Converging Evidence of Similar Symptomatology of ME/CFS and PASC Indicating Multisystemic Dyshomeostasis
Abstract
:1. Introduction
2. ME/CFS
- Pain—very common, but highly variable in presence, nature, and severity
- Certain infections may act as triggers
- Gastrointestinal and genitourinary problems
- Sore throat or scratchy throat
- Painful or tender axillary/cervical lymph nodes
- Sensitivity to external stimuli
- ‘Flu-like fatigue/exhaustion’
- ‘I feel like a battery that is never able to be recharged fully despite resting a lot and limiting my activities to only the bare essentials needed to get by’
- ‘Thinking takes a lot more work than it used to’
- ‘My arms, legs, body feel heavy and harder to move’
- Severe limitations in personal and household management
- Loss of job, medical insurance, and career
- Being predominantly housebound
- Decreased social interaction and increased isolation
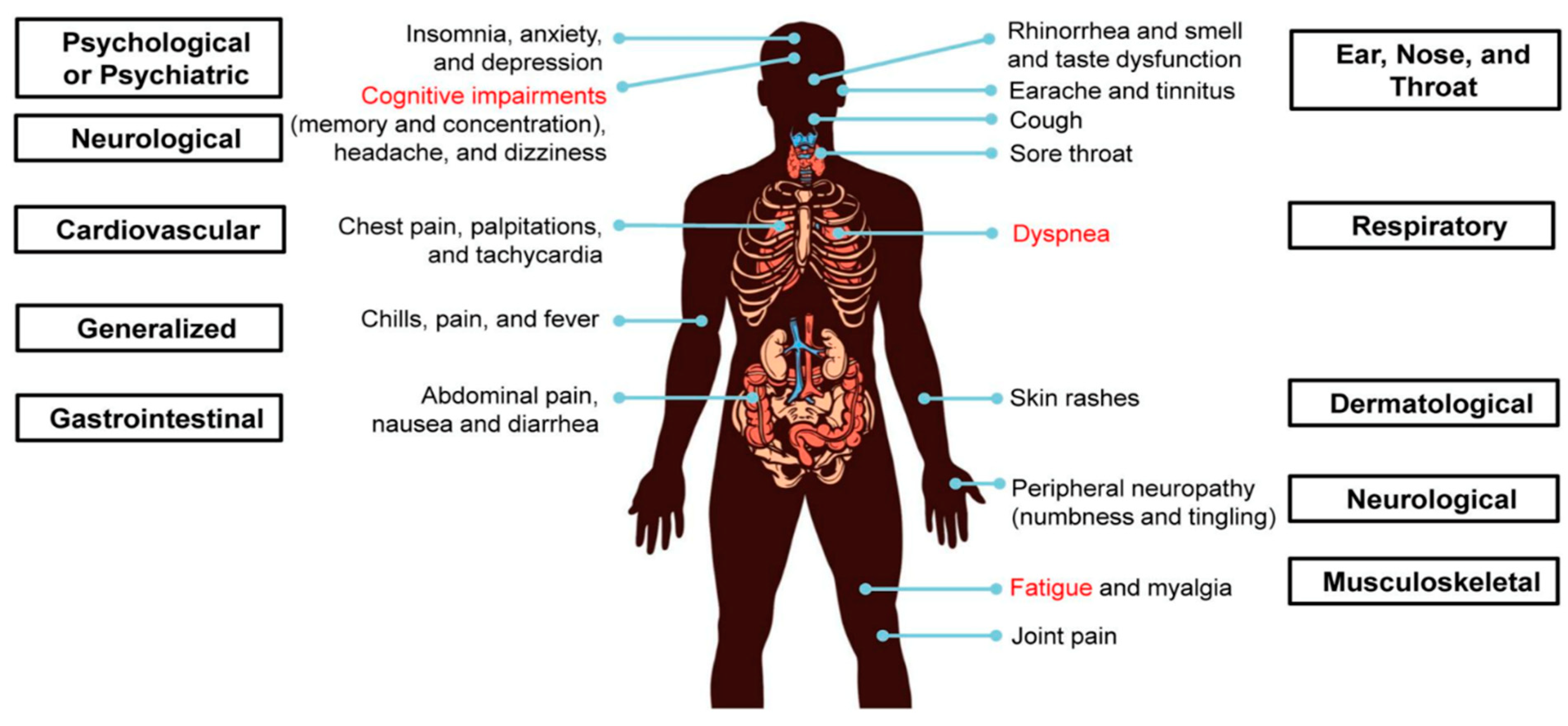
3. PASC
4. Controlled Study of PASC and ME/CFS Symptomatology
5. Multisystemic Dyshomeostasis, ME/CFS and PASC
6. Central Homeostasis Network
7. Examples of Dyshomeostasis in ME/CFS and PASC
“Following activation of a systemic immune/inflammatory response to an infection or severe stress event, abnormal transport of signals or molecules into the CNS occurs through neurovascular pathways or a disrupted BBB. If the initial stressor is not resolved, this leads to fluctuating chronic neuroinflammation that sustains and controls the complex neurological symptoms of ME/CFS and long-COVID and facilitates frequent, more serious relapses in response to life stress, as evidenced from a comprehensive disruption to the cellular molecular biology and body’s physiological pathways.”
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Komaroff, A.L.; Lipkin, W.I. Insights from myalgic encephalomyelitis/chronic fatigue syndrome may help unravel the pathogenesis of postacute COVID-19 syndrome. Trends Mol. Med. 2021, 27, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Kedor, C.; Freitag, H.; Meyer-Arndt, L.; Wittke, K.; Zoller, T.; Steinbeis, F.; Haffke, M.; Rudolf, G.; Heidecker, B.; Volk, H.D.; et al. Chronic COVID-19 Syndrome and Chronic Fatigue Syndrome (ME/CFS) following the first pandemic wave in Germany–a first analysis of a prospective observational study. medRxiv 2021. [Google Scholar] [CrossRef]
- Mantovani, E.; Mariotto, S.; Gabbiani, D.; Dorelli, G.; Bozzetti, S.; Federico, A.; Zanzoni, S.; Girelli, D.; Crisafulli, E.; Ferrari, S.; et al. Chronic fatigue syndrome: An emerging sequela in COVID-19 survivors? J. NeuroVirol. 2021, 27, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Sukocheva, O.A.; Maksoud, R.; Beeraka, N.M.; Madhunapantula, S.V.; Sinelnikov, M.; Nikolenko, V.N.; Neganova, M.E.; Klochkov, S.G.; Kamal, M.A.; Staines, D.R.; et al. Analysis of post COVID-19 condition and its overlap with myalgic encephalomyelitis/chronic fatigue syndrome. J. Adv. Res. 2021, 40, 179–196. [Google Scholar] [CrossRef]
- Tate, W.; Walker, M.; Sweetman, E.; Helliwell, A.; Peppercorn, K.; Edgar, C.; Blair, A.; Chatterjee, A. Molecular Mechanisms of Neuroinflammation in ME/CFS and Long COVID to Sustain Disease and Promote Relapses. Front. Neurol. 2022, 13, 877772. [Google Scholar] [CrossRef]
- Tziastoudi, M.; Cholevas, C.; Stefanidis, I.; Theoharides, T.C. Genetics of COVID-19 and myalgic encephalomyelitis/chronic fatigue syndrome: A systematic review. Ann. Clin. Transl. Neurol. 2022, 9, 1838–1857. [Google Scholar] [CrossRef]
- Lim, E.J.; Ahn, Y.C.; Jang, E.S.; Lee, S.W.; Lee, S.H.; Son, C.G. Systematic review and meta-analysis of the prevalence of chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME). J. Transl. Med. 2020, 18, 100. [Google Scholar] [CrossRef] [PubMed]
- Jason, L.A.; Mirin, A.A. Updating the National Academy of Medicine ME/CFS prevalence and economic impact figures to account for population growth and inflation. Fatigue Biomed. Health Behav. 2021, 9, 9–13. [Google Scholar] [CrossRef]
- Chen, C.; Haupert, S.R.; Zimmermann, L.; Shi, X.; Fritsche, L.G.; Mukherjee, B. Global Prevalence of Post COVID-19 Condition or Post-COVID-19 syndrome: A Meta-Analysis and Systematic Review. J. Infect. Dis. 2022, 226, 1593–1607. [Google Scholar] [CrossRef]
- Institute of Medicine Beyond. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness; The National Academies Press: Washington, DC, USA, 2015. [Google Scholar] [CrossRef]
- Carruthers, B.M.; van de Sande, M.I. Myalgic encephalomyelitis/chronic fatigue syndrome. A clinical case definition and guidelines for medical practitioners. In An Overview of the Canadian Consensus Document; The National Library of Canada: Ottawa, ON, Canada, 2005. [Google Scholar]
- Wessely, S.; David, A.; Butler, S.; Chalder, T. Management of chronic (post-viral) fatigue syndrome. J. R. Coll. Gen. Pract. 1989, 39, 26–29. [Google Scholar]
- Marks, D.F. The rise and fall of the psychosomatic approach to Medically Unexplained Symptoms, Myalgic Encaphalomyelitis and Chronic Fatigue Syndrome. Arch. Epidemiol. Public Health Res. 2022, 1, 97–144. [Google Scholar]
- Yong, S.J.; Liu, S. Proposed subtypes of post-COVID-19 syndrome (or long-COVID) and their respective potential therapies. Rev. Med. Virol. 2022, 32, e2315. [Google Scholar] [CrossRef] [PubMed]
- Jason, L.A.; Islam, M.F.; Conroy, K.; Cotler, J.; Torres, C.; Johnson, M.; Mabie, B. COVID-19 symptoms over time: Comparing long-haulers to ME/CFS. Fatigue Biomed. Health Behav. 2021, 9, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Friedman, K.J.; Bateman, L.; Bested, A.; Nahle, Z. Advances in ME/CFS Research and Clinical Care. Front. Pediatr. 2019, 7, 370. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.; Anderson, G.; Galecki, P.; Berk, M.; Maes, M. A narrative review on the similarities and dissimilarities between myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and sickness behavior. BMC Med. 2013, 11, 64. [Google Scholar] [CrossRef] [Green Version]
- Morris, G.; Maes, M. Mitochondrial dysfunctions in myalgic encephalomyelitis/chronic fatigue syndrome explained by activated immuno-inflammatory, oxidative and nitrosative stress pathways. Metab. Brain Dis. 2014, 29, 19–36. [Google Scholar] [CrossRef]
- Schei, T.; Angelsen, A. The Course of the Illness for ME Patients in Norway: What Are the Typical Courses of the Illness, and What Worsen or Improve Them? Norwegian ME Association. 2021. Available online: https://www.me-foreningen.no/wp-content/uploads/2021/03/Norwegian-ME-Association-2021-Report-on-the-course-of-illness-English-summary.pdf (accessed on 10 January 2022.).
- Rodebaugh, T.L.; Frumkin, M.R.; Reiersen, A.M.; Lenze, E.J.; Avidan, M.S.; Miller, J.P.; Piccirillo, J.F.; Zorumski, C.F.; Mattar, C. Acute symptoms of mild to moderate COVID-19 are highly heterogeneous across individuals and over time. In Open Forum Infectious Diseases; Oxford University Press: Oxford, UK, 2021; Volume 8, p. ofab090. [Google Scholar]
- Laracy, J.C.; Kamboj, M.; Vardhana, S.A. Long and persistent COVID-19 in patients with hematologic malignancies: From bench to bedside. Curr. Opin. Infect. Dis. 2022, 35, 271–279. [Google Scholar] [CrossRef]
- Kim, Y.; Kwon, O.; Paek, J.H.; Park, W.Y.; Jin, K.; Hyun, M.; Lee, J.Y.; Kim, H.A.; Han, S. Two distinct cases with COVID-19 in kidney transplant recipients. Am. J. Transp. 2020, 20, 2269–2275. [Google Scholar] [CrossRef]
- Spector, N.H.; Korneva, E.A. Neurophysiology, Immunophysiology, and Neuroimmunomodulation; Academic Press: New York, NY, USA, 1981; Volume 449. [Google Scholar]
- Arroll, M.A. Allostatic overload in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Med. Hypotheses 2013, 81, 506–508. [Google Scholar] [CrossRef]
- Hatziagelaki, E.; Adamaki, M.; Tsilioni, I.; Dimitriadis, G.; Theoharides, T.C. Myalgic encephalomyelitis/chronic fatigue syndrome—Metabolic disease or disturbed homeostasis due to focal inflammation in the hypothalamus? J. Pharmacol. Exp. Ther. 2018, 367, 155–167. [Google Scholar] [CrossRef] [Green Version]
- Nacul, L.; O’Boyle, S.; Palla, L.; Nacul, F.E.; Mudie, K.; Kingdon, C.C.; Cliff, J.M.; Clark, T.G.; Dockrell, H.M.; Lacerda, E.M. How myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) progresses: The natural history of ME/CFS. Front. Neurol. 2020, 11, 826. [Google Scholar] [CrossRef]
- Kotas, M.E.; Medzhitov, R. Homeostasis, inflammation, and disease susceptibility. Cell 2015, 160, 816–827. [Google Scholar] [CrossRef] [Green Version]
- Tomas, C.; Newton, J.; Watson, S. A review of hypothalamic–pituitary–adrenal axis function in chronic fatigue syndrome. ISRN Neurosci. 2013, 2013, 784520. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Yang, Y.; Wang, D.; Li, C.; Qu, Y.; Guo, J.; Shi, T.; Bo, W.; Sun, Z.; Asakawa, T. The clinical value of cytokines in chronic fatigue syndrome. J. Transl. Med. 2019, 17, 1–12. [Google Scholar] [CrossRef]
- Marks, D.F. Homeostatic theory of obesity. Health Psychol. Open 2015, 2, 2055102915590692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, G.; Maes, M.; Berk, M.; Puri, B.K. Myalgic encephalomyelitis or chronic fatigue syndrome: How could the illness develop? Metab. Brain Dis. 2019, 34, 385–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldstein, D.S. The extended autonomic system, dyshomeostasis, and COVID-19. Clin. Auton. Res. 2020, 30, 299–315. [Google Scholar] [CrossRef]
- Edlow, B.L.; McNab, J.A.; Witzel, T.; Kinney, H.C. The structural connectome of the human central homeostatic network. Brain Connect. 2016, 6, 187–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glassford, J.A. The neuroinflammatory etiopathology of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Front. Physiol. 2017, 8, 88. [Google Scholar] [CrossRef] [Green Version]
- Mackay, A.; Tate, W.P. A compromised paraventricular nucleus within a dysfunctional hypothalamus: A novel neuroinflammatory paradigm for ME/CFS. Int. J. Immunopathol. Pharmacol. 2018, 32, 2058738418812342. [Google Scholar] [CrossRef] [Green Version]
- Xanthos, D.N.; Sandkühler, J. Neurogenic neuroinflammation: Inflammatory CNS reactions in response to neuronal activity. Nat. Rev. Neurosci. 2014, 15, 43–53. [Google Scholar] [CrossRef] [PubMed]
- VanElzakker, M.B.; Brumfield, S.A.; Lara Mejia, P.S. Neuroinflammation and cytokines in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): A critical review of research methods. Front. Neurol. 2019, 9, 1033. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, P.C.; Liu, K.K.; Lin, A.; Bartsch, R.P. Network physiology: From neural plasticity to organ network interactions. In Emergent Complexity from Nonlinearity, in Physics, Engineering and the Life Sciences; Springer: Cham, Switzerland, 2017; pp. 145–165. [Google Scholar]
- McCarthy, M.J. Circadian rhythm disruption in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Implications for the post-acute sequelae of COVID-19. Brain Behav. Immun. Health 2022, 20, 100412. [Google Scholar] [CrossRef]
- Gay, C.W.; Robinson, M.E.; Lai, S.; O’Shea, A.; Craggs, J.G.; Price, D.D.; Staud, R. Abnormal resting-state functional connectivity in patients with chronic fatigue syndrome: Results of seed and data-driven analyses. Brain Connect. 2016, 6, 48–56. [Google Scholar] [CrossRef] [Green Version]
- Boissoneault, J.; Letzen, J.; Lai, S.; O’Shea, A.; Craggs, J.; Robinson, M.E.; Staud, R. Abnormal resting state functional connectivity in patients with chronic fatigue syndrome: An arterial spin-labeling fMRI study. Magn. Reason. Imaging 2016, 34, 603–608. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Loggia, M.L.; Cahalan, C.M.; Harris, R.E.; Beissner, F.; Garcia, R.G.; Kim, H.; Barbieri, R.; Wasan, A.D.; Edwards, R.R.; et al. The somatosensory link in fibromyalgia: Functional connectivity of the primary somatosensory cortex is altered by sustained pain and is associated with clinical/autonomic dysfunction. Arthritis Rheumatol. 2015, 67, 1395–1405. [Google Scholar] [CrossRef] [Green Version]
- Wortinger, L.A.; Endestad, T.; Melinder, A.M.D.; Øie, M.G.; Sevenius, A.; Wyller, V.B. Aberrant resting-state functional connectivity in the salience network of adolescent chronic fatigue syndrome. PLoS ONE 2016, 11, e0159351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnden, L.R.; Crouch, B.; Kwiatek, R.; Burnet, R.; Del Fante, P. Evidence in chronic fatigue syndrome for severity-dependent upregulation of prefrontal myelination that is independent of anxiety and depression. NMR Biomed. 2015, 28, 404–413. [Google Scholar] [CrossRef] [Green Version]
- Finkelmeyer, A.; He, J.; Maclachlan, L.; Watson, S.; Gallagher, P.; Newton, J.L.; Blamire, A.M. Grey and white matter differences in chronic fatigue syndrome—A voxel-based morphometry study. NeuroImage Clin. 2018, 17, 24–30. [Google Scholar] [CrossRef] [Green Version]
- Puri, B.K.; Counsell, S.; Zaman, R.; Main, J.; Collins, A.; Hajnal, J.; Davey, N. Relative increase in choline in the occipital cortex in chronic fatigue syndrome. Acta Psychiatr. Scand. 2002, 106, 224–226. [Google Scholar] [CrossRef]
- Shan, Z.Y.; Kwiatek, R.; Burnet, R.; Del Fante, P.; Staines, D.R.; Marshall-Gradisnik, S.M.; Barnden, L.R. Progressive brain changes in patients with chronic fatigue syndrome: A longitudinal MRI study. J. Magn. Reason. Imaging 2016, 44, 1301–1311. [Google Scholar] [CrossRef] [PubMed]
- Thapaliya, K.; Staines, D.; Marshall-Gradisnik, S.; Su, J.; Barnden, L. Volumetric differences in hippocampal subfields and associations with clinical measures in myalgic encephalomyelitis/chronic fatigue syndrome. J. Neurosci. Res. 2022, 100, 1476–1486. [Google Scholar] [CrossRef]
- Barnden, L.R.; Shan, Z.Y.; Staines, D.R.; Marshall-Gradisnik, S.; Finegan, K.; Ireland, T.; Bhuta, S. Intra brainstem connectivity is impaired in chronic fatigue syndrome. NeuroImage Clin. 2019, 24, 102045. [Google Scholar] [CrossRef]
- Zinn, M.A.; Zinn, M.L.; Valencia, I.; Jason, L.A.; Montoya, J.G. Cortical hypoactivation during resting EEG suggests central nervous system pathology in patients with Chronic Fatigue Syndrome. Biol. Psychol. 2018, 136, 87–99. [Google Scholar] [CrossRef] [PubMed]
- De Lange, F.P.; Kalkman, J.S.; Bleijenberg, G.; Hagoort, P.; Van der Meer, J.W.; Toni, I. Gray matter volume reduction in the chronic fatigue syndrome. Neuroimage 2005, 26, 777–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okada, T.; Tanak, M.; Kuratsune, H.; Watanabe, Y.; Sadato, N. Mechanisms underlying fatigue: A voxel-based morphometric study of chronic fatigue syndrome. BMC Neurol. 2004, 4, 14. [Google Scholar] [CrossRef]
- Zinn, M.A.; Jason, L.A. Cortical autonomic network connectivity predicts symptoms in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Int. J. Psychophysiol. 2021, 170, 89–101. [Google Scholar] [CrossRef]
- Jason, L. The Energy Envelope Theory and myalgic encephalomyelitis/chronic fatigue syndrome. Aaohn J. 2008, 56, 189–195. [Google Scholar] [CrossRef] [Green Version]
- Sweetman, E.; Noble, A.; Edgar, C.; Mackay, A.; Helliwell, A.; Vallings, R.; Ryan, M.; Tate, W. Current research provides insight into the biological basis and diagnostic potential for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Diagnostics 2019, 9, 73. [Google Scholar] [CrossRef] [Green Version]
- Sweetman, E.; Kleffmann, T.; Edgar, C.; de Lange, M.; Vallings, R.; Tate, W. A SWATH-MS analysis of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome peripheral blood mononuclear cell proteomes reveals mitochondrial dysfunction. J. Transl. Med. 2020, 18, 1–18. [Google Scholar] [CrossRef]
- Shukla, S.K.; Cook, D.; Meyer, J.; Vernon, S.D.; Le, T.; Clevidence, D.; Robertson, C.E.; Schrodi, S.J.; Yale, S.; Frank, D.N. Changes in gut and plasma microbiome following exercise challenge in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). PLoS ONE 2015, 10, e0145453. [Google Scholar] [CrossRef] [PubMed]
- Cortes Rivera, M.; Mastronardi, C.; Silva-Aldana, C.T.; Arcos-Burgos, M.; Lidbury, B.A. Myalgic encephalomyelitis/chronic fatigue syndrome: A comprehensive review. Diagnostics 2019, 9, 91. [Google Scholar] [CrossRef] [Green Version]
- Mandarano, A.H.; Giloteaux, L.; Keller, B.A.; Levine, S.M.; Hanson, M.R. Eukaryotes in the gut microbiota in myalgic encephalomyelitis/chronic fatigue syndrome. PeerJ 2018, 6, e4282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, G.; Berk, M.; Galecki, P.; Maes, M. The emerging role of autoimmunity in myalgic encephalomyelitis/chronic fatigue syndrome (ME/cfs). Mol. Neurobiol. 2014, 49, 741–756. [Google Scholar] [CrossRef]
- Dhabhar, F.S. Enhancing versus suppressive effects of stress on immune function: Implications for immunoprotection and immunopathology. Neuroimmunomodulation 2009, 16, 300–317. [Google Scholar] [CrossRef] [Green Version]
- Antoni, M.H.; Weiss, D.E. Stress, immunity and chronic fatigue syndrome: A conceptual model to guide the development of treatment and research. In Handbook of Chronic Fatigue Syndrome and Fatiguing Illnesses; Fennell, P., Jason, L.A., Taylor, R., Eds.; John Wiley Sons, Inc.: New York, NY, USA, 2003. [Google Scholar]
- Tønnesen, E.; Christensen, N.J.; Brinkløv, M.M. Natural killer cell activity during cortisol and adrenaline infusion in healthy volunteers. Eur. J. Clin. Investig. 1987, 17, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, D.C.; Chaudhuri, D.A. ME/CFS/PVFS: An Exploration of the Key Clinical Issues; ME Association: Gawcott, UK, 2019. [Google Scholar]
- Chu, L.; Valencia, I.J.; Garvert, D.W.; Montoya, J.G. Deconstructing post-exertional malaise in myalgic encephalomyelitis/chronic fatigue syndrome: A patient-centered, cross-sectional survey. PLoS ONE 2018, 13, e0197811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jais, A.; Brüning, J.C. Hypothalamic inflammation in obesity and metabolic disease. J. Clin. Investig. 2017, 127, 24–32. [Google Scholar] [CrossRef]





| Diagnosis requires that the patient have the following three symptoms: |
|
|
|
| At least one of the two following manifestations is also required: |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marks, D.F. Converging Evidence of Similar Symptomatology of ME/CFS and PASC Indicating Multisystemic Dyshomeostasis. Biomedicines 2023, 11, 180. https://doi.org/10.3390/biomedicines11010180
Marks DF. Converging Evidence of Similar Symptomatology of ME/CFS and PASC Indicating Multisystemic Dyshomeostasis. Biomedicines. 2023; 11(1):180. https://doi.org/10.3390/biomedicines11010180
Chicago/Turabian StyleMarks, David F. 2023. "Converging Evidence of Similar Symptomatology of ME/CFS and PASC Indicating Multisystemic Dyshomeostasis" Biomedicines 11, no. 1: 180. https://doi.org/10.3390/biomedicines11010180
APA StyleMarks, D. F. (2023). Converging Evidence of Similar Symptomatology of ME/CFS and PASC Indicating Multisystemic Dyshomeostasis. Biomedicines, 11(1), 180. https://doi.org/10.3390/biomedicines11010180






