Pancreatic Cancer Resistance to Treatment: The Role of Microbiota
Abstract
:1. Pancreatic Cancer
1.1. Therapies for Pancreatic Cancer
1.2. Microbiota and Pancreatic Cancer
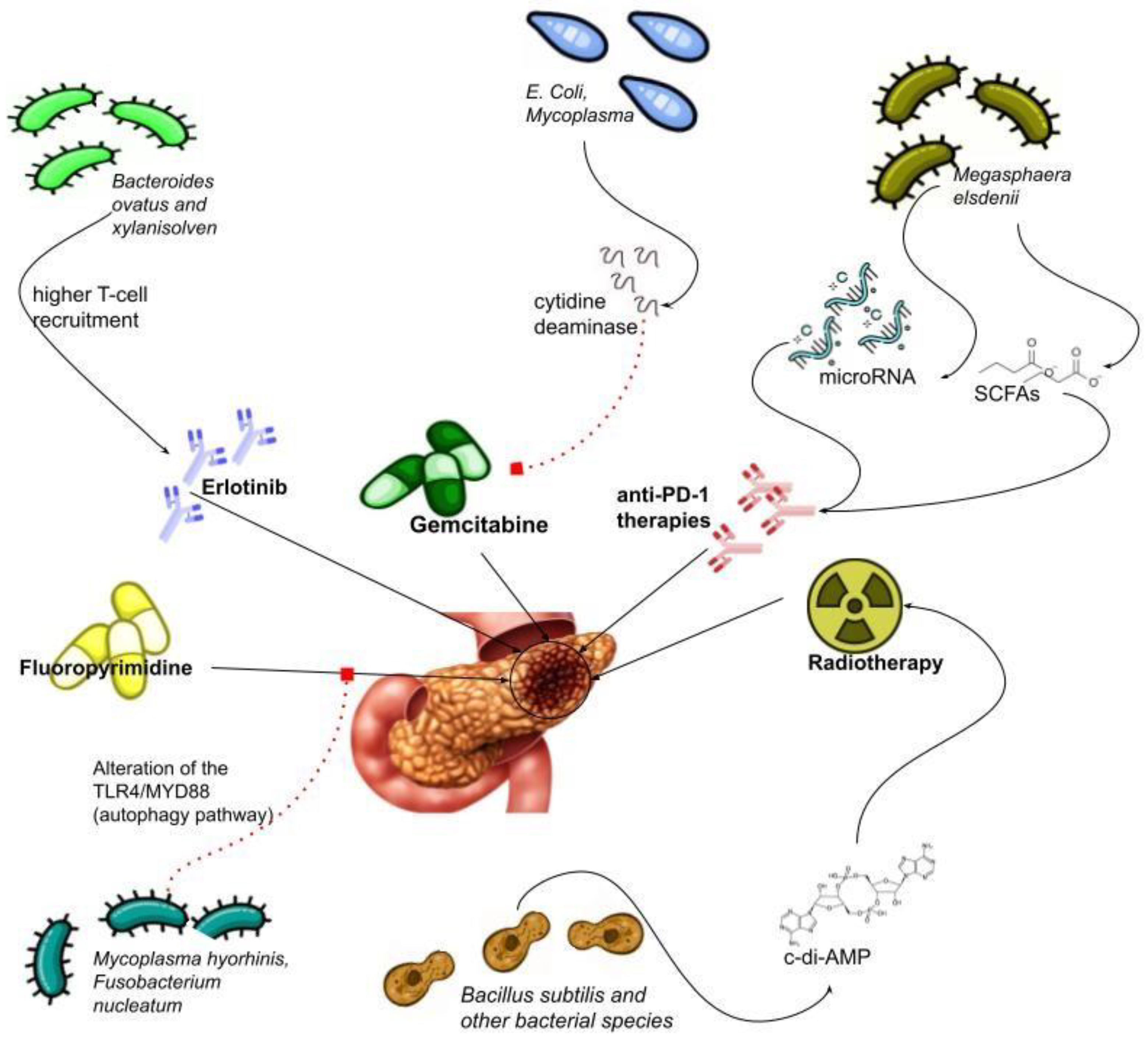
1.3. Microbiota and Chemotherapy
1.4. Microbiota and Immunotherapy
1.5. Microbiota and Radiotherapy
1.6. Bidirectional Relationship between Therapy and Microbiota
2. Conclusions and Future Perspectives
3. Materials and Methods
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics. CA: Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Laurent, L.; Vullierme, M.; Rebours, V.; Maire, F.; Hentic, O.; Francoz, C.; Durand, F.; Ruszniewski, P.; Lévy, P. Estimation of the prevalence of intraductal papillary mucinous neoplasm of the pancreas in the French population through patients waiting for liver transplantation. United Eur. Gastroenterol. J. 2017, 5, 499–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizrahi, J.D.; Surana, R.; Valle, J.W.; Shroff, R.T. Pancreatic cancer. Lancet 2020, 395, 2008–2020. [Google Scholar] [CrossRef] [PubMed]
- Ben, Q.-W.; Liu, J.; Sun, Y.-W.; Wang, L.-F.; Zou, D.-W.; Yuan, Y.-Z. Cigarette Smoking and Mortality in Patients with Pan-creatic Cancer: A Systematic Review and Meta-Analysis. Pancreas 2019, 48, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P.; Sunkara, T.; Gaduputi, V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J. Oncol. 2019, 10, 10–27. [Google Scholar] [CrossRef]
- Sammallahti, H.; Kokkola, A.; Rezasoltani, S.; Ghanbari, R.; Aghdaei, H.A.; Knuutila, S.; Puolakkainen, P.; Sarhadi, V.K. Microbiota Alterations and Their Association with Oncogenomic Changes in Pancreatic Cancer Patients. Int. J. Mol. Sci. 2021, 22, 12978. [Google Scholar] [CrossRef]
- Zhang, J.-J.; Jia, J.-P.; Shao, Q.; Wang, Y.-K. Diabetes mellitus and risk of pancreatic cancer in China: A meta-analysis based on 26 case-control studies. Prim. Care Diabetes 2019, 13, 276–282. [Google Scholar] [CrossRef]
- Gandhi, S.; de la Fuente, J.; Murad, M.H.; Majumder, S. Chronic Pancreatitis Is a Risk Factor for Pancreatic Cancer, and Incidence Increases with Duration of Disease: A Systematic Review and Meta-analysis. Clin. Transl. Gastroenterol. 2022, 13, e00463. [Google Scholar] [CrossRef]
- Bae, J.-M.; Shim, S.R. Coffee Consumption and Pancreatic Cancer Risk: A Meta-Epidemiological Study of Population-based Cohort Studies. Asian Pac. J. Cancer Prev. 2020, 21, 2793–2798. [Google Scholar] [CrossRef] [PubMed]
- Ilic, M.; Milicic, B.; Ilic, I. Association between oral contraceptive use and pancreatic cancer risk: A systematic review and meta-analysis. World J. Gastroenterol. 2021, 27, 2643–2656. [Google Scholar] [CrossRef] [PubMed]
- Laoveeravat, P.; Thavaraputta, S.; Vutthikraivit, W.; Suchartlikitwong, S.; Mingbunjerdsuk, T.; Motes, A.; Nugent, K.; Rakvit, A.; Islam, E.; Islam, S. Proton Pump Inhibitors and Histamine-2 Receptor Antagonists on the Risk of Pancreatic Cancer: A Systematic Review and Meta-analysis. J. Assoc. Physicians 2020, 113, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Park, W.; Chawla, A.; O’Reilly, E.M. Pancreatic Cancer. A Review. J. Am. Med. Assoc. 2021, 326, 851–862. [Google Scholar] [CrossRef] [PubMed]
- Sohal, D.P.; Mangu, P.B.; Khorana, A.; Shah, M.A.; Philip, P.A.; O’Reilly, E.M.; Uronis, H.E.; Ramanathan, R.K.; Crane, C.H.; Engebretson, A.; et al. Metastatic Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2016, 34, 2784–2796. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, M.; Abdelrahim, M. The Latest Advancement in Pancreatic Ductal Adenocarcinoma Therapy: A Review Article for the Latest Guidelines and Novel Therapies. Biomedicines 2021, 9, 389. [Google Scholar] [CrossRef]
- Khorana, A.; Mangu, P.B.; Berlin, J.; Engebretson, A.; Hong, T.S.; Maitra, A.; Mohile, S.G.; Mumber, M.; Schulick, R.; Shapiro, M.; et al. Potentially Curable Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2016, 34, 2541–2556. [Google Scholar] [CrossRef]
- Tempero, M.A.; Malafa, M.P.; Al-Hawary, M.; Behrman, S.W.; Benson, A.B.; Cardin, D.B.; Chiorean, E.G.; Chung, V.; Czito, B.; Del Chiaro, M.; et al. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 439–457. [Google Scholar] [CrossRef]
- Tas, F.; Sen, F.; Keskin, S.; Kilic, L.; Yildiz, I. Prognostic factors in metastatic pancreatic cancer: Older patients are associated with reduced overall survival. Mol. Clin. Oncol. 2013, 1, 788–792. [Google Scholar] [CrossRef] [Green Version]
- Pushalkar, S.; Hundeyin, M.; Daley, D.; Zambirinis, C.P.; Kurz, E.; Mishra, A.; Mohan, N.; Aykut, B.; Usyk, M.; Torres, L.E.; et al. The Pancreatic Cancer Microbiome Promotes Oncogenesis by Induction of Innate and Adaptive Immune Suppression. Cancer Discov. 2018, 8, 403–416. [Google Scholar] [CrossRef] [Green Version]
- Oh, J.-K.; Weiderpass, E. Infection and Cancer: Global Distribution and Burden of Diseases. Ann. Glob. Health 2014, 80, 384–392. [Google Scholar] [CrossRef]
- Sarhadi, V.; Mathew, B.; Kokkola, A.; Karla, T.; Tikkanen, M.; Rautelin, H.; Lahti, L.; Puolakkainen, P.; Knuutila, S. Gut microbiota of patients with different subtypes of gastric cancer and gastrointestinal stromal tumors. Gut Pathog. 2021, 13, 11. [Google Scholar] [CrossRef]
- Schepis, T.; De Lucia, S.S.; Nista, E.C.; Manilla, V.; Pignataro, G.; Ojetti, V.; Piccioni, A.; Gasbarrini, A.; Franceschi, F.; Candelli, M. Microbiota in Pancreatic Diseases: A Review of the Literature. J. Clin. Med. 2021, 10, 5920. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, M.; Schwartz, D.; Tandon, M.; Son, A.; Zeng, M.; Swaim, W.; Eckhaus, M.; Hoffman, V.; Cui, Y.; Xiao, B.; et al. Orai1-Mediated Antimicrobial Secretion from Pancreatic Acini Shapes the Gut Microbiome and Regulates Gut Innate Immunity. Cell Metab. 2017, 25, 635–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitsuhashi, K.; Nosho, K.; Sukawa, Y.; Matsunaga, Y.; Ito, M.; Kurihara, H.; Kanno, S.; Igarashi, H.; Naito, T.; Adachi, Y.; et al. Association of Fusobacteriums pecies in pancreatic cancer tissues with molecular features and prognosis. Oncotarget 2015, 6, 7209–7220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres, P.J.; Fletcher, E.M.; Gibbons, S.M.; Bouvet, M.; Doran, K.S.; Kelley, S.T. Characterization of the salivary microbiome in patients with pancreatic cancer. Peerj 2015, 3, e1373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, H.; Ren, Z.; Li, A.; Li, J.; Xu, S.; Zhang, H.; Jiang, J.; Yang, J.; Luo, Q.; Zhou, K.; et al. Tongue coating microbiome data distinguish patients with pancreatic head cancer from healthy controls. J. Oral Microbiol. 2019, 11, 1563409. [Google Scholar] [CrossRef] [PubMed]
- Shirazi, M.S.R.; Al-Alo, K.Z.K.; Al-Yasiri, M.H.; Lateef, Z.M.; Ghasemian, A. Microbiome Dysbiosis and Predominant Bacterial Species as Human Cancer Biomarkers. J. Gastrointest. Cancer 2020, 51, 725–728. [Google Scholar] [CrossRef] [PubMed]
- Öğrendik, M. Periodontal Pathogens in the Etiology of Pancreatic Cancer. Gastrointest. Tumors 2017, 3, 125–127. [Google Scholar] [CrossRef] [Green Version]
- Gnanasekaran, J.; Gallimidi, A.B.; Saba, E.; Pandi, K.; Berchoer, L.E.; Hermano, E.; Angabo, S.; Makkawi, H.; Khashan, A.; Daoud, A.; et al. Intracellular Porphyromonas gingivalis Promotes the Tumorigenic Behavior of Pancreatic Carcinoma Cells. Cancers 2020, 12, 2331. [Google Scholar] [CrossRef]
- Geller, L.T.; Barzily-Rokni, M.; Danino, T.; Jonas, O.H.; Shental, N.; Nejman, D.; Gavert, N.; Zwang, Y.; Cooper, Z.A.; Shee, K.; et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 2017, 357, 1156–1160. [Google Scholar] [CrossRef] [Green Version]
- Guo, W.; Zhang, Y.; Guo, S.; Mei, Z.; Liao, H.; Dong, H.; Wu, K.; Ye, H.; Zhang, Y.; Zhu, Y.; et al. Tumor microbiome contributes to an aggressive phenotype in the basal-like subtype of pancreatic cancer. Commun. Biol. 2021, 4, 1019. [Google Scholar] [CrossRef]
- Del Castillo, E.; Meier, R.; Chung, M.; Koestler, D.C.; Chen, T.; Paster, B.J.; Charpentier, K.P.; Kelsey, K.T.; Izard, J.; Michaud, D.S. The Microbiomes of Pancreatic and Duodenum Tissue Overlap and Are Highly Subject Specific but Differ between Pan-creatic Cancer and Noncancer Subjects. Cancer Epidemiol. Biomark. Prev. 2019, 28, 370–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nejman, D.; Livyatan, I.; Fuks, G.; Gavert, N.; Zwang, Y.; Geller, L.T.; Rotter-Maskowitz, A.; Weiser, R.; Mallel, G.; Gigi, E.; et al. The human tumor microbiome is composed of tumor type–specific intracellular bacteria. Science 2020, 368, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Gaiser, R.A.; Halimi, A.; Alkharaan, H.; Lu, L.; Davanian, H.; Healy, K.; Hugerth, L.W.; Ateeb, Z.; Valente, R.; Moro, C.F.; et al. Enrichment of oral microbiota in early cystic precursors to invasive pancreatic cancer. Gut 2019, 68, 2186–2194. [Google Scholar] [CrossRef] [Green Version]
- Ren, Z.; Jiang, J.; Xie, H.; Li, A.; Lu, H.; Xu, S.; Zhou, L.; Zhang, H.; Cui, G.; Chen, X.; et al. Gut microbial profile analysis by MiSeq sequencing of pancreatic carcinoma patients in China. Oncotarget 2017, 8, 95176–95191. [Google Scholar] [CrossRef] [Green Version]
- Candelli, M.; Franza, L.; Pignataro, G.; Ojetti, V.; Covino, M.; Piccioni, A.; Gasbarrini, A.; Franceschi, F. Interaction between Lipopolysaccharide and Gut Microbiota in Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2021, 22, 6242. [Google Scholar] [CrossRef] [PubMed]
- Kadosh, E.; Snir-Alkalay, I.; Venkatachalam, A.; May, S.; Lasry, A.; Elyada, E.; Zinger, A.; Shaham, M.; Vaalani, G.; Mernberger, M.; et al. The gut microbiome switches mutant p53 from tumour-suppressive to oncogenic. Nature 2020, 586, 133–138. [Google Scholar] [CrossRef]
- Casero, R.A., Jr.; Stewart, T.M.; Pegg, A.E. Polyamine metabolism and cancer: Treatments, challenges and opportunities. Nat. Rev. Cancer 2018, 18, 681–695. [Google Scholar] [CrossRef]
- Alonso-Curbelo, D.; Ho, Y.-J.; Burdziak, C.; Maag, J.L.V.; Iv, J.P.M.; Chandwani, R.; Chen, H.-A.; Tsanov, K.M.; Barriga, F.M.; Luan, W.; et al. A gene–environment-induced epigenetic program initiates tumorigenesis. Nature 2021, 590, 642–648. [Google Scholar] [CrossRef]
- Aykut, B.; Pushalkar, S.; Chen, R.; Li, Q.; Abengozar, R.; Kim, J.I.; Shadaloey, S.A.; Wu, D.; Preiss, P.; Verma, N.; et al. The fungal mycobiome promotes pancreatic oncogenesis via activation of MBL. Nature 2019, 574, 264–267. [Google Scholar] [CrossRef]
- Riquelme, E.; Zhang, Y.; Zhang, L.; Montiel, M.; Zoltan, M.; Dong, W.; Quesada, P.; Sahin, I.; Chandra, V.; Lucas, A.S.; et al. Tumor Microbiome Diversity and Composition Influence Pancreatic Cancer Outcomes. Cell 2019, 178, 795–806.e12. [Google Scholar] [CrossRef]
- Alam, A.; Levanduski, E.; Denz, P.; Villavicencio, H.S.; Bhatta, M.; Alhorebi, L.; Zhang, Y.; Gomez, E.C.; Morreale, B.; Senchanthisai, S.; et al. Fungal mycobiome drives IL-33 secretion and type 2 immunity in pancreatic cancer. Cancer Cell 2022, 40, 153–167.e11. [Google Scholar] [CrossRef] [PubMed]
- Chakladar, J.; Kuo, S.Z.; Castaneda, G.; Li, W.T.; Gnanasekar, A.; Yu, M.A.; Chang, E.Y.; Wang, X.Q.; Ongkeko, W.M. The Pancreatic Microbiome Is Associated with Carcinogenesis and Worse Prognosis in Males and Smokers. Cancers 2020, 12, 2672. [Google Scholar] [CrossRef] [PubMed]
- Kartal, E.; Schmidt, T.S.B.; Molina-Montes, E.; Rodríguez-Perales, S.; Wirbel, J.; Maistrenko, O.M.; Akanni, W.; Alhamwe, B.A.; Alves, R.J.; Carrato, A.; et al. A faecal microbiota signature with high specificity for pancreatic cancer. Gut 2022, 71, 1359–1372. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Ricciardiello, F.; Yang, G.; Qiu, J.; Huang, H.; Xiao, J.; Cao, Z.; Zhao, F.; Liu, Y.; Luo, W.; et al. The Role of Mitochondria in the Chemoresistance of Pancreatic Cancer Cells. Cells 2021, 10, 497. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Li, M.; Hou, S.; Tian, B. Role of the microbiome in systemic therapy for pancreatic ductal adenocarcinoma (Review). Int. J. Oncol. 2021, 59, 101. [Google Scholar] [CrossRef]
- Lehouritis, P.; Cummins, J.; Stanton, M.; Murphy, C.T.; McCarthy, F.; Reid, G.; Urbaniak, C.; Byrne, W.L.; Tangney, M. Local bacteria affect the efficacy of chemotherapeutic drugs. Sci. Rep. 2015, 5, 14554. [Google Scholar] [CrossRef] [Green Version]
- Voorde, J.V.; Sabuncuoğlu, S.; Noppen, S.; Hofer, A.; Ranjbarian, F.; Fieuws, S.; Balzarini, J.; Liekens, S. Nucleoside-catabolizing Enzymes in Mycoplasma-infected Tumor Cell Cultures Compromise the Cytostatic Activity of the Anticancer Drug Gemcitabine. J. Biol. Chem. 2014, 289, 13054–13065. [Google Scholar] [CrossRef] [Green Version]
- Tijeras-Raballand, A.; Hilmi, M.; Astorgues-Xerri, L.; Nicolle, R.; Bièche, I.; Neuzillet, C. Microbiome and pancreatic ductal adenocarcinoma. Clin. Res. Hepatol. Gastroenterol. 2021, 45, 101589. [Google Scholar] [CrossRef]
- Weniger, M.; Hank, T.; Qadan, M.; Ciprani, D.; Michelakos, T.; Niess, H.; Heiliger, C.; Ilmer, M.; D’Haese, J.G.; Ferrone, C.R.; et al. Influence of Klebsiella pneumoniae and quinolone treatment on prognosis in patients with pancreatic cancer. Br. J. Surg. 2021, 108, 709–716. [Google Scholar] [CrossRef]
- Kesh, K.; Mendez, R.; Abdelrahman, L.; Banerjee, S.; Banerjee, S. Type 2 diabetes induced microbiome dysbiosis is associated with therapy resistance in pancreatic adenocarcinoma. Microb. Cell Factories 2020, 19, 75. [Google Scholar] [CrossRef]
- Arsenijevic, T.; Nicolle, R.; Bouchart, C.; D’Haene, N.; Demetter, P.; Puleo, F.; Van Laethem, J.-L. Pancreatic Cancer Meets Human Microbiota: Close Encounters of the Third Kind. Cancers 2021, 13, 1231. [Google Scholar] [CrossRef] [PubMed]
- Bronckaers, A.; Balzarini, J.; Liekens, S. The cytostatic activity of pyrimidine nucleosides is strongly modulated by Mycoplasma hyorhinis infection: Implications for cancer therapy. Biochem. Pharmacol. 2008, 76, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, Y.; Weng, W.; Guo, B.; Cai, G.; Ma, Y.; Cai, S. Fusobacterium nucleatum promotes chemoresistance to 5-fluorouracil by upregulation of BIRC3 expression in colorectal cancer. J. Exp. Clin. Cancer Res. 2019, 38, 14. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Guo, F.; Yu, Y.; Sun, T.; Ma, D.; Han, J.; Qian, Y.; Kryczek, I.; Sun, D.; Nagarsheth, N.; et al. Fusobacterium nucleatum Promotes Chemoresistance to Colorectal Cancer by Modulating Autophagy. Cell 2017, 170, 548–563.e16. [Google Scholar] [CrossRef] [Green Version]
- Heshiki, Y.; Vazquez-Uribe, R.; Li, J.; Ni, Y.; Quainoo, S.; Imamovic, L.; Li, J.; Sørensen, M.; Chow, B.K.C.; Weiss, G.J.; et al. Predictable modulation of cancer treatment outcomes by the gut microbiota. Microbiome 2020, 8, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz-Cordero, R.; Devine, W.P. Targeted Therapy and Checkpoint Immunotherapy in Lung Cancer. Surg. Pathol. Clin. 2020, 13, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, L.; Daud, A. Immunotherapy for melanoma. Semin. Cutan. Med. Surg. 2018, 37, 127–131. [Google Scholar] [CrossRef]
- Balachandran, V.P.; Beatty, G.L.; Dougan, S.K. Broadening the Impact of Immunotherapy to Pancreatic Cancer: Challenges and Opportunities. Gastroenterology 2019, 156, 2056–2072. [Google Scholar] [CrossRef]
- Feig, C.; Gopinathan, A.; Neesse, A.; Chan, D.S.; Cook, N.; Tuveson, D.A. The Pancreas Cancer Microenvironment. Clin. Cancer Res. 2012, 18, 4266–4276. [Google Scholar] [CrossRef] [Green Version]
- Elkrief, A.; Derosa, L.; Zitvogel, L.; Kroemer, G.; Routy, B. The intimate relationship between gut microbiota and cancer immunotherapy. Gut Microbes 2019, 10, 424–428. [Google Scholar] [CrossRef]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Lei, Y.M.; Jabri, B.; Alegre, M.-L.; et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef] [Green Version]
- Sethi, V.; Kurtom, S.; Tarique, M.; Lavania, S.; Malchiodi, Z.; Hellmund, L.; Zhang, L.; Sharma, U.; Giri, B.; Garg, B.; et al. Gut Microbiota Promotes Tumor Growth in Mice by Modulating Immune Response. Gastroenterology 2018, 155, 33–37.e6. [Google Scholar] [CrossRef]
- Dong, J.; Gao, H.-L.; Wang, W.-Q.; Yu, X.-J.; Liu, L. Bidirectional and dynamic interaction between the microbiota and therapeutic resistance in pancreatic cancer. Biochim. Biophys. Acta Rev. Cancer 2021, 1875, 188484. [Google Scholar] [CrossRef]
- Virtue, A.; Wang, H.; Yang, X.-F. MicroRNAs and Toll-like Receptor/Interleukin-1 Receptor Signaling. J. Hematol. Oncol. 2012, 5, 66. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Zhu, N.; Zheng, X.; Liu, Y.; Lu, H.; Yin, X.; Hao, H.; Tan, Y.; Wang, D.; Hu, H.; et al. Intratumor Microbiome Analysis Identifies Positive Association Between Megasphaera and Survival of Chinese Patients With Pancreatic Ductal Adenocarcinomas. Front. Immunol. 2022, 13, 785422. [Google Scholar] [CrossRef]
- Roy, S.; Trinchieri, S.R.G. Microbiota: A key orchestrator of cancer therapy. Nat. Rev. Cancer 2017, 17, 271–285. [Google Scholar] [CrossRef]
- Garajová, I.; Balsano, R.; Wang, H.; Leonardi, F.; Giovannetti, E.; Deng, D.; Peters, G.J. The role of the microbiome in drug resistance in gastrointestinal cancers. Expert Rev. Anticancer. Ther. 2021, 21, 165–176. [Google Scholar] [CrossRef]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Baskar, R.; Dai, J.; Wenlong, N.; Yeo, R.; Yeoh, K.-W. Biological response of cancer cells to radiation treatment. Front. Mol. Biosci. 2014, 1, 24. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Zhang, Y.; Hong, W.; Wang, B.; Chen, Y.; Yang, P.; Zhou, J.; Fan, J.; Zeng, Z.; Du, S. Gut microbiota modulate radiotherapy-associated antitumor immune responses against hepatocellular carcinoma Via STING signaling. Gut Microbes 2022, 14, 2119055. [Google Scholar] [CrossRef]
- Shen, S.; Lim, G.; You, Z.; Ding, W.; Huang, P.; Ran, C.; Doheny, J.; Caravan, P.; Tate, S.; Hu, K.; et al. Gut microbiota is critical for the induction of chemotherapy-induced pain. Nat. Neurosci. 2017, 20, 1213–1216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, T.-S.; Xie, L.-W.; Cai, S.; Xu, J.-Y.; Zhou, H.; Tang, L.-F.; Yang, C.; Fang, S.; Li, M.; Tian, Y. Dysbiosis of Gut Microbiota Is Associated with the Progression of Radiation-Induced Intestinal Injury and Is Alleviated by Oral Compound Probiotics in Mouse Model. Front. Cell. Infect. Microbiol. 2021, 11, 717636. [Google Scholar] [CrossRef]
- Jian, Y.; Zhang, D.; Liu, M.; Wang, Y.; Xu, Z.-X. The Impact of Gut Microbiota on Radiation-Induced Enteritis. Front. Cell. Infect. Microbiol. 2021, 11, 586392. [Google Scholar] [CrossRef]
- Yuan, L.; Zhang, S.; Li, H.; Yang, F.; Mushtaq, N.; Ullah, S.; Shi, Y.; An, C.; Xu, J. The influence of gut microbiota dysbiosis to the efficacy of 5-Fluorouracil treatment on colorectal cancer. Biomed. Pharmacother. 2018, 108, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Alekseyenko, A.V.; Wu, J.; Peters, B.A.; Jacobs, E.J.; Gapstur, S.M.; Purdue, M.P.; Abnet, C.C.; Stolzenberg-Solomon, R.; Miller, G.; et al. Human oral microbiome and prospective risk for pancreatic cancer: A population-based nested case-control study. Gut 2018, 67, 120–127. [Google Scholar] [CrossRef] [Green Version]
- García-González, A.P.; Ritter, A.D.; Shrestha, S.; Andersen, E.C.; Yilmaz, L.S.; Walhout, A.J. Bacterial Metabolism Affects the C. elegans Response to Cancer Chemotherapeutics. Cell 2017, 169, 431–441.e8. [Google Scholar] [CrossRef] [Green Version]
- Peretz, A.; Ben Shlomo, I.; Nitzan, O.; Bonavina, L.; Schaffer, P.M.; Schaffer, M. Clostridium difficile Infection: Associations with Chemotherapy, Radiation Therapy, and Targeting Therapy Treatments. Curr. Med. Chem. 2016, 23, 4442–4449. [Google Scholar] [CrossRef]
- Hato, S.V.; Khong, A.; de Vries, I.J.M.; Lesterhuis, W.J. Molecular Pathways: The Immunogenic Effects of Platinum-Based Chemotherapeutics. Clin. Cancer Res. 2014, 20, 2831–2837. [Google Scholar] [CrossRef] [Green Version]
- Yeung, C.; Chiau, J.C.; Cheng, M.; Chan, W.; Chang, S.; Chang, Y.; Jiang, C.; Lee, H. Modulations of probiotics on gut microbiota in a 5-fluorouracil-induced mouse model of mucositis. J. Gastroenterol. Hepatol. 2020, 35, 806–814. [Google Scholar] [CrossRef]
- Kato, S.; Hamouda, N.; Kano, Y.; Oikawa, Y.; Tanaka, Y.; Matsumoto, K.; Amagase, K.; Shimakawa, M. Probiotic Bifidobacterium bifidum G9-1 attenuates 5-fluorouracil-induced intestinal mucositis in mice via suppression of dysbiosis-related secondary inflammatory responses. Clin. Exp. Pharmacol. Physiol. 2017, 44, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-W.; Lee, H.-C.; Li, L.-H.; Chiau, J.-S.C.; Wang, T.-E.; Chuang, W.-H.; Chen, M.-J.; Wang, H.-Y.; Shih, S.-C.; Liu, C.-Y.; et al. Fecal Microbiota Transplantation Prevents Intestinal Injury, Upregulation of Toll-Like Receptors, and 5-Fluorouracil/Oxaliplatin-Induced Toxicity in Colorectal Cancer. Int. J. Mol. Sci. 2020, 21, 386. [Google Scholar] [CrossRef] [Green Version]
- Chamseddine, A.N.; Ducreux, M.; Armand, J.-P.; Paoletti, X.; Satar, T.; Paci, A.; Mir, O. Intestinal bacterial β-glucuronidase as a possible predictive biomarker of irinotecan-induced diarrhea severity. Pharmacol. Ther. 2019, 199, 1–15. [Google Scholar] [CrossRef]
- Wallace, B.D.; Wang, H.; Lane, K.T.; Scott, J.E.; Orans, J.; Koo, J.S.; Venkatesh, M.; Jobin, C.; Yeh, L.-A.; Mani, S.; et al. Alleviating Cancer Drug Toxicity by Inhibiting a Bacterial Enzyme. Science 2010, 330, 831–835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, M.; Xiao, H.; Li, Y.; Zhou, L.; Zhao, S.; Luo, D.; Zheng, Q.; Dong, J.; Zhao, Y.; Zhang, X.; et al. Faecal microbiota transplantation protects against radiation-induced toxicity. EMBO Mol. Med. 2017, 9, 448–461. [Google Scholar] [CrossRef] [PubMed]
- Nomura, T.; Tsuchiya, Y.; Nashimoto, A.; Yabusaki, H.; Takii, Y.; Nakagawa, S.; Sato, N.; Kanbayashi, C.; Tanaka, O. Probi-otics reduce infectious complications after pancreaticoduodenectomy. Hepatogastroenterology 2007, 54, 661–663. [Google Scholar] [PubMed]
- Ijiri, M.; Fujiya, M.; Konishi, H.; Tanaka, H.; Ueno, N.; Kashima, S.; Moriichi, K.; Sasajima, J.; Ikuta, K.; Okumura, T. Ferri-chrome Identified from Lactobacillus Casei ATCC334 Induces Apoptosis through Its Iron-Binding Site in Gastric Cancer Cells. Tumor Biol. 2017, 39, 1010428317711311. [Google Scholar] [CrossRef] [Green Version]
- Konishi, H.; Fujiya, M.; Tanaka, H.; Ueno, N.; Moriichi, K.; Sasajima, J.; Ikuta, K.; Akutsu, H.; Tanabe, H.; Kohgo, Y. Probiotic-derived ferrichrome inhibits colon cancer progression via JNK-mediated apoptosis. Nat. Commun. 2016, 7, 12365. [Google Scholar] [CrossRef]
- Chaib, M.; Hafeez, B.B.; Mandil, H.; Daria, D.; Pingili, A.K.; Kumari, S.; Sikander, M.; Kashyap, V.K.; Chen, G.-Y.; Anning, E.; et al. Reprogramming of pancreatic adenocarcinoma immunosurveillance by a microbial probiotic siderophore. Commun. Biol. 2022, 5, 1181. [Google Scholar] [CrossRef]
- Chang, C.-J.; Lin, T.-L.; Tsai, Y.-L.; Wu, T.-R.; Lai, W.-F.; Lu, C.-C.; Lai, H.-C. Next generation probiotics in disease amelioration. J. Food Drug Anal. 2019, 27, 615–622. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.-N.; Zhang, L.; Dong, X.-Y.; Liu, M.; Cheng, G.; Zhang, X.-L.; He, F.; Wang, G.-Q. Effects of Akkermansia muciniphila on the Proliferation, Apoptosis and Insulin Secretion of Rat Islet Cell Tumor Cells. Sichuan Da Xue Xue Bao Yi Xue Ban 2020, 51, 13–17. [Google Scholar]
- Mohindroo, C.; Rogers, J.E.; Hasanov, M.; Mizrahi, J.; Overman, M.J.; Varadhachary, G.R.; Wolff, R.A.; Javle, M.M.; Fogelman, D.R.; Pant, S.; et al. A retrospective analysis of antibiotics usage and effect on overall survival and progressive free survival in patients with metastatic pancreatic cancer. J. Clin. Oncol. 2019, 37, e15781. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Min, J.-J. Salmonella-Mediated Cancer Therapy: Roles and Potential. Nucl. Med. Mol. Imaging 2017, 51, 118–126. [Google Scholar] [CrossRef]
- Live Biotherapeutic Product MRx0518 and Pembrolizumab Combination Study in Solid Tumors—Full Text View—Clinical-Trials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03637803 (accessed on 7 November 2022).
- Nivolumab (Anti-PD1), Tadalafil and Oral Vancomycin in People with Refractory Primary Hepatocellular Carcinoma or Liver Dominant Metastatic Cancer from Colorectal or Pancreatic Cancers—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03785210 (accessed on 9 November 2022).
- Amara, S.; Yang, L.V.; Tiriveedhi, V.; Muzaffar, M. Complex Role of Microbiome in Pancreatic Tumorigenesis: Potential Therapeutic Implications. Cells 2022, 11, 1900. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Picozzi, V.J.; Ko, A.H.; Wainberg, Z.A.; Kindler, H.; Wang-Gillam, A.; Oberstein, P.E.; Morse, M.A.; Zeh, H.J.; Weekes, C.D.; et al. Results from a Phase IIb, Randomized, Multicenter Study of GVAX Pancreas and CRS-207 Compared with Chemotherapy in Adults with Previously Treated Metastatic Pancreatic Adenocarcinoma (ECLIPSE Study). Clin. Cancer Res. 2019, 25, 5493–5502. [Google Scholar] [CrossRef] [PubMed]
- Frances, A.; Cordelier, P. The Emerging Role of Cytidine Deaminase in Human Diseases: A New Opportunity for Therapy? Mol. Ther. 2020, 28, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Wang-Gillam, A.; Picozzi, V.; Greten, T.F.; Crocenzi, T.; Springett, G.; Morse, M.; Zeh, H.; Cohen, D.; Fine, R.L.; et al. Safety and Survival with GVAX Pancreas Prime and Listeria Monocytogenes–Expressing Mesothelin (CRS-207) Boost Vaccines for Metastatic Pancreatic Cancer. J. Clin. Oncol. 2015, 33, 1325–1333. [Google Scholar] [CrossRef] [PubMed]
| Pathway Inhibitors | Targeting DNA Damage Response (DDR) | Targeting Immune System | Targeting Tumor Metabolism | Targeting Tumor Stroma Fibrosis, and Extracellular Matrix |
|---|---|---|---|---|
| KRAS | The Poly (ADP-ribose) polymerase (PARP) Inhibitor | Immune Checkpoint Inhibitors (PD-L1/PD-1, CTLA-4) | Targeting Tricarboxylic acid (TCA) enzymes | Targeting Hyaluronic Acid |
| Neurotrophic Tyrosine Receptor Kinase (NTRK) fusion | Vaccines | Targeting Immune Cells/Signals inside the stroma | ||
| Neuregulin-1 gene (NRG1) Fusion | CAR-t Cells Transfusion CD-40, IL-10 (failed to provide benefit) |
| Tumour-Associated Microbiota in Pancreatic Cancer | ||
|---|---|---|
| Increased Presence | Reduced Presence | |
| Phylum | Euryarchaeota | Firmicutes and Proteobacteria |
| Genus |
|
|
| Species | Fusobacterium nucleatum, Beutenbergia cavernae DSM 12333, Mycoplasma hypopneumoniae, Tolypothrix sp. PCC 7601, Acidovorax ebreuus TPSY, Agrobacterium radiobacter K84, Aggregatibacter aphorophilus NJ8700, Shigella sonnei Ss046, Salmonella enterica, Citrobacter freundii |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nista, E.C.; Del Gaudio, A.; Del Vecchio, L.E.; Mezza, T.; Pignataro, G.; Piccioni, A.; Gasbarrini, A.; Franceschi, F.; Candelli, M. Pancreatic Cancer Resistance to Treatment: The Role of Microbiota. Biomedicines 2023, 11, 157. https://doi.org/10.3390/biomedicines11010157
Nista EC, Del Gaudio A, Del Vecchio LE, Mezza T, Pignataro G, Piccioni A, Gasbarrini A, Franceschi F, Candelli M. Pancreatic Cancer Resistance to Treatment: The Role of Microbiota. Biomedicines. 2023; 11(1):157. https://doi.org/10.3390/biomedicines11010157
Chicago/Turabian StyleNista, Enrico Celestino, Angelo Del Gaudio, Livio Enrico Del Vecchio, Teresa Mezza, Giulia Pignataro, Andrea Piccioni, Antonio Gasbarrini, Francesco Franceschi, and Marcello Candelli. 2023. "Pancreatic Cancer Resistance to Treatment: The Role of Microbiota" Biomedicines 11, no. 1: 157. https://doi.org/10.3390/biomedicines11010157
APA StyleNista, E. C., Del Gaudio, A., Del Vecchio, L. E., Mezza, T., Pignataro, G., Piccioni, A., Gasbarrini, A., Franceschi, F., & Candelli, M. (2023). Pancreatic Cancer Resistance to Treatment: The Role of Microbiota. Biomedicines, 11(1), 157. https://doi.org/10.3390/biomedicines11010157










