Immuno-Modulatory Effects of Dexamethasone in Severe COVID-19—A Swedish Cohort Study
Abstract
1. Introduction
2. Methods
2.1. Patients
2.2. Exposure
2.3. Primary Outcome
2.4. Baseline Characteristics
2.5. Clinical Variables and Biochemistry
2.6. Cytokine Assay
2.7. COVID-19 Antibodies
2.8. Statistics
3. Results
3.1. Study Population
3.2. Organ Support and Crude Outcomes
3.3. Laboratory and Biochemical Parameters
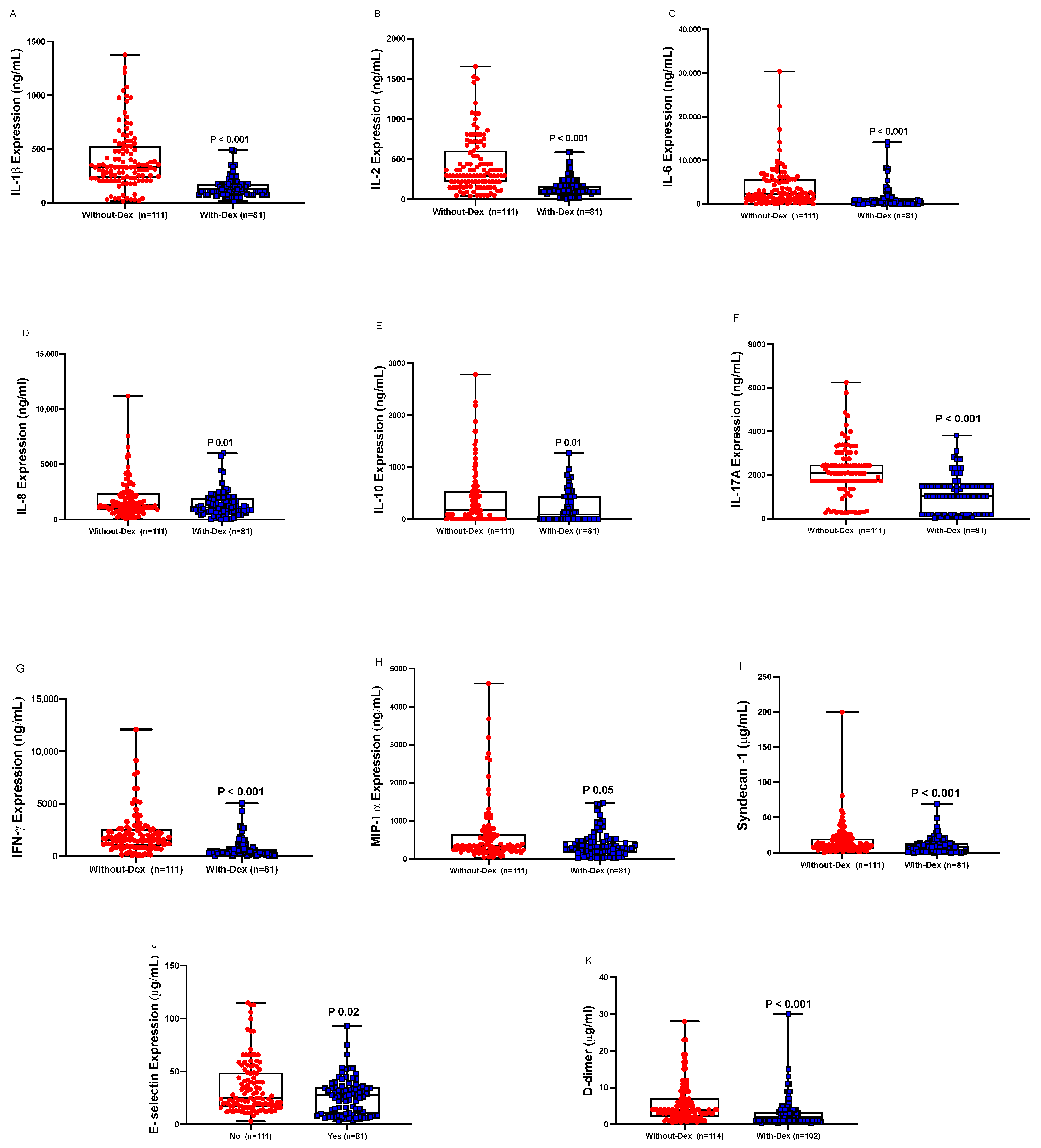
3.4. Cytokines and Endothelial Markers
3.5. COVID-19 Antibodies
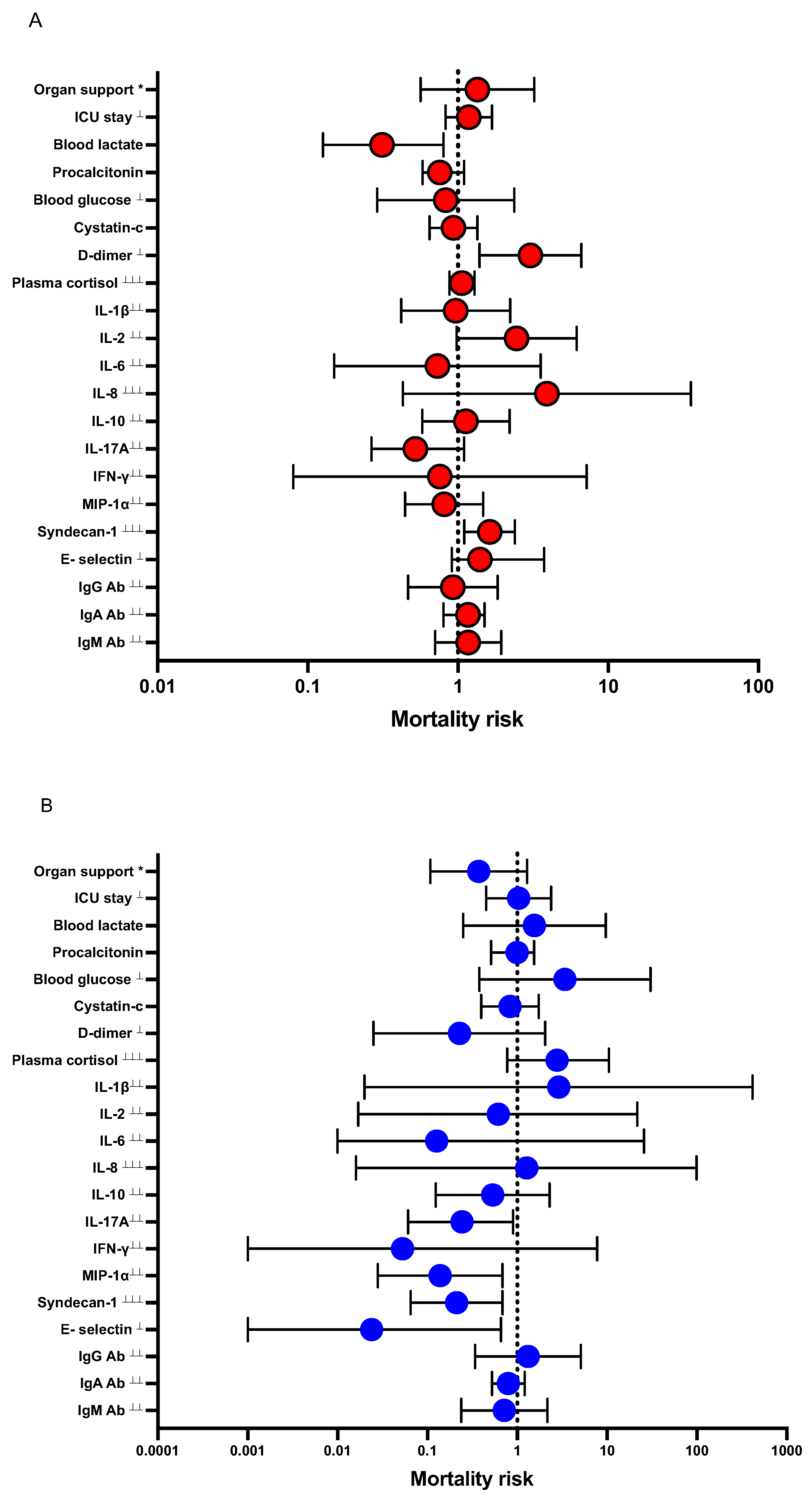
3.6. Logistic Regression Models
4. Discussion
4.1. Strengths and Limitations
4.2. Clinical Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmed, M.H.; Hassan, A. Dexamethasone for the Treatment of Coronavirus Disease (COVID-19): A Review. SN Compr. Clin. Med. 2020, 2, 2637–2646. [Google Scholar] [CrossRef]
- Giamarellos-Bourboulis, E.J.; Netea, M.G.; Rovina, N.; Akinosoglou, K.; Antoniadou, A.; Antonakos, N.; Damoraki, G.; Gkavogianni, T.; Adami, M.E.; Katsaounou, P.; et al. Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host Microb. 2020, 27, 992–1000.e3. [Google Scholar] [CrossRef]
- van de Veerdonk, F.L.; Giamarellos-Bourboulis, E.; Pickkers, P.; Derde, L.; Leavis, H.; van Crevel, R.; Engel, J.J.; Wiersinga, W.J.; Vlaar, A.P.J.; Shankar-Hari, M.; et al. A guide to immunotherapy for COVID-19. Nat. Med. 2022, 28, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Group, R.C.; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef]
- Martinez-Guerra, B.A.; Gonzalez-Lara, M.F.; Roman-Montes, C.M.; Tamez-Torres, K.M.; Dardon-Fierro, F.E.; Rajme-Lopez, S.; Medrano-Borromeo, C.; Martinez-Valenzuela, A.; Ortiz-Brizuela, E.; Sifuentes-Osornio, J.; et al. Outcomes of patients with severe and critical COVID-19 treated with dexamethasone: A prospective cohort study. Emerg. Microb. Infect. 2022, 11, 50–59. [Google Scholar] [CrossRef]
- Tomazini, B.M.; Maia, I.S.; Cavalcanti, A.B.; Berwanger, O.; Rosa, R.G.; Veiga, V.C.; Avezum, A.; Lopes, R.D.; Bueno, F.R.; Silva, M.; et al. Effect of Dexamethasone on Days Alive and Ventilator-Free in Patients With Moderate or Severe Acute Respiratory Distress Syndrome and COVID-19: The CoDEX Randomized Clinical Trial. JAMA 2020, 324, 1307–1316. [Google Scholar] [CrossRef]
- Angus, D.C.; Derde, L.; Al-Beidh, F.; Annane, D.; Arabi, Y.; Beane, A.; van Bentum-Puijk, W.; Berry, L.; Bhimani, Z.; Bonten, M.; et al. Effect of Hydrocortisone on Mortality and Organ Support in Patients With Severe COVID-19: The REMAP-CAP COVID-19 Corticosteroid Domain Randomized Clinical Trial. JAMA 2020, 324, 1317–1329. [Google Scholar] [CrossRef]
- Johns, M.; George, S.; Taburyanskaya, M.; Poon, Y.K. A Review of the Evidence for Corticosteroids in COVID-19. J. Pharm. Pract. 2022, 35, 626–637. [Google Scholar] [CrossRef]
- Wang, J.; Yang, W.; Chen, P.; Guo, J.; Liu, R.; Wen, P.; Li, K.; Lu, Y.; Ma, T.; Li, X.; et al. The proportion and effect of corticosteroid therapy in patients with COVID-19 infection: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0249481. [Google Scholar] [CrossRef]
- Kocks, J.; Kerkhof, M.; Scherpenisse, J.; van de Maat, A.; van Geer-Postmus, I.; le Rutte, T.; Schaart, J.; Gans, R.O.B.; Kerstjens, H.A.M. A potential harmful effect of dexamethasone in non-severe COVID-19: Results from the COPPER-pilot study. ERJ Open Res. 2022, 8, 23120541. [Google Scholar] [CrossRef]
- Kino, T.; Burd, I.; Segars, J.H. Dexamethasone for Severe COVID-19: How Does It Work at Cellular and Molecular Levels? Int. J. Mol. Sci. 2021, 22, 6764. [Google Scholar] [CrossRef]
- Strehl, C.; Ehlers, L.; Gaber, T.; Buttgereit, F. Glucocorticoids-All-Rounders Tackling the Versatile Players of the Immune System. Front. Immunol. 2019, 10, 1744. [Google Scholar] [CrossRef]
- Bernard, I.; Limonta, D.; Mahal, L.K.; Hobman, T.C. Endothelium Infection and Dysregulation by SARS-CoV-2: Evidence and Caveats in COVID-19. Viruses 2020, 13, 29. [Google Scholar] [CrossRef]
- Perlman, S. COVID-19 poses a riddle for the immune system. Nature 2020, 584, 345–346. [Google Scholar] [CrossRef]
- Wyler, E.; Adler, J.M.; Eschke, K.; Teixeira Alves, G.; Peidli, S.; Pott, F.; Kazmierski, J.; Michalick, L.; Kershaw, O.; Bushe, J.; et al. Key benefits of dexamethasone and antibody treatment in COVID-19 hamster models revealed by single-cell transcriptomics. Mol. Ther. 2022, 30, 1952–1965. [Google Scholar] [CrossRef]
- Higashikuni, Y.; Liu, W.; Obana, T.; Sata, M. Pathogenic Basis of Thromboinflammation and Endothelial Injury in COVID-19: Current Findings and Therapeutic Implications. Int. J. Mol. Sci. 2021, 22, 12081. [Google Scholar] [CrossRef]
- Kim, W.Y.; Kweon, O.J.; Cha, M.J.; Baek, M.S.; Choi, S.H. Dexamethasone may improve severe COVID-19 via ameliorating endothelial injury and inflammation: A preliminary pilot study. PLoS ONE 2021, 16, e0254167. [Google Scholar] [CrossRef]
- Jensterle, M.; Herman, R.; Janez, A.; Mahmeed, W.A.; Al-Rasadi, K.; Al-Alawi, K.; Banach, M.; Banerjee, Y.; Ceriello, A.; Cesur, M.; et al. The Relationship between COVID-19 and Hypothalamic-Pituitary-Adrenal Axis: A Large Spectrum from Glucocorticoid Insufficiency to Excess-The CAPISCO International Expert Panel. Int. J. Mol. Sci. 2022, 23, 7326. [Google Scholar] [CrossRef]
- Song, P.; Xu, X.; Zhao, Y.; Gu, M.; Chen, X.; Zhang, H.; Wu, X.; Yu, C.; Niu, J.; Ding, W.; et al. Different stages of chronic kidney disease are associated with physical performance in adults over 60 years. Front. Publ. Health 2022, 10, 963913. [Google Scholar] [CrossRef]
- Metnitz, P.G.; Moreno, R.P.; Almeida, E.; Jordan, B.; Bauer, P.; Campos, R.A.; Iapichino, G.; Edbrooke, D.; Capuzzo, M.; Le Gall, J.R.; et al. SAPS 3--From evaluation of the patient to evaluation of the intensive care unit. Part 1: Objectives, methods and cohort description. Intens. Care Med. 2005, 31, 1336–1344. [Google Scholar] [CrossRef]
- Force, A.D.T.; Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar] [CrossRef]
- Zhang, D.; Li, L.; Chen, Y.; Ma, J.; Yang, Y.; Aodeng, S.; Cui, Q.; Wen, K.; Xiao, M.; Xie, J.; et al. Syndecan-1, an indicator of endothelial glycocalyx degradation, predicts outcome of patients admitted to an ICU with COVID-19. Mol. Med. 2021, 27, 151. [Google Scholar] [CrossRef]
- Patterson, E.K.; Cepinskas, G.; Fraser, D.D. Endothelial Glycocalyx Degradation in Critical Illness and Injury. Front. Med. 2022, 9, 898592. [Google Scholar] [CrossRef]
- Astapenko, D.; Tomasova, A.; Ticha, A.; Hyspler, R.; Chua, H.S.; Manzoor, M.; Skulec, R.; Lehmann, C.; Hahn, R.G.; Malbrain, M.L.; et al. Endothelial glycocalyx damage in patients with severe COVID-19 on mechanical ventilation - A prospective observational pilot study. Clin. Hemorheol. Microcirc. 2022, 81, 205–219. [Google Scholar] [CrossRef]
- Zha, D.; Fu, M.; Qian, Y. Vascular Endothelial Glycocalyx Damage and Potential Targeted Therapy in COVID-19. Cells 2022, 11, 1972. [Google Scholar] [CrossRef]
- Codina, H.; Vieitez, I.; Gutierrez-Valencia, A.; Skouridou, V.; Martinez, C.; Patino, L.; Botero-Gallego, M.; Trujillo-Rodriguez, M.; Serna-Gallego, A.; Munoz-Muela, E.; et al. Elevated Anti-SARS-CoV-2 Antibodies and IL-6, IL-8, MIP-1beta, Early Predictors of Severe COVID-19. Microorganisms 2021, 9, 2259. [Google Scholar] [CrossRef]
- Fadlallah, S.; Sham Eddin, M.S.; Rahal, E.A. IL-17A in COVID-19 Cases: A meta-analysis. J. Infect. Dev. Ctries 2021, 15, 1630–1639. [Google Scholar] [CrossRef]
- Raucci, F.; Mansour, A.A.; Casillo, G.M.; Saviano, A.; Caso, F.; Scarpa, R.; Mascolo, N.; Iqbal, A.J.; Maione, F. Interleukin-17A (IL-17A), a key molecule of innate and adaptive immunity, and its potential involvement in COVID-19-related thrombotic and vascular mechanisms. Autoimmun. Rev. 2020, 19, 102572. [Google Scholar] [CrossRef]
- Pallikkuth, S.; Micci, L.; Ende, Z.S.; Iriele, R.I.; Cervasi, B.; Lawson, B.; McGary, C.S.; Rogers, K.A.; Else, J.G.; Silvestri, G.; et al. Maintenance of intestinal Th17 cells and reduced microbial translocation in SIV-infected rhesus macaques treated with interleukin (IL)-21. PLoS Pathog. 2013, 9, e1003471. [Google Scholar] [CrossRef]
- Schramm, R.; Liu, Q.; Thorlacius, H. Expression and function of MIP-2 are reduced by dexamethasone treatment in vivo. Br. J. Pharmacol. 2000, 131, 328–334. [Google Scholar] [CrossRef]
- Franchimont, D. Overview of the actions of glucocorticoids on the immune response: A good model to characterize new pathways of immunosuppression for new treatment strategies. Ann. N. Y. Acad. Sci. 2004, 1024, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Muhlemann, B.; Thibeault, C.; Hillus, D.; Helbig, E.T.; Lippert, L.J.; Tober-Lau, P.; Schwarz, T.; Muller, M.A. Impact of dexamethasone on SARS-CoV-2 concentration kinetics and antibody response in hospitalized COVID-19 patients: Results from a prospective observational study. Clin. Microbiol. Infect. 2021, 27, 1520.e7–1520.e10. [Google Scholar] [CrossRef] [PubMed]
- Masia, M.; Fernandez-Gonzalez, M.; Garcia, J.A.; Padilla, S.; Gutierrez, F. Lack of detrimental effect of corticosteroids on antibody responses to SARS-CoV-2 and viral clearance in patients hospitalized with COVID-19. J. Infect. 2021, 82, 414–451. [Google Scholar] [CrossRef] [PubMed]
- Swiatkowska-Stodulska, R.; Berlinska, A.; Puchalska-Reglinska, E. Cortisol as an Independent Predictor of Unfavorable Outcomes in Hospitalized COVID-19 Patients. Biomedicines 2022, 10, 1527. [Google Scholar] [CrossRef] [PubMed]
- Falcinelli, E.; Petito, E.; Gresele, P. The role of platelets, neutrophils and endothelium in COVID-19 infection. Exp. Rev. Hematol. 2022, 10, 19. [Google Scholar] [CrossRef]
- Six, I.; Guillaume, N.; Jacob, V.; Mentaverri, R.; Kamel, S.; Boullier, A.; Slama, M. The Endothelium and COVID-19: An Increasingly Clear Link Brief Title: Endotheliopathy in COVID-19. Int. J. Mol. Sci. 2022, 23, 6196. [Google Scholar] [CrossRef]
- Lee, H.W.; Park, J.; Lee, J.K.; Park, T.Y.; Heo, E.Y. The Effect of the Timing of Dexamethasone Administration in Patients with COVID-19 Pneumonia. Tuberc. Respir. Dis. 2021, 84, 217–225. [Google Scholar] [CrossRef]
- Rothe, K.; Lahmer, T.; Rasch, S.; Schneider, J.; Spinner, C.D.; Wallnofer, F.; Wurst, M.; Schmid, R.M.; Waschulzik, B.; Fuest, K.; et al. Dexamethasone therapy and rates of secondary pulmonary and bloodstream infections in critically ill COVID-19 patients. Multidiscip. Respir. Med. 2021, 16, 793. [Google Scholar] [CrossRef]
- Brooks, D.; Schulman-Rosenbaum, R.; Griff, M.; Lester, J.; Low Wang, C.C. Glucocorticoid-Induced Hyperglycemia Including Dexamethasone-Associated Hyperglycemia in COVID-19 Infection: A Systematic Review. Endocr. Pract. 2022, 7, 14. [Google Scholar] [CrossRef]
- Zhai, J.L.; Ge, N.; Zhen, Y.; Zhao, Q.; Liu, C. Corticosteroids Significantly Increase Serum Cystatin C Concentration without Affecting Renal Function in Symptomatic Heart Failure. Clin. Lab. 2016, 62, 203–207. [Google Scholar] [CrossRef]
- Larsson, A.; Campbell, A.; Eriksson, M. Chicken antibodies are highly suitable for particle enhanced turbidimetric assays. Front. Immunol. 2022, 13, 1016781. [Google Scholar] [CrossRef] [PubMed]
- Ritter, L.A.; Britton, N.; Heil, E.L.; Teeter, W.A.; Murthi, S.B.; Chow, J.H.; Ricotta, E.; Chertow, D.S.; Grazioli, A.; Levine, A.R. The Impact of Corticosteroids on Secondary Infection and Mortality in Critically Ill COVID-19 Patients. J. Intens. Care Med. 2021, 36, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Reyes, L.F.; Rodriguez, A.; Bastidas, A.; Parra-Tanoux, D.; Fuentes, Y.V.; Garcia-Gallo, E.; Moreno, G.; Ospina-Tascon, G.; Hernandez, G.; Silva, E.; et al. Dexamethasone as risk-factor for ICU-acquired respiratory tract infections in severe COVID-19. J. Crit. Care 2022, 69, 154014. [Google Scholar] [CrossRef] [PubMed]
- Ahlstrom, B.; Frithiof, R.; Hultstrom, M.; Larsson, I.M.; Strandberg, G.; Lipcsey, M. The swedish COVID-19 intensive care cohort: Risk factors of ICU admission and ICU mortality. Acta Anaesthesiol. Scand. 2021, 12, 13781. [Google Scholar] [CrossRef] [PubMed]
| Without-Dex n = 114 (52%) | With-Dex n = 102 (47%) | p Value e | ||
|---|---|---|---|---|
| Cohort characteristics | ||||
| Age (years) | 62 ± 15 | 66 ± 12 | 0.09 | |
| BMI a (kg/m2) | 29 ± 5.8 | 30 ± 7.1 | 0.47 | |
| Sex | Male | 88 (77) | 72 (71) | 0.38 |
| COVID-19 day b on ICU c admission | 11 ± 4.4 | 12 ± 4.1 | 0.10 | |
| SAPS3 d | 53 ± 10 | 55 ± 8 | 0.43 | |
| Comorbidities | Hypertension | 61 (53) | 77 (76) | 0.001 |
| Cardiovascular disease | 5 (4) | 17 (17) | 0.003 | |
| Pulmonary disease | 30 (26) | 20 (20) | 0.24 | |
| Diabetes mellitus | 32 (28) | 35 (34) | 0.32 | |
| Chronic kidney disease (stage 3–5) | 40 (35) | 42 (42) | 0.30 | |
| Without-Dex (n = 114) | With-Dex (n = 102) | p Value h | |
|---|---|---|---|
| Disease severity, n (%) | |||
| Vasopressors | 64 (56) | 35 (34) | 0.001 |
| CRRT a | 15 (13) | 5 (5) | 0.04 |
| Mechanical ventilation | 65 (57) | 43 (42) | 0.04 |
| Hospital-acquired infections | 56 (55) | 50 (44) | 0.10 |
| Length of ICU stay (days) | 11 ± 10 | 8.7 ± 7.0 | 0.01 |
| Mortality (30 days) b | 30 (26) | 21 (21) | 0.32 |
| Laboratory parameters on admission (mean ± SD) | |||
| PaO2/FiO2 ratio c | 20 ± 8.0 | 20 ± 19 | 0.61 |
| Blood lactate (mmol/L) | 1.5 ± 1.1 | 1.2 ± 0.5 | 0.01 |
| hsCRP d (mg/L) | 172 ± 82 | 154 ± 82 | 0.08 |
| Pro-calcitonin (ng/mL) | 1.3 ± 1.7 | 2.2 ± 1.8 | 0.001 |
| Blood sodium (mmol/L) | 136 ± 4.3 | 137 ± 3.2 | 0.13 |
| Blood potassium (mmol/L) | 3.8 ± 0.4 | 3.8 ± 0.5 | 0.39 |
| Blood glucose (mmol/L) | 9.0 ± 3.3 | 10 ± 3.2 | 0.004 |
| Blood creatinine (mg/dL) | 100 ± 98 | 108 ± 125 | 0.60 |
| Laboratory parameters measured on seven days after ICU admission median (interquartile range) | |||
| Cystatin-C e (mg/L) | 1.1 (0.6–5.2) | 1.2 (0.6–6.1) | 0.004 |
| Plasma cortisol f (ng/mL) | 6.9 (0.05–189) | 1.6 (0.01–23) | 0.005 |
| Plasma ferritin (µg/L) | 2255 (107–36,018) | 1572 (120–3866) | 0.94 |
| Fibrinogen D-dimer (µg/mL) | 4.0 (0.5–23) | 1.4 (0.3–30) | 0.001 |
| COVID-19 antibodies g (n = 190) | |||
| IgG (mg/L) | 3.5 (0.007–23) | 1.9 (0.001–28) | 0.07 |
| IgA (mg/L) | 0.45 (0.003–24) | 0.33 (0.001–7) | 0.13 |
| IgM (mg/L) | 3.4 (0.006–47) | 1.3 (0.00–25) | 0.001 |
| (A) All Patients (n = 216) Admitted to Intensive Care (With-Dex, n = 110 vs. Without-Dex, n = 114), and Mortality at 30 Days. | ||
| Crude | Adjusted c | |
| OR (95% CI) | OR (95% CI) | |
| With-Dex | 0.7 (0.4–1.4) | 0.7 (0.3–1.3) |
| Comorbidities a | 0.5 (0.3–1.1) | |
| SAPS3 b | 1.1 (0.9–1.1) | |
| (B) Sub-analysis among COVID-19 patients With-Dex (Early-Dex received Dex treatment within < 14 days of COVID-19 and Late-Dex received ≥ 14 days) vs. Without-Dex and mortality at 30 days. | ||
| Early-Dex (n = 83) | Adjusted b | |
| With-Dex | 0.4 (0.2–0.8) | |
| Comorbidities a | 0.6 (0.2–1.3) | |
| SAPS | 1.1 (0.9–1.1) | |
| Late-Dex (n = 19) | Adjusted c | |
| With-Dex | 2.2 (0.4–12) | |
| Comorbidities | 0.4 (0.1–2.5) | |
| SAPS | 1.1 (0.9–1.2) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asif, S.; Frithiof, R.; Larsson, A.; Franzén, S.; Anderberg, S.B.; Kristensen, B.; Hultström, M.; Lipcsey, M. Immuno-Modulatory Effects of Dexamethasone in Severe COVID-19—A Swedish Cohort Study. Biomedicines 2023, 11, 164. https://doi.org/10.3390/biomedicines11010164
Asif S, Frithiof R, Larsson A, Franzén S, Anderberg SB, Kristensen B, Hultström M, Lipcsey M. Immuno-Modulatory Effects of Dexamethasone in Severe COVID-19—A Swedish Cohort Study. Biomedicines. 2023; 11(1):164. https://doi.org/10.3390/biomedicines11010164
Chicago/Turabian StyleAsif, Sana, Robert Frithiof, Anders Larsson, Stephanie Franzén, Sara Bülow Anderberg, Bjarne Kristensen, Michael Hultström, and Miklos Lipcsey. 2023. "Immuno-Modulatory Effects of Dexamethasone in Severe COVID-19—A Swedish Cohort Study" Biomedicines 11, no. 1: 164. https://doi.org/10.3390/biomedicines11010164
APA StyleAsif, S., Frithiof, R., Larsson, A., Franzén, S., Anderberg, S. B., Kristensen, B., Hultström, M., & Lipcsey, M. (2023). Immuno-Modulatory Effects of Dexamethasone in Severe COVID-19—A Swedish Cohort Study. Biomedicines, 11(1), 164. https://doi.org/10.3390/biomedicines11010164







