Sci. 2022, 37, e146. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 601. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Miyazaki, S.; Tamaki, A.; Yoshimura, Y.; Arai, H.; Fujiwara, D.; Katsura, H.; Kawagoshi, A.; Kozu, R.; Maeda, K.; et al. Respiratory sarcopenia: A position paper by four professional organizations. Geriatr. Gerontol. Int. 2022, 23, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, S.K. Sarcopenia: A Contemporary Health Problem among Older Adult Populations. Nutrients 2020, 12, 1293. [Google Scholar] [CrossRef]
- Gueugneau, M. The value of dietary plant protein in older people. Curr. Opin. Clin. Nutr. Metab. Care 2023, 26, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh Pahlavani, H.; Laher, I.; Knechtle, B.; Zouhal, H. Exercise and mitochondrial mechanisms in patients with sarcopenia. Front. Physiol. 2022, 13, 1040381. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ (Clin. Res. Ed.) 2021, 372, n71. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Amasene, M.; Cadenas-Sanchez, C.; Echeverria, I.; Sanz, B.; Alonso, C.; Tobalina, I.; Irazusta, J.; Labayen, I.; Besga, A. Effects of Resistance Training Intervention along with Leucine-Enriched Whey Protein Supplementation on Sarcopenia and Frailty in Post-Hospitalized Older Adults: Preliminary Findings of a Randomized Controlled Trial. J. Clin. Med. 2021, 11, 97. [Google Scholar] [CrossRef]
- Martínez-Arnau, F.M.; Fonfría-Vivas, R.; Buigues, C.; Castillo, Y.; Molina, P.; Hoogland, A.J.; van Doesburg, F.; Pruimboom, L.; Fernández-Garrido, J.; Cauli, O. Effects of Leucine Administration in Sarcopenia: A Randomized and Placebo-controlled Clinical Trial. Nutrients 2020, 12, 932. [Google Scholar] [CrossRef]
- Mohta, S.; Anand, A.; Sharma, S.; Qamar, S.; Agarwal, S.; Gunjan, D.; Singh, N.; Madhusudhan, K.S.; Pandey, R.M.; Saraya, A. Randomised clinical trial: Effect of adding branched chain amino acids to exercise and standard-of-care on muscle mass in cirrhotic patients with sarcopenia. Hepatol. Int. 2022, 16, 680–690. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Yoshioka, K. Quick and effective improvement of leucine enriched dietary supplement on malnutrition in acute stroke patients receiving enteral tube feeding. BMC Emerg. Med. 2020, 20, 56. [Google Scholar] [CrossRef]
- Ikeda, T.; Morotomi, N.; Kamono, A.; Ishimoto, S.; Miyazawa, R.; Kometani, S.; Sako, R.; Kaneko, N.; Iida, M.; Kawate, N. The Effects of Timing of a Leucine-Enriched Amino Acid Supplement on Body Composition and Physical Function in Stroke Patients: A Randomized Controlled Trial. Nutrients 2020, 12, 1928. [Google Scholar] [CrossRef] [PubMed]
- Dalle, S.; Van Roie, E.; Hiroux, C.; Vanmunster, M.; Coudyzer, W.; Suhr, F.; Bogaerts, S.; Van Thienen, R.; Koppo, K. Omega-3 Supplementation Improves Isometric Strength But Not Muscle Anabolic and Catabolic Signaling in Response to Resistance Exercise in Healthy Older Adults. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2021, 76, 406–414. [Google Scholar] [CrossRef]
- Bagheri, A.; Hashemi, R.; Heshmat, R.; Motlagh, A.D.; Esmaillzadeh, A. Patterns of Nutrient Intake in Relation to Sarcopenia and Its Components. Front. Nutr. 2021, 8, 645072. [Google Scholar] [CrossRef]
- Jabbour, J.; Rahme, M.; Mahfoud, Z.R.; El-Hajj Fuleihan, G. Effect of high dose vitamin D supplementation on indices of sarcopenia and obesity assessed by DXA among older adults: A randomized controlled trial. Endocrine 2022, 76, 162–171. [Google Scholar] [CrossRef]
- Rousseau, A.F.; Foidart-Desalle, M.; Ledoux, D.; Remy, C.; Croisier, J.L.; Damas, P.; Cavalier, E. Effects of cholecalciferol supplementation and optimized calcium intakes on vitamin D status, muscle strength and bone health: A one-year pilot randomized controlled trial in adults with severe burns. Burn. J. Int. Soc. Burn Inj. 2015, 41, 317–325. [Google Scholar] [CrossRef]
- Stout, J.R.; Smith-Ryan, A.E.; Fukuda, D.H.; Kendall, K.L.; Moon, J.R.; Hoffman, J.R.; Wilson, J.M.; Oliver, J.S.; Mustad, V.A. Effect of calcium β-hydroxy-β-methylbutyrate (CaHMB) with and without resistance training in men and women 65+ yrs: A randomized, double-blind pilot trial. Exp. Gerontol. 2013, 48, 1303–1310. [Google Scholar] [CrossRef]
- Scott, D.; Blizzard, L.; Fell, J.; Giles, G.; Jones, G. Associations Between Dietary Nutrient Intake and Muscle Mass and Strength in Community-Dwelling Older Adults: The Tasmanian Older Adult Cohort Study. J. Am. Geriatr. Soc. 2010, 58, 2129–2134. [Google Scholar] [CrossRef]
- Sasaki, T.; Sugawara, M.; Fukuda, M. Sodium-glucose cotransporter 2 inhibitor-induced changes in body composition and simultaneous changes in metabolic profile: 52-week prospective LIGHT (Luseogliflozin: The Components of Weight Loss in Japanese Patients with Type 2 Diabetes Mellitus) Study. J. Diabetes Investig. 2019, 10, 108–117. [Google Scholar] [CrossRef]
- Adrian, S.; Scherzinger, A.; Sanyal, A.; Lake, J.E.; Falutz, J.; Dubé, M.P.; Stanley, T.; Grinspoon, S.; Mamputu, J.C.; Marsolais, C.; et al. The Growth Hormone Releasing Hormone Analogue, Tesamorelin, Decreases Muscle Fat and Increases Muscle Area in Adults with HIV. J. Frailty Aging 2019, 8, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Perna, S.; Guido, D.; Bologna, C.; Solerte, S.B.; Guerriero, F.; Isu, A.; Rondanelli, M. Liraglutide and obesity in elderly: Efficacy in fat loss and safety in order to prevent sarcopenia. A perspective case series study. Aging Clin. Exp. Res. 2016, 28, 1251–1257. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Xu, S.; Wang, Y.; Chen, F.; Cao, L.; Liu, T.; Huang, T.; Wei, Q.; Ma, G.; Zhao, Y.; et al. Risk Factors for Sarcopenia in the Elderly with Type 2 Diabetes Mellitus and the Effect of Metformin. J. Diabetes Res. 2020, 2020, 3950404. [Google Scholar] [CrossRef] [PubMed]
- Laksmi, P.W.; Setiati, S.; Tamin, T.Z.; Soewondo, P.; Rochmah, W.; Nafrialdi, N.; Prihartono, J. Effect of Metformin on Handgrip Strength, Gait Speed, Myostatin Serum Level, and Health-related Quality of Life: A Double Blind Randomized Controlled Trial among Non-diabetic Pre-frail Elderly Patients. Acta Med. Indones 2017, 49, 118–127. [Google Scholar] [PubMed]
- Lindström, I.; Protto, S.; Khan, N.; Väärämäki, S.; Oksala, N.; Hernesniemi, J. Statin use, development of sarcopenia, and long-term survival after endovascular aortic repair. J. Vasc. Surg. 2021, 74, 1651–1658.e1651. [Google Scholar] [CrossRef]
- Lin, M.H.; Chiu, S.Y.; Chang, P.H.; Lai, Y.L.; Chen, P.C.; Ho, W.C. Hyperlipidemia and Statins Use for the Risk of New Diagnosed Sarcopenia in Patients with Chronic Kidney: A Population-Based Study. Int. J. Env. Res. Public Health 2020, 17, 1494. [Google Scholar] [CrossRef]
- Witham, M.D.; Syddall, H.E.; Dennison, E.; Cooper, C.; McMurdo, M.E.; Sayer, A.A. ACE inhibitors, statins and thiazides: No association with change in grip strength among community dwelling older men and women from the Hertfordshire Cohort Study. Age Ageing 2014, 43, 661–666. [Google Scholar] [CrossRef]
- Sencan, C.; Dost, F.S.; Ates Bulut, E.; Isik, A.T. DPP4 inhibitors as a potential therapeutic option for sarcopenia: A 6-month follow-up study in diabetic older patients. Exp. Gerontol. 2022, 164, 111832. [Google Scholar] [CrossRef]
- Bouchi, R.; Fukuda, T.; Takeuchi, T.; Nakano, Y.; Murakami, M.; Minami, I.; Izumiyama, H.; Hashimoto, K.; Yoshimoto, T.; Ogawa, Y. Dipeptidyl peptidase 4 inhibitors attenuates the decline of skeletal muscle mass in patients with type 2 diabetes. Diabetes Metab. Res. Rev. 2018, 34, e2957. [Google Scholar] [CrossRef]
- Rizzo, M.R.; Barbieri, M.; Fava, I.; Desiderio, M.; Coppola, C.; Marfella, R.; Paolisso, G. Sarcopenia in Elderly Diabetic Patients: Role of Dipeptidyl Peptidase 4 Inhibitors. J. Am. Med. Dir. Assoc. 2016, 17, 896–901. [Google Scholar] [CrossRef]
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.I.; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-Related Loss of Muscle Mass and Function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, E.; Troutman, A.D.; Moorthi, R.N.; Avin, K.G.; Coggan, A.R.; Lim, K. Klotho: An Emerging Factor with Ergogenic Potential. Front. Rehabil. Sci. 2021, 2, 807123. [Google Scholar] [CrossRef] [PubMed]
- Qaisar, R.; Karim, A.; Muhammad, T.; Shah, I. Circulating Biomarkers of Accelerated Sarcopenia in Respiratory Diseases. Biology 2020, 9, 322. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Ruiz, M.E.; Guarner-Lans, V.; Pérez-Torres, I.; Soto, M.E. Mechanisms Underlying Metabolic Syndrome-Related Sarcopenia and Possible Therapeutic Measures. Int. J. Mol. Sci. 2019, 20, 647. [Google Scholar] [CrossRef]
- Püschel, G.P.; Klauder, J.; Henkel, J. Macrophages, Low-Grade Inflammation, Insulin Resistance and Hyperinsulinemia: A Mutual Ambiguous Relationship in the Development of Metabolic Diseases. J. Clin. Med. 2022, 11, 4358. [Google Scholar] [CrossRef]
- Manríquez-Núñez, J.; Ramos-Gómez, M. Bioactive Compounds and Adipocyte Browning Phenomenon. Curr. Issues Mol. Biol. 2022, 44, 3039–3052. [Google Scholar] [CrossRef]
- de Oliveira Dos Santos, A.R.; de Oliveira Zanuso, B.; Miola, V.F.B.; Barbalho, S.M.; Santos Bueno, P.C.; Flato, U.A.P.; Detregiachi, C.R.P.; Buchaim, D.V.; Buchaim, R.L.; Tofano, R.J.; et al. Adipokines, Myokines, and Hepatokines: Crosstalk and Metabolic Repercussions. Int. J. Mol. Sci. 2021, 22, 2639. [Google Scholar] [CrossRef]
- Barbalho, S.M.; Flato, U.A.P.; Tofano, R.J.; Goulart, R.d.A.; Guiguer, E.L.; Detregiachi, C.R.P.; Buchaim, D.V.; Araújo, A.C.; Buchaim, R.L.; Reina, F.T.R.; et al. Physical exercise and myokines: Relationships with sarcopenia and cardiovascular complications. Int. J. Mol. Sci. 2020, 21, 3607. [Google Scholar] [CrossRef]
- Silveira Rossi, J.L.; Barbalho, S.M.; Reverete de Araujo, R.; Bechara, M.D.; Sloan, K.P.; Sloan, L.A.J.D.M.R. Metabolic syndrome and cardiovascular diseases: Going beyond traditional risk factors. Diabetes/Metab. Res. Rev. 2022, 38, e3502. [Google Scholar] [CrossRef]
- Barbalho, S.M.; Bechara, M.D.; Quesada, K.; Gabaldi, M.R.; Goulart, R.d.A.; Tofano, R.J.; Gasparini, R.G.J.J.V.B. Metabolic syndrome, atherosclerosis and inflammation: An inseparable triad? J. Vasc. Bras. 2015, 14, 319–327. [Google Scholar] [CrossRef]
- Chen, M.; Wang, Y.; Deng, S.; Lian, Z.; Yu, K. Skeletal muscle oxidative stress and inflammation in aging: Focus on antioxidant and anti-inflammatory therapy. Front. Cell Dev. Biol. 2022, 10, 964130. [Google Scholar] [CrossRef]
- Salucci, S.; Bartoletti-Stella, A.; Bavelloni, A.; Aramini, B.; Blalock, W.L.; Fabbri, F.; Vannini, I.; Sambri, V.; Stella, F.; Faenza, I. Extra Virgin Olive Oil (EVOO), a Mediterranean Diet Component, in the Management of Muscle Mass and Function Preservation. Nutrients 2022, 14, 3567. [Google Scholar] [CrossRef] [PubMed]
- Belenguer-Varea, Á.; Tarazona-Santabalbina, F.J.; Avellana-Zaragoza, J.A.; Martínez-Reig, M.; Mas-Bargues, C.; Inglés, M. Oxidative stress and exceptional human longevity: Systematic review. Free Radic. Biol. Med. 2020, 149, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.; Pender, C.L.; Bar-Ziv, R.; Zhang, H.; Wickham, K.; Willey, E.; Durieux, J.; Ahmad, Q.; Dillin, A. Mitochondria as Cellular and Organismal Signaling Hubs. Annu. Rev. Cell Dev. Biol. 2022, 38, 179–218. [Google Scholar] [CrossRef] [PubMed]
- Conte, M.; Giuliani, C.; Chiariello, A.; Iannuzzi, V.; Franceschi, C.; Salvioli, S. GDF15, an emerging key player in human aging. Ageing Res. Rev. 2022, 75, 101569. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Nezu, Y.; Tagawa, R.; Higami, Y. Mitochondrial Unfolded Protein Responses in White Adipose Tissue: Lipoatrophy, Whole-Body Metabolism and Lifespan. Int. J. Mol. Sci. 2021, 22, 2854. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Fernández, J.; Fernández-Montero, A.; Córdova-Martínez, A.; Pastor, D.; Martínez-Rodríguez, A.; Roche, E. Sarcopenia: Molecular Pathways and Potential Targets for Intervention. Int. J. Mol. Sci. 2020, 21, 8844. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Shimizu, T.; Kato, S.; Nara, M.; Suganuma, Y.; Sato, T.; Morii, T.; Yamada, Y.; Fujita, H. Reduction of Superoxide Dismutase 1 Delays Regeneration of Cardiotoxin-Injured Skeletal Muscle in KK/Ta-Ins2(Akita) Mice with Progressive Diabetic Nephropathy. Int. J. Mol. Sci. 2021, 22, 5491. [Google Scholar] [CrossRef]
- Antuña, E.; Cachán-Vega, C.; Bermejo-Millo, J.C.; Potes, Y.; Caballero, B.; Vega-Naredo, I.; Coto-Montes, A.; Garcia-Gonzalez, C. Inflammaging: Implications in Sarcopenia. Int. J. Mol. Sci. 2022, 23, 15039. [Google Scholar] [CrossRef]
- Flor-Rufino, C.; Barrachina-Igual, J.; Pérez-Ros, P.; Pablos-Monzó, A.; Sanz-Requena, R.; Martínez-Arnau, F.M. Fat infiltration and muscle hydration improve after high-intensity resistance training in women with sarcopenia. A randomized clinical trial. Maturitas 2022, 168, 29–36. [Google Scholar] [CrossRef]
- Dozio, E.; Vettoretti, S.; Lungarella, G.; Messa, P.; Corsi Romanelli, M.M. Sarcopenia in Chronic Kidney Disease: Focus on Advanced Glycation End Products as Mediators and Markers of Oxidative Stress. Biomedicines 2021, 9, 405. [Google Scholar] [CrossRef]
- Hu, S.; Wan, X.; Li, X.; Wang, X. Aerobic exercise alleviates pyroptosis-related diseases by regulating NLRP3 inflammasome. Front. Physiol. 2022, 13, 965366. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.Y.; Meng, X.F.; Han, Y.P.; Yan, J.L.; Xiao, C.; Qian, L.B. Profile of crosstalk between glucose and lipid metabolic disturbance and diabetic cardiomyopathy: Inflammation and oxidative stress. Front. Endocrinol. 2022, 13, 983713. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, L.A.C.; Dos Santos, J.M.; Parentoni, A.N.; Lima, L.P.; Duarte, T.C.; Brant, F.P.; Neves, C.D.C.; Pereira, F.S.M.; Avelar, N.C.P.; Danielewicz, A.L.; et al. Adiponectin Is a Contributing Factor of Low Appendicular Lean Mass in Older Community-Dwelling Women: A Cross-Sectional Study. J. Clin. Med. 2022, 11, 7175. [Google Scholar] [CrossRef]
- Kochlik, B.; Franz, K.; Henning, T.; Weber, D.; Wernitz, A.; Herpich, C.; Jannasch, F.; Aykaç, V.; Müller-Werdan, U.; Schulze, M.B.; et al. Frailty is characterized by biomarker patterns reflecting inflammation or muscle catabolism in multi-morbid patients. J. Cachexia Sarcopenia Muscle 2022. [Google Scholar] [CrossRef] [PubMed]
- Bernabeu-Wittel, M.; Gómez-Díaz, R.; González-Molina, Á.; Vidal-Serrano, S.; Díez-Manglano, J.; Salgado, F.; Soto-Martín, M.; Ollero-Baturone, M.; on Behalf of the Proteo, R. Oxidative Stress, Telomere Shortening, and Apoptosis Associated to Sarcopenia and Frailty in Patients with Multimorbidity. J. Clin. Med. 2020, 9, 2669. [Google Scholar] [CrossRef] [PubMed]
- Prasun, P. Mitochondrial dysfunction in metabolic syndrome. Biochim. Biophys. Acta Mol. Basis. Dis. 2020, 1866, 165838. [Google Scholar] [CrossRef] [PubMed]
- Eldehni, M.T. Frailty, multimorbidity and sarcopaenia in haemodialysis patients. Curr. Opin. Nephrol. Hypertens. 2022, 31, 560–565. [Google Scholar] [CrossRef]
- Pizzimenti, M.; Meyer, A.; Charles, A.L.; Giannini, M.; Chakfé, N.; Lejay, A.; Geny, B. Sarcopenia and peripheral arterial disease: A systematic review. J. Cachexia Sarcopenia Muscle 2020, 11, 866–886. [Google Scholar] [CrossRef]
- Ferreira, J.M.M.; Cunha, P.; Carneiro, A.; Vila, I.; Cunha, C.; Silva, C.; Longatto-Filho, A.; Mesquita, A.; Cotter, J.; Mansilha, A.; et al. Sarcopenia as a Prognostic Factor in Peripheral Arterial Disease: Descriptive Review. Ann. Vasc. Surg. 2021, 74, 460–474. [Google Scholar] [CrossRef]
- Sharpe, M.; Okoye, E.; Antoniou, G.A. Prognostic review and time-to-event data meta-analysis of low skeletal muscle mass in patients with peripheral arterial disease of the lower limbs undergoing revascularization. Int. Angiol. A J. Int. Union Angiol. 2020, 39, 50–59. [Google Scholar] [CrossRef]
- McDermott, M.M.; Ferrucci, L.; Guralnik, J.M.; Tian, L.; Green, D.; Liu, K.; Tan, J.; Liao, Y.; Pearce, W.H.; Schneider, J.R.; et al. Elevated levels of inflammation, d-dimer, and homocysteine are associated with adverse calf muscle characteristics and reduced calf strength in peripheral arterial disease. J. Am. Coll. Cardiol. 2007, 50, 897–905. [Google Scholar] [CrossRef] [PubMed]
- McDermott, M.M.; Guralnik, J.M.; Corsi, A.; Albay, M.; Macchi, C.; Bandinelli, S.; Ferrucci, L. Patterns of inflammation associated with peripheral arterial disease: The InCHIANTI study. Am. Heart J. 2005, 150, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Addison, O.; Prior, S.J.; Kundi, R.; Serra, M.C.; Katzel, L.I.; Gardner, A.W.; Ryan, A.S. Sarcopenia in Peripheral Arterial Disease: Prevalence and Effect on Functional Status. Arch. Phys. Med. Rehabil. 2018, 99, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Barbalho, S.M.; Tofano, R.J.; Chagas, E.F.B.; Detregiachi, C.R.P.; de Alvares Goulart, R.; Flato, U.A.P. Benchside to the bedside of frailty and cardiovascular aging: Main shared cellular and molecular mechanisms. Exp. Gerontol. 2021, 148, 111302. [Google Scholar] [CrossRef]
- Bjarnason-Wehrens, B.; Schwaab, B.; Reiss, N.; Schmidt, T. Resistance Training in Patients with Coronary Artery Disease, Heart Failure, and Valvular Heart Disease: A Review with Special Emphasis on Old Age, Frailty, and Physical Limitations. J. Cardiopulm. Rehabil. Prev. 2022, 42, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Prokopidis, K.; Isanejad, M.; Akpan, A.; Stefil, M.; Tajik, B.; Giannos, P.; Venturelli, M.; Sankaranarayanan, R. Exercise and nutritional interventions on sarcopenia and frailty in heart failure: A narrative review of systematic reviews and meta-analyses. ESC Heart Fail. 2022, 9, 2787–2799. [Google Scholar] [CrossRef]
- Anagnostis, P.; Gkekas, N.K.; Achilla, C.; Pananastasiou, G.; Taouxidou, P.; Mitsiou, M.; Kenanidis, E.; Potoupnis, M.; Tsiridis, E.; Goulis, D.G. Type 2 Diabetes Mellitus is Associated with Increased Risk of Sarcopenia: A Systematic Review and Meta-analysis. Calcif. Tissue Int. 2020, 107, 453–463. [Google Scholar] [CrossRef]
- Mesinovic, J.; Zengin, A.; De Courten, B.; Ebeling, P.R.; Scott, D. Sarcopenia and type 2 diabetes mellitus: A bidirectional relationship. Diabetes Metab. Syndr. Obes. 2019, 12, 1057–1072. [Google Scholar] [CrossRef]
- Daniels, C.C.; Isaacs, Z.; Finelli, R.; Leisegang, K. The efficacy of Zingiber officinale on dyslipidaemia, blood pressure, and inflammation as cardiovascular risk factors: A systematic review. Clin. Nutr. ESPEN 2022, 51, 72–82. [Google Scholar] [CrossRef]
- Wang, M.; Tan, Y.; Shi, Y.; Wang, X.; Liao, Z.; Wei, P. Diabetes and Sarcopenic Obesity: Pathogenesis, Diagnosis, and Treatments. Front. Endocrinol. 2020, 11, 568. [Google Scholar] [CrossRef]
- Izzo, A.; Massimino, E.; Riccardi, G.; Della Pepa, G. A Narrative Review on Sarcopenia in Type 2 Diabetes Mellitus: Prevalence and Associated Factors. Nutrients 2021, 13, 183. [Google Scholar] [CrossRef] [PubMed]
- Gugliucci, A. Biomarkers of dysfunctional visceral fat. Adv. Clin. Chem. 2022, 109, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Aljafary, M.A.; Al-Suhaimi, E.A. Adiponectin System (Rescue Hormone): The Missing Link between Metabolic and Cardiovascular Diseases. Pharmaceutics 2022, 14, 1430. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Shi, Z.; Ji, X.; Zhang, W.; Luan, J.; Zahr, T.; Qiang, L. Adipokines, adiposity, and atherosclerosis. Cell. Mol. Life Sci. CMLS 2022, 79, 272. [Google Scholar] [CrossRef]
- Yarla, N.S.; Polito, A.; Peluso, I. Effects of Olive Oil on TNF-α and IL-6 in Humans: Implication in Obesity and Frailty. Endocr. Metab. Immune Disord. Drug Targets 2018, 18, 63–74. [Google Scholar] [CrossRef]
- Nunan, E.; Wright, C.L.; Semola, O.A.; Subramanian, M.; Balasubramanian, P.; Lovern, P.C.; Fancher, I.S.; Butcher, J.T. Obesity as a premature aging phenotype—Implications for sarcopenic obesity. GeroScience 2022, 44, 1393–1405. [Google Scholar] [CrossRef]
- Kim, S.H.; Shin, M.J.; Shin, Y.B.; Kim, K.U. Sarcopenia Associated with Chronic Obstructive Pulmonary Disease. J. Bone Metab. 2019, 26, 65–74. [Google Scholar] [CrossRef]
- Bone, A.E.; Hepgul, N.; Kon, S.; Maddocks, M. Sarcopenia and frailty in chronic respiratory disease. Chron. Respir. Dis. 2017, 14, 85–99. [Google Scholar] [CrossRef]
- Yin, J.; Yang, L.; Xie, Y.; Liu, Y.; Li, S.; Yang, W.; Xu, B.; Ji, H.; Ding, L.; Wang, K.; et al. Dkk3 dependent transcriptional regulation controls age related skeletal muscle atrophy. Nat. Commun. 2018, 9, 1752. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Kitamura, A.; Seino, S.; Kim, H.; Obuchi, S.; Kawai, H.; Hirano, H.; Watanabe, Y.; Motokawa, K.; Narita, M.; et al. Association of nutrient-derived dietary patterns with sarcopenia and its components in community-dwelling older Japanese: A cross-sectional study. Nutr. J. 2021, 20, 7. [Google Scholar] [CrossRef]
- Rondanelli, M.; Nichetti, M.; Peroni, G.; Faliva, M.A.; Naso, M.; Gasparri, C.; Perna, S.; Oberto, L.; Di Paolo, E.; Riva, A.; et al. Where to Find Leucine in Food and How to Feed Elderly with Sarcopenia in Order to Counteract Loss of Muscle Mass: Practical Advice. Front. Nutr. 2020, 7, 622391. [Google Scholar] [CrossRef] [PubMed]
- Cereda, E.; Pisati, R.; Rondanelli, M.; Caccialanza, R. Whey Protein, Leucine- and Vitamin-D-Enriched Oral Nutritional Supplementation for the Treatment of Sarcopenia. Nutrients 2022, 14, 1524. [Google Scholar] [CrossRef] [PubMed]
- Hey, P.; Gow, P.; Testro, A.G.; Apostolov, R.; Chapman, B.; Sinclair, M. Nutraceuticals for the treatment of sarcopenia in chronic liver disease. Clin. Nutr. ESPEN 2021, 41, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Le Couteur, D.G.; Solon-Biet, S.M.; Cogger, V.C.; Ribeiro, R.; de Cabo, R.; Raubenheimer, D.; Cooney, G.J.; Simpson, S.J. Branched chain amino acids, aging and age-related health. Ageing Res. Rev. 2020, 64, 101198. [Google Scholar] [CrossRef] [PubMed]
- McKendry, J.; Currier, B.S.; Lim, C.; McLeod, J.C.; Thomas, A.C.Q.; Phillips, S.M. Nutritional Supplements to Support Resistance Exercise in Countering the Sarcopenia of Aging. Nutrients 2020, 12, 2057. [Google Scholar] [CrossRef]
- Dolan, E.; Artioli, G.G.; Pereira, R.M.R.; Gualano, B. Muscular Atrophy and Sarcopenia in the Elderly: Is There a Role for Creatine Supplementation? Biomolecules 2019, 9, 642. [Google Scholar] [CrossRef] [PubMed]
- Candow, D.G.; Forbes, S.C.; Chilibeck, P.D.; Cornish, S.M.; Antonio, J.; Kreider, R.B. Effectiveness of Creatine Supplementation on Aging Muscle and Bone: Focus on Falls Prevention and Inflammation. J. Clin. Med. 2019, 8, 488. [Google Scholar] [CrossRef]
- Candow, D.G.; Forbes, S.C.; Chilibeck, P.D.; Cornish, S.M.; Antonio, J.; Kreider, R.B. Variables Influencing the Effectiveness of Creatine Supplementation as a Therapeutic Intervention for Sarcopenia. Front. Nutr. 2019, 6, 124. [Google Scholar] [CrossRef]
- Troesch, B.; Eggersdorfer, M.; Laviano, A.; Rolland, Y.; Smith, A.D.; Warnke, I.; Weimann, A.; Calder, P.C. Expert Opinion on Benefits of Long-Chain Omega-3 Fatty Acids (DHA and EPA) in Aging and Clinical Nutrition. Nutrients 2020, 12, 2555. [Google Scholar] [CrossRef]
- Ganapathy, A.; Nieves, J.W. Nutrition and Sarcopenia—What Do We Know? Nutrients 2020, 12, 1755. [Google Scholar] [CrossRef]
- Dupont, J.; Dedeyne, L.; Dalle, S.; Koppo, K.; Gielen, E. The role of omega-3 in the prevention and treatment of sarcopenia. Aging Clin. Exp. Res. 2019, 31, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Noce, A.; Marrone, G.; Ottaviani, E.; Guerriero, C.; Di Daniele, F.; Pietroboni Zaitseva, A.; Di Daniele, N. Uremic Sarcopenia and Its Possible Nutritional Approach. Nutrients 2021, 13, 147. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Lu, Y.; Yang, X.; Pan, D.; Wang, Y.; Yin, S.; Wang, S.; Sun, G. Effects of fish oil-derived n-3 polyunsaturated fatty acid on body composition, muscle strength and physical performance in older people: A secondary analysis of a randomised, double-blind, placebo-controlled trial. Age Ageing 2022, 51. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Jang, G.; Park, J.W.; Lee, Y.K.; Koo, K.H. Vitamin D Deficiency and Sarcopenia in Hip Fracture Patients. J. Bone Metab. 2021, 28, 79–83. [Google Scholar] [CrossRef]
- Palmese, F.; Del Toro, R.; Di Marzio, G.; Cataleta, P.; Sama, M.G.; Domenicali, M. Sarcopenia and Vitamin D Deficiency in Patients with Crohn’s Disease: Pathological Conditions That Should Be Linked Together. Nutrients 2021, 13, 1378. [Google Scholar] [CrossRef]
- Hata, R.; Miyamoto, K.; Abe, Y.; Sasaki, T.; Oguma, Y.; Tajima, T.; Arai, Y.; Matsumoto, M.; Nakamura, M.; Kanaji, A.; et al. Osteoporosis and sarcopenia are associated with each other and reduced IGF1 levels are a risk for both diseases in the very old elderly. Bone 2022, 166, 116570. [Google Scholar] [CrossRef]
- Torii, M.; Itaya, T.; Minamino, H.; Katsushima, M.; Fujita, Y.; Tanaka, H.; Oshima, Y.; Watanabe, R.; Ito, H.; Arai, H.; et al. Management of Sarcopenia in Patients with Rheumatoid Arthritis. Mod. Rheumatol. 2022, roac095. [Google Scholar] [CrossRef]
- Papadopoulou, S.K.; Voulgaridou, G.; Kondyli, F.S.; Drakaki, M.; Sianidou, K.; Andrianopoulou, R.; Rodopaios, N.; Pritsa, A. Nutritional and Nutrition-Related Biomarkers as Prognostic Factors of Sarcopenia, and Their Role in Disease Progression. Diseases 2022, 10, 42. [Google Scholar] [CrossRef]
- Uchitomi, R.; Oyabu, M.; Kamei, Y. Vitamin D and Sarcopenia: Potential of Vitamin D Supplementation in Sarcopenia Prevention and Treatment. Nutrients 2020, 12, 3189. [Google Scholar] [CrossRef]
- Agergaard, J.; Trøstrup, J.; Uth, J.; Iversen, J.V.; Boesen, A.; Andersen, J.L.; Schjerling, P.; Langberg, H.J.N. Does vitamin-D intake during resistance training improve the skeletal muscle hypertrophic and strength response in young and elderly men?—A randomized controlled trial. Nutr. Metab. 2015, 12, 1–14. [Google Scholar] [CrossRef]
- Gkekas, N.K.; Anagnostis, P.; Paraschou, V.; Stamiris, D.; Dellis, S.; Kenanidis, E.; Potoupnis, M.; Tsiridis, E.; Goulis, D.G. The effect of vitamin D plus protein supplementation on sarcopenia: A systematic review and meta-analysis of randomized controlled trials. Maturitas 2021, 145, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.H.; Chen, K.H.; Chen, C.; Chu, W.C.; Kang, Y.N. The Optimal Strategy of Vitamin D for Sarcopenia: A Network Meta-Analysis of Randomized Controlled Trials. Nutrients 2021, 13, 3589. [Google Scholar] [CrossRef] [PubMed]
- Kupisz-Urbańska, M.; Płudowski, P.; Marcinowska-Suchowierska, E. Vitamin D Deficiency in Older Patients-Problems of Sarcopenia, Drug Interactions, Management in Deficiency. Nutrients 2021, 13, 1247. [Google Scholar] [CrossRef] [PubMed]
- Rizzoli, R. Vitamin D supplementation: Upper limit for safety revisited? Aging Clin. Exp. Res. 2021, 33, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Prokopidis, K.; Giannos, P.; Katsikas Triantafyllidis, K.; Kechagias, K.S.; Mesinovic, J.; Witard, O.C.; Scott, D. The authors’ reply: ‘Comment on: “Effect of vitamin D monotherapy on indices of sarcopenia in community-dwelling older adults: A systematic review and meta-analysis” by Prokopidis et al. J. Cachexia Sarcopenia Muscle 2022, 13, 2757–2758. [Google Scholar] [CrossRef]
- Kirk, B.; Zanker, J.; Duque, G. Osteosarcopenia: Epidemiology, diagnosis, and treatment-facts and numbers. J. Cachexia Sarcopenia Muscle 2020, 11, 609–618. [Google Scholar] [CrossRef]
- van Dronkelaar, C.; van Velzen, A.; Abdelrazek, M.; van der Steen, A.; Weijs, P.J.M.; Tieland, M. Minerals and Sarcopenia; The Role of Calcium, Iron, Magnesium, Phosphorus, Potassium, Selenium, Sodium, and Zinc on Muscle Mass, Muscle Strength, and Physical Performance in Older Adults: A Systematic Review. J. Am. Med. Dir. Assoc. 2018, 19, 6–11.e13. [Google Scholar] [CrossRef]
- Tang, B.M.; Eslick, G.D.; Nowson, C.; Smith, C.; Bensoussan, A. Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: A meta-analysis. Lancet 2007, 370, 657–666. [Google Scholar] [CrossRef]
- Harvey, N.C.; Biver, E.; Kaufman, J.M.; Bauer, J.; Branco, J.; Brandi, M.L.; Bruyère, O.; Coxam, V.; Cruz-Jentoft, A.; Czerwinski, E.; et al. The role of calcium supplementation in healthy musculoskeletal ageing: An expert consensus meeting of the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) and the International Foundation for Osteoporosis (IOF). Osteoporos Int. 2017, 28, 447–462. [Google Scholar] [CrossRef]
- Hall, J.E.; Hall, M.E. Guyton and Hall Textbook of Medical Physiology; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Thabit, H.; Barry, M.; Sreenan, S.; Smith, D. Proximal myopathy in lacto-vegetarian Asian patients responding to Vitamin D and calcium supplement therapy—Two case reports and review of the literature. J. Med. Case Rep. 2011, 5, 178. [Google Scholar] [CrossRef]
- Hirata, D.; Nagashima, T.; Saito, S.; Okazaki, H.; Kano, S.; Minota, S. Elevated muscle enzymes in a patient with severe hypocalcemia mimicking polymyositis. Mod. Rheumatol. 2002, 12, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Policepatil, S.M.; Caplan, R.H.; Dolan, M. Hypocalcemic myopathy secondary to hypoparathyroidism. WMJ 2012, 111, 173–175. [Google Scholar] [PubMed]
- Bennett, B.T.; Mohamed, J.S.; Alway, S.E. The Effects of Calcium-β-Hydroxy-β-Methylbutyrate on Aging-Associated Apoptotic Signaling and Muscle Mass and Function in Unloaded but Nonatrophied Extensor Digitorum Longus Muscles of Aged Rats. Oxid. Med. Cell. Longev. 2020, 2020, 3938672. [Google Scholar] [CrossRef] [PubMed]
- Onder, G.; Della Vedova, C.; Landi, F. Validated treatments and therapeutics prospectives regarding pharmacological products for sarcopenia. J. Nutr. Health Aging 2009, 13, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Campins, L.; Camps, M.; Riera, A.; Pleguezuelos, E.; Yebenes, J.C.; Serra-Prat, M. Oral Drugs Related with Muscle Wasting and Sarcopenia. A Review. Pharmacology 2017, 99, 1–8. [Google Scholar] [CrossRef]
- Vallon, V.; Verma, S. Effects of SGLT2 Inhibitors on Kidney and Cardiovascular Function. Annu. Rev. Physiol. 2021, 83, 503–528. [Google Scholar] [CrossRef]
- Cowie, M.R.; Fisher, M. SGLT2 inhibitors: Mechanisms of cardiovascular benefit beyond glycaemic control. Nat. Rev. Cardiol. 2020, 17, 761–772. [Google Scholar] [CrossRef]
- Bamba, R.; Okamura, T.; Hashimoto, Y.; Majima, S.; Senmaru, T.; Ushigome, E.; Nakanishi, N.; Asano, M.; Yamazaki, M.; Takakuwa, H.; et al. Extracellular lipidome change by an SGLT2 inhibitor, luseogliflozin, contributes to prevent skeletal muscle atrophy in db/db mice. J. Cachexia Sarcopenia Muscle 2022, 13, 574–588. [Google Scholar] [CrossRef]
- Otsuka, H.; Yokomizo, H.; Nakamura, S.; Izumi, Y.; Takahashi, M.; Obara, S.; Nakao, M.; Ikeda, Y.; Sato, N.; Sakamoto, R.; et al. Differential effect of canagliflozin, a sodium-glucose cotransporter 2 (SGLT2) inhibitor, on slow and fast skeletal muscles from nondiabetic mice. Biochem. J. 2022, 479, 425–444. [Google Scholar] [CrossRef]
- Siebert, D.M.; Rao, A.L. The Use and Abuse of Human Growth Hormone in Sports. Sport. Health 2018, 10, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Graber, E.; Reiter, E.O.; Rogol, A.D. Human Growth and Growth Hormone: From Antiquity to the Recominant Age to the Future. Front. Endocrinol. 2021, 12, 709936. [Google Scholar] [CrossRef] [PubMed]
- Bian, A.; Ma, Y.; Zhou, X.; Guo, Y.; Wang, W.; Zhang, Y.; Wang, X. Association between sarcopenia and levels of growth hormone and insulin-like growth factor-1 in the elderly. BMC Musculoskelet. Disord. 2020, 21, 214. [Google Scholar] [CrossRef] [PubMed]
- Marcell, T.J.; Harman, S.M.; Urban, R.J.; Metz, D.D.; Rodgers, B.D.; Blackman, M.R. Comparison of GH, IGF-I, and testosterone with mRNA of receptors and myostatin in skeletal muscle in older men. Am. J. Physiol. Endocrinol. Metab. 2001, 281, E1159–E1164. [Google Scholar] [CrossRef] [PubMed]
- Laurindo, L.F.; Barbalho, S.M.; Guiguer, E.L.; da Silva Soares de Souza, M.; de Souza, G.A.; Fidalgo, T.M.; Araújo, A.C.; de Souza Gonzaga, H.F.; de Bortoli Teixeira, D.; de Oliveira Silva Ullmann, T.; et al. GLP-1a: Going beyond Traditional Use. Int. J. Mol. Sci. 2022, 23, 739. [Google Scholar] [CrossRef]
- Hong, Y.; Lee, J.H.; Jeong, K.W.; Choi, C.S.; Jun, H.S. Amelioration of muscle wasting by glucagon-like peptide-1 receptor agonist in muscle atrophy. J. Cachexia Sarcopenia Muscle 2019, 10, 903–918. [Google Scholar] [CrossRef]
- Khin, P.P.; Hong, Y.; Yeon, M.; Lee, D.H.; Lee, J.H.; Jun, H.S. Dulaglutide improves muscle function by attenuating inflammation through OPA-1-TLR-9 signaling in aged mice. Aging 2021, 13, 21962–21974. [Google Scholar] [CrossRef]
- Podhorecka, M.; Ibanez, B.; Dmoszyńska, A. Metformin—Its potential anti-cancer and anti-aging effects. Postep. Hig Med. Dosw. 2017, 71, 170–175. [Google Scholar] [CrossRef]
- McCreight, L.J.; Bailey, C.J.; Pearson, E.R. Metformin and the gastrointestinal tract. Diabetologia 2016, 59, 426–435. [Google Scholar] [CrossRef]
- Kang, M.J.; Moon, J.W.; Lee, J.O.; Kim, J.H.; Jung, E.J.; Kim, S.J.; Oh, J.Y.; Wu, S.W.; Lee, P.R.; Park, S.H.; et al. Metformin induces muscle atrophy by transcriptional regulation of myostatin via HDAC6 and FoxO3a. J. Cachexia Sarcopenia Muscle 2022, 13, 605–620. [Google Scholar] [CrossRef]
- Toledo-Pérez, R.; Lopéz-Cervantes, S.P.; Hernández-Álvarez, D.; Mena-Montes, B.; Pedraza-Vázquez, G.; Sánchez-Garibay, C.; López-Diazguerrero, N.E.; Königsberg, M.; Luna-López, A. Metformin and tBHQ Treatment Combined with an Exercise Regime Prevents Osteosarcopenic Obesity in Middle-Aged Wistar Female Rats. Oxid. Med. Cell. Longev. 2021, 2021, 5294266. [Google Scholar] [CrossRef]
- Yang, Y.; Liao, Z.; Xiao, Q. Metformin ameliorates skeletal muscle atrophy in Grx1 KO mice by regulating intramuscular lipid accumulation and glucose utilization. Biochem. Biophys. Res. Commun. 2020, 533, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Zakeri, M.; Fatemi, I.; Kaeidi, A.; Zakeri, M.A.; Hakimizadeh, E.; Hassanipour, M.; Rahmani, M.; Hassanshahi, J.; Ayoobi, F.; Allahtavakoli, M. Pro-neurocognitive and anti-sarcopenic benefits of one-year metformin therapy in ovariectomized aged mice. Clin. Exp. Pharm. Physiol. 2019, 46, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Dungan, C.M.; Li, Z.; Wright, D.C.; Williamson, D.L. Hyperactive mTORC1 signaling is unaffected by metformin treatment in aged skeletal muscle. Muscle Nerve 2016, 53, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Álvarez, D.; Mena-Montes, B.; Toledo-Pérez, R.; Pedraza-Vázquez, G.; López-Cervantes, S.P.; Morales-Salazar, A.; Hernández-Cruz, E.; Lazzarini-Lechuga, R.; Vázquez-Cárdenas, R.R.; Vilchis-DeLaRosa, S.; et al. Long-Term Moderate Exercise Combined with Metformin Treatment Induces an Hormetic Response That Prevents Strength and Muscle Mass Loss in Old Female Wistar Rats. Oxid. Med. Cell. Longev. 2019, 2019, 3428543. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.J.; Mancini, G.B.; Genest, J., Jr.; Grégoire, J.; Lonn, E.M.; Hegele, R.A. The new dyslipidemia guidelines: What is the debate? Can. J. Cardiol. 2015, 31, 605–612. [Google Scholar] [CrossRef]
- Adhyaru, B.B.; Jacobson, T.A. Safety and efficacy of statin therapy. Nat. Rev. Cardiol. 2018, 15, 757–769. [Google Scholar] [CrossRef]
- Sahebkar, A.; Cicero, A.F.G.; Di Giosia, P.; Pomilio, I.; Stamerra, C.A.; Giorgini, P.; Ferri, C.; von Haehling, S.; Banach, M.; Jamialahmadi, T. Pathophysiological mechanisms of statin-associated myopathies: Possible role of the ubiquitin-proteasome system. J. Cachexia Sarcopenia Muscle 2020, 11, 1177–1186. [Google Scholar] [CrossRef]
- Bielecka-Dabrowa, A.; Fabis, J.; Mikhailidis, D.P.; von Haehling, S.; Sahebkar, A.; Rysz, J.; Banach, M. Prosarcopenic Effects of Statins May Limit Their Effectiveness in Patients with Heart Failure. Trends Pharm. Sci. 2018, 39, 331–353. [Google Scholar] [CrossRef]
- Clarke, A.; Ladha, C.; Wright, A.; Pattinson, K. Losartan may attenuate altitude-related sleep disturbance. BMJ Mil. Health 2021, 167, 424–428. [Google Scholar] [CrossRef]
- Al-Majed, A.R.; Assiri, E.; Khalil, N.Y.; Abdel-Aziz, H.A. Losartan: Comprehensive Profile. Profiles Drug Subst. Excip. Relat. Methodol. 2015, 40, 159–194. [Google Scholar] [CrossRef]
- Burks, T.N.; Andres-Mateos, E.; Marx, R.; Mejias, R.; Van Erp, C.; Simmers, J.L.; Walston, J.D.; Ward, C.W.; Cohn, R.D. Losartan restores skeletal muscle remodeling and protects against disuse atrophy in sarcopenia. Sci. Transl. Med. 2011, 3, 82ra37. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Chang, P.C.; Chu, P.H.; Chuang, Y.F.; Huang, R.C.; Chen, C.N. Effects of losartan and exercise on muscle mass and exercise endurance of old mice. Exp. Gerontol. 2022, 165, 111869. [Google Scholar] [CrossRef] [PubMed]
- Pahor, M.; Anton, S.D.; Beavers, D.P.; Cauley, J.A.; Fielding, R.A.; Kritchevsky, S.B.; Leeuwenburgh, C.; Lewis, K.H.; Liu, C.K.; Lovato, L.C.; et al. Effect of Losartan and Fish Oil on Plasma IL-6 and Mobility in Older Persons. The ENRGISE Pilot Randomized Clinical Trial. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 1612–1619. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S.; Kaji, K.; Nishimura, N.; Enomoto, M.; Fujimoto, Y.; Murata, K.; Takaya, H.; Kawaratani, H.; Moriya, K.; Namisaki, T.; et al. Angiotensin Receptor Blockers Potentiate the Protective Effect of Branched-Chain Amino Acids on Skeletal Muscle Atrophy in Cirrhotic Rats. Mol. Nutr. Food Res. 2021, 65, e2100526. [Google Scholar] [CrossRef]
- Gallwitz, B. Clinical Use of DPP-4 Inhibitors. Front. Endocrinol. 2019, 10, 389. [Google Scholar] [CrossRef]
- Deacon, C.F. Dipeptidyl peptidase 4 inhibitors in the treatment of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2020, 16, 642–653. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mellen, R.H.; Girotto, O.S.; Marques, E.B.; Laurindo, L.F.; Grippa, P.C.; Mendes, C.G.; Garcia, L.N.H.; Bechara, M.D.; Barbalho, S.M.; Sinatora, R.V.; et al. Insights into Pathogenesis, Nutritional and Drug Approach in Sarcopenia: A Systematic Review. Biomedicines 2023, 11, 136. https://doi.org/10.3390/biomedicines11010136
Mellen RH, Girotto OS, Marques EB, Laurindo LF, Grippa PC, Mendes CG, Garcia LNH, Bechara MD, Barbalho SM, Sinatora RV, et al. Insights into Pathogenesis, Nutritional and Drug Approach in Sarcopenia: A Systematic Review. Biomedicines. 2023; 11(1):136. https://doi.org/10.3390/biomedicines11010136
Chicago/Turabian StyleMellen, Rodrigo Haber, Otávio Simões Girotto, Eduarda Boni Marques, Lucas Fornari Laurindo, Paulo Cesar Grippa, Claudemir Gregório Mendes, Lorena Natalino Haber Garcia, Marcelo Dib Bechara, Sandra Maria Barbalho, Renata Vargas Sinatora, and et al. 2023. "Insights into Pathogenesis, Nutritional and Drug Approach in Sarcopenia: A Systematic Review" Biomedicines 11, no. 1: 136. https://doi.org/10.3390/biomedicines11010136
APA StyleMellen, R. H., Girotto, O. S., Marques, E. B., Laurindo, L. F., Grippa, P. C., Mendes, C. G., Garcia, L. N. H., Bechara, M. D., Barbalho, S. M., Sinatora, R. V., Haber, J. F. d. S., Flato, U. A. P., Bueno, P. C. d. S., Detregiachi, C. R. P., & Quesada, K. (2023). Insights into Pathogenesis, Nutritional and Drug Approach in Sarcopenia: A Systematic Review. Biomedicines, 11(1), 136. https://doi.org/10.3390/biomedicines11010136








