Effects of Transient Administration of the NMDA Receptor Antagonist MK-801 in Drosophila melanogaster Activity, Sleep, and Negative Geotaxis
Abstract
1. Introduction
2. Materials and Methods
2.1. Fly Strains and Maintenance
2.2. Pharmacology
2.3. General Activity and Forced Climbing Assays
2.4. Statistical Analyses
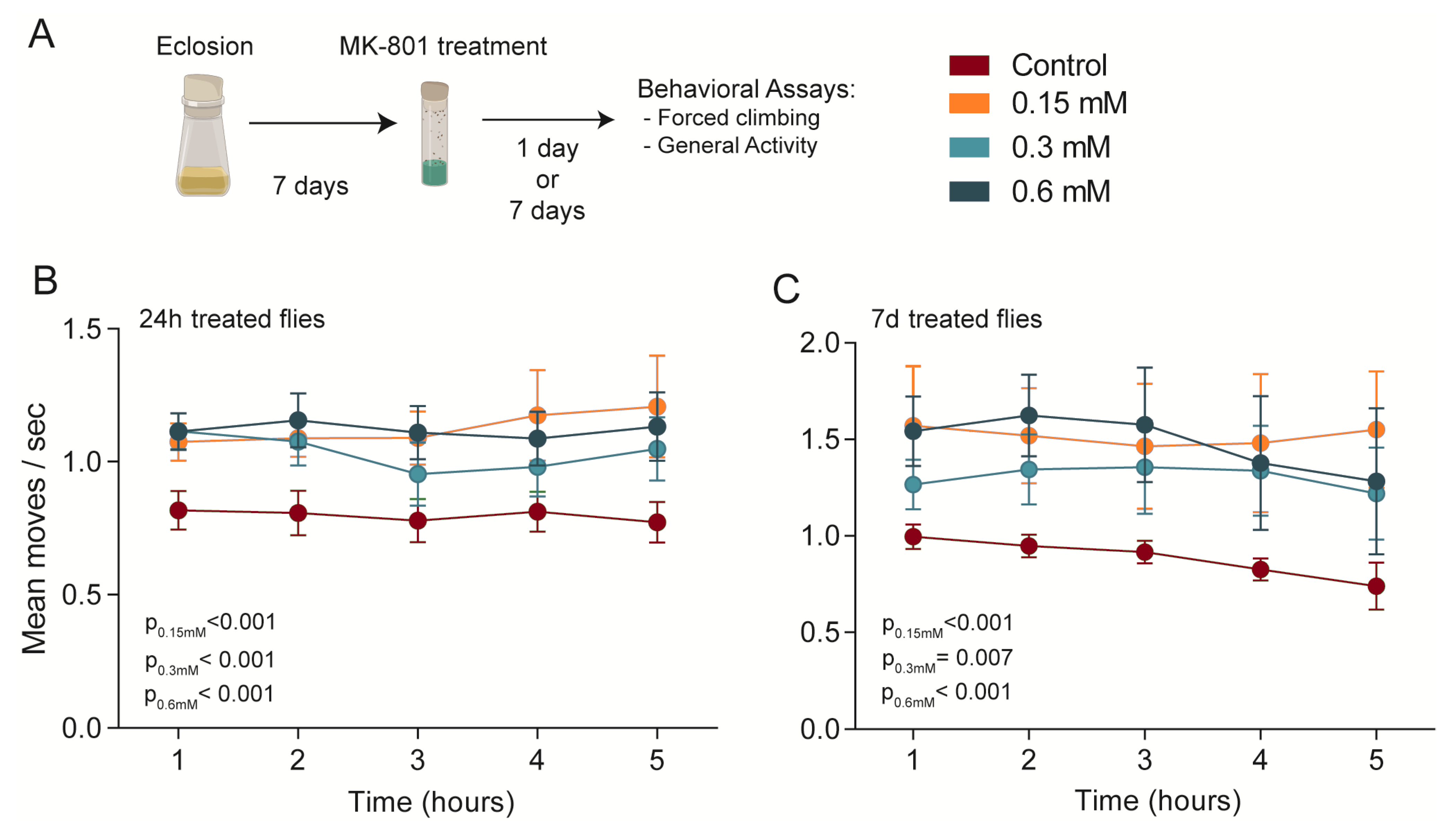
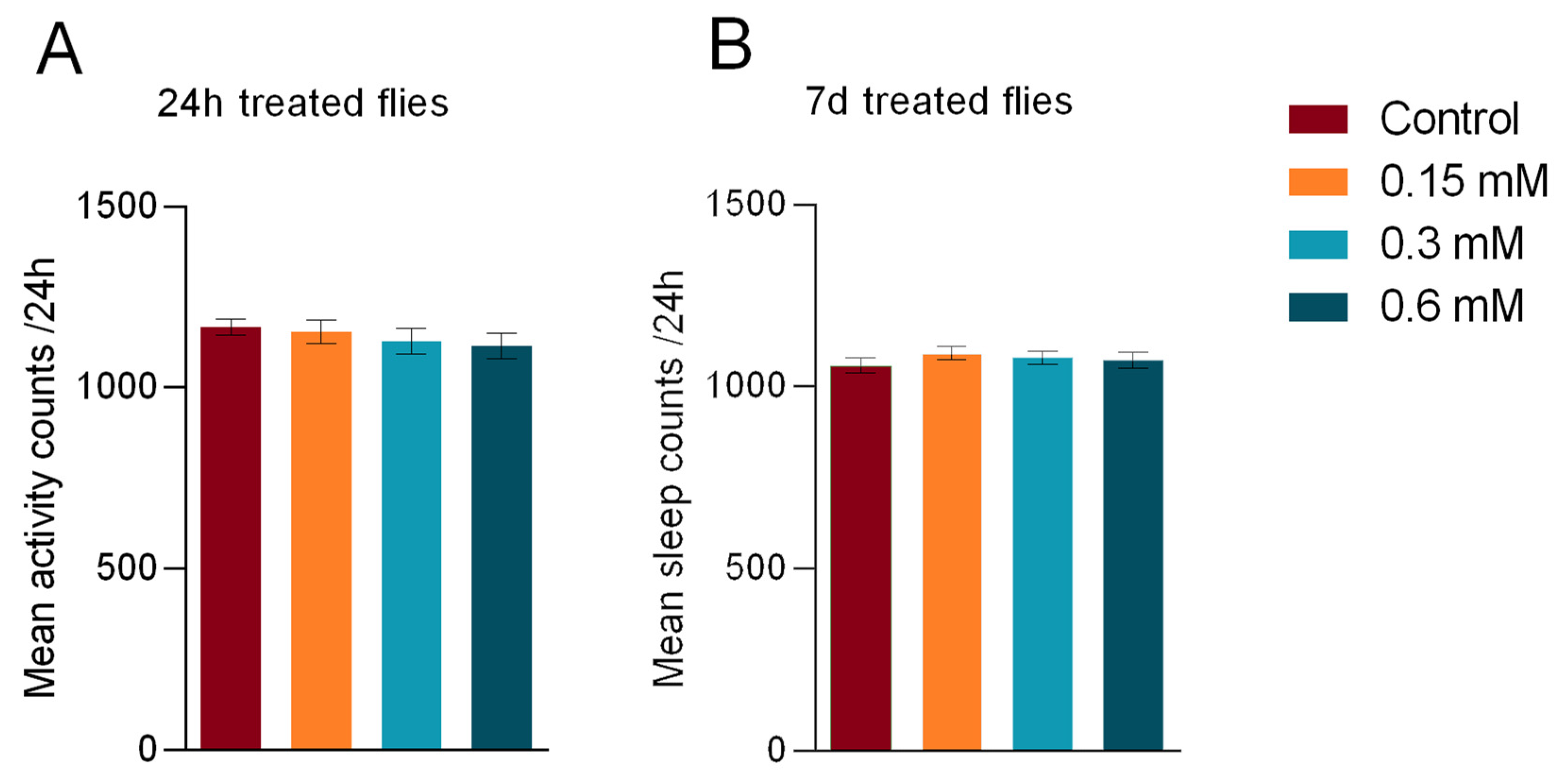
3. Results
3.1. Forced Climbing Assay
3.2. General Activity
3.3. Sleep Duration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- O’Kane, C.J. Drosophila as a Model Organism for the Study of Neuropsychiatric Disorders. Curr. Top Behav. Neurosci. 2011, 7, 37–60. [Google Scholar] [CrossRef]
- Kasture, A.S.; Hummel, T.; Sucic, S.; Freissmuth, M. Big Lessons from Tiny Flies: Drosophila Melanogaster as a Model to Explore Dysfunction of Dopaminergic and Serotonergic Neurotransmitter Systems. Int. J. Mol. Sci. 2018, 19, 1788. [Google Scholar] [CrossRef]
- Reiter, L.T.; Potocki, L.; Chien, S.; Gribskov, M.; Bier, E. A Systematic Analysis of Human Disease-Associated Gene Sequences In Drosophila Melanogaster. Genome Res. 2001, 11, 1114–1125. [Google Scholar] [CrossRef]
- van Alphen, B.; van Swinderen, B. Drosophila Strategies to Study Psychiatric Disorders. Brain Res. Bull. 2013, 92, 1–11. [Google Scholar] [CrossRef]
- Moulin, T.C.; Covill, L.E.; Itskov, P.M.; Williams, M.J.; Schiöth, H.B. Rodent and Fly Models in Behavioral Neuroscience: An Evaluation of Methodological Advances, Comparative Research, and Future Perspectives. Neurosci. Biobehav. Rev. 2021, 120, 1–12. [Google Scholar] [CrossRef]
- Moulin, T.C.; Ferro, F.; Hoyer, A.; Cheung, P.; Williams, M.J.; Schiöth, H.B. The Drosophila Melanogaster Levodopa-Induced Depression Model Exhibits Negative Geotaxis Deficits and Differential Gene Expression in Males and Females. Front. Neurosci. 2021, 15, 653470. [Google Scholar] [CrossRef]
- Ries, A.-S.; Hermanns, T.; Poeck, B.; Strauss, R. Serotonin Modulates a Depression-like State in Drosophila Responsive to Lithium Treatment. Nat. Commun. 2017, 8, 15738. [Google Scholar] [CrossRef]
- Dockendorff, T.C.; Su, H.S.; McBride, S.M.J.; Yang, Z.; Choi, C.H.; Siwicki, K.K.; Sehgal, A.; Jongens, T.A. Drosophila Lacking Dfmr1 Activity Show Defects in Circadian Output and Fail to Maintain Courtship Interest. Neuron 2002, 34, 973–984. [Google Scholar] [CrossRef]
- Bhogal, B.; Jongens, T.A. Fragile X Syndrome and Model Organisms: Identifying Potential Routes of Therapeutic Intervention. Dis. Model. Mech. 2010, 3, 693–700. [Google Scholar] [CrossRef][Green Version]
- Peru Y Colón de Portugal, R.L.; Ojelade, S.A.; Penninti, P.S.; Dove, R.J.; Nye, M.J.; Acevedo, S.F.; Lopez, A.; Rodan, A.R.; Rothenfluh, A. Long-Lasting, Experience-Dependent Alcohol Preference in Drosophila. Addict. Biol. 2014, 19, 392–401. [Google Scholar] [CrossRef]
- Bozler, J.; Kacsoh, B.Z.; Bosco, G. Transgenerational Inheritance of Ethanol Preference Is Caused by Maternal NPF Repression. Elife 2019, 8, e45391. [Google Scholar] [CrossRef]
- Hsu, C.T.; Bhandawat, V. Organization of Descending Neurons in Drosophila Melanogaster. Sci. Rep. 2016, 6, 20259. [Google Scholar] [CrossRef]
- Greer, J.B.; Khuri, S.; Fieber, L.A. Phylogenetic Analysis of Ionotropic L-Glutamate Receptor Genes in the Bilateria, with Special Notes on Aplysia Californica. BMC Evol. Biol. 2017, 17, 11. [Google Scholar] [CrossRef]
- Wang, T.-T.; Si, F.-L.; He, Z.-B.; Chen, B. Genome-Wide Identification, Characterisation and Classification of Ionotropic Glutamate Receptor Genes (IGluRs) in the Malaria Vector Anopheles Sinensis (Diptera: Culicidae). Parasit Vectors 2018, 11, 34. [Google Scholar] [CrossRef]
- Brockie, P.J.; Madsen, D.M.; Zheng, Y.; Mellem, J.; Maricq, A.v. Differential Expression of Glutamate Receptor Subunits in the Nervous System of Caenorhabditis Elegans and Their Regulation by the Homeodomain Protein UNC-42. J. Neurosci. 2001, 21, 1510–1522. [Google Scholar] [CrossRef]
- Moriyoshi, K.; Masu, M.; Ishii, T.; Shigemoto, R.; Mizuno, N.; Nakanishi, S. Molecular Cloning and Characterization of the Rat NMDA Receptor. Nature 1991, 354, 31–37. [Google Scholar] [CrossRef]
- Dingledine, R.; Borges, K.; Bowie, D.; Traynelis, S.F. The Glutamate Receptor Ion Channels. Pharmacol. Rev. 1999, 51, 7–61. [Google Scholar]
- Cull-Candy, S.G.; Leszkiewicz, D.N. Role of Distinct NMDA Receptor Subtypes at Central Synapses. Sci. STKE 2004, 2004, re16. [Google Scholar] [CrossRef]
- Xia, S.; Chiang, A.S. NMDA Receptors in Drosophila. Biol. NMDA Recept. 2009, 213–233. [Google Scholar] [CrossRef]
- Zannat, M.T.; Locatelli, F.; Rybak, J.; Menzel, R.; Leboulle, G. Identification and Localisation of the NR1 Sub-Unit Homologue of the NMDA Glutamate Receptor in the Honeybee Brain. Neurosci. Lett. 2006, 398, 274–279. [Google Scholar] [CrossRef]
- Xia, S.; Miyashita, T.; Fu, T.F.; Lin, W.Y.; Wu, C.L.; Pyzocha, L.; Lin, I.R.; Saitoe, M.; Tully, T.; Chiang, A.S. NMDA Receptors Mediate Olfactory Learning and Memory in Drosophila. Curr. Biol. 2005, 15, 603–615. [Google Scholar] [CrossRef]
- Ha, T.J.; Kohn, A.B.; Bobkova, Y.v.; Moroz, L.L. Molecular Characterization of NMDA-like Receptors in Aplysia and Lymnaea: Relevance to Memory Mechanisms. Biol. Bull. 2006, 210, 255–270. [Google Scholar] [CrossRef]
- Chiang, A.S.; Lin, W.Y.; Liu, H.P.; Pszczolkowski, M.A.; Fu, T.F.; Chiu, S.L.; Holbrook, G.L. Insect NMDA Receptors Mediate Juvenile Hormone Biosynthesis. Proc. Natl. Acad. Sci. USA 2002, 99, 37–42. [Google Scholar] [CrossRef]
- Kovacic, P.; Somanathan, R. Clinical Physiology and Mechanism of Dizocilpine (MK-801): Electron Transfer, Radicals, Redox Metabolites and Bioactivity. Oxid. Med. Cell. Longev. 2010, 3, 13. [Google Scholar] [CrossRef]
- Horváth, Z.C.; Czopf, J.; Buzsáki, G. MK-801-Induced Neuronal Damage in Rats. Brain Res. 1997, 753, 181–195. [Google Scholar] [CrossRef]
- Swerdlow, N.R.; Braff, D.L.; Geyer, M.A. Sensorimotor Gating of the Startle Reflex: What We Said 25 Years Ago, What Has Happened since Then, and What Comes Next. J. Psychopharmacol. 2016, 30, 1072–1081. [Google Scholar] [CrossRef]
- Geyer, M.A.; Krebs-Thomson, K.; Braff, D.L.; Swerdlow, N.R. Pharmacological Studies of Prepulse Inhibition Models of Sensorimotor Gating Deficits in Schizophrenia: A Decade in Review. Psychopharmacology 2001, 156, 117–154. [Google Scholar] [CrossRef]
- Seibt, K.J.; Piato, A.L.; da Luz Oliveira, R.; Capiotti, K.M.; Vianna, M.R.; Bonan, C.D. Antipsychotic Drugs Reverse MK-801-Induced Cognitive and Social Interaction Deficits in Zebrafish (Danio Rerio). Behav. Brain Res. 2011, 224, 135–139. [Google Scholar] [CrossRef]
- Maj, J.; Rogóż, Z.; Skuza, G.; Sowińska, H. Effects of MK-801 and Antidepressant Drugs in the Forced Swimming Test in Rats. Eur. Neuropsychopharmacol. 1992, 2, 37–41. [Google Scholar] [CrossRef]
- Carey, R.J.; Dai, H.; Gui, J. Effects of Dizocilpine (MK-801) on Motor Activity and Memory. Psychopharmacology 1998, 137, 241–246. [Google Scholar] [CrossRef]
- Babcock, A.M.; Wright, J.; Bjerkness, D.; Hartman, H.; Tall Bear, N. Effects of Prior Apparatus Experience and Novelty of Testing Environment on Locomotor Activity Following MK-801. Physiol. Behav. 2002, 77, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.W.; Kang, H.S.; Park, J.K.; Lee, S.J.; Baek, S.-B.; Kim, C.J. Voluntary Wheel Running Ameliorates Symptoms of MK-801-Induced Schizophrenia in Mice. Mol. Med. Rep. 2014, 10, 2924–2930. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Si, A.; Helliwell, P.; Maleszka, R. Effects of NMDA Receptor Antagonists on Olfactory Learning and Memory in the Honeybee (Apis Mellifera). Pharmacol. Biochem. Behav. 2004, 77, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Cattaert, D.; Birman, S. Blockade of the Central Generator of Locomotor Rhythm by Noncompetitive NMDA Receptor Antagonists in Drosophila Larvae. J. Neurobiol. 2001, 48, 58–73. [Google Scholar] [CrossRef]
- Brockie, P.J.; Mellem, J.E.; Hills, T.; Madsen, D.M.; Maricq, A.v. The C. Elegans Glutamate Receptor Subunit NMR-1 Is Required for Slow NMDA-Activated Currents That Regulate Reversal Frequency during Locomotion. Neuron 2001, 31, 617–630. [Google Scholar] [CrossRef]
- Olney, J.W.; Farber, N.B. Glutamate Receptor Dysfunction and Schizophrenia. Arch. Gen. Psychiatry 1995, 52, 998–1007. [Google Scholar] [CrossRef]
- Kehrer, C. Altered Excitatory-Inhibitory Balance in the NMDA-Hypofunction Model of Schizophrenia. Front. Mol. Neurosci. 2008, 1, 6. [Google Scholar] [CrossRef]
- Tomita, J.; Ueno, T.; Mitsuyoshi, M.; Kume, S.; Kume, K. The NMDA Receptor Promotes Sleep in the Fruit Fly, Drosophila Melanogaster. PLoS ONE 2015, 10, e0128101. [Google Scholar] [CrossRef]
- Bubeníková-Valešová, V.; Horáček, J.; Vrajová, M.; Höschl, C. Models of Schizophrenia in Humans and Animals Based on Inhibition of NMDA Receptors. Neurosci. Biobehav. Rev. 2008, 32, 1014–1023. [Google Scholar] [CrossRef]
- Moulin, T.C.; Ferro, F.; Berkins, S.; Hoyer, A.; Williams, M.J.; Schiöth, H.B. Transient Administration of Dopaminergic Precursor Causes Inheritable Overfeeding Behavior in Young Drosophila Melanogaster Adults. Brain Sci. 2020, 10, 487. [Google Scholar] [CrossRef]
- Williams, M.J.; Alsehli, A.M.; Gartner, S.N.; Clemensson, L.E.; Liao, S.; Eriksson, A.; Isgrove, K.; Thelander, L.; Khan, Z.; Itskov, P.M.; et al. The Statin Target Hmgcr Regulates Energy Metabolism and Food Intake through Central Mechanisms. Cells 2022, 11, 970. [Google Scholar] [CrossRef]
- Turrigiano, G.G.; Leslie, K.R.; Desai, N.S.; Rutherford, L.C.; Nelson, S.B. Activity-Dependent Scaling of Quantal Amplitude in Neocortical Neurons. Nature 1998, 391, 892–896. [Google Scholar] [CrossRef] [PubMed]
- Moulin, T.C.; Petiz, L.L.; Rayêe, D.; Winne, J.; Maia, R.G.; Lima da Cruz, R.V.; Amaral, O.B.; Leão, R.N. Chronic in Vivo Optogenetic Stimulation Modulates Neuronal Excitability, Spine Morphology, and Hebbian Plasticity in the Mouse Hippocampus. Hippocampus 2019, 29, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Stone, W.S.; Walker, D.L.; Gold, P.E. Sleep deficits in rats after NMDA receptor blockade. Physiol. Behav. 1992, 52, 609–612. [Google Scholar] [CrossRef] [PubMed]
- Campbell, I.G.; Feinberg, I. Comparison of MK-801 and sleep deprivation effects on NREM, REM, and waking spectra in the rat. Sleep 1999, 22, 423–432. [Google Scholar] [CrossRef]
- Gorter, J.A.; Veerman, M.; Mirmiran, M.; Bos, N.P.; Corner, M.A. Spectral analysis of the electroencephalogram in neonatal rats chronically treated with the NMDA antagonist MK-801. Brain Res. Dev. Brain Res. 1991, 64, 37–41. [Google Scholar] [CrossRef]
- Prospero-García, O.; Criado, J.R.; Henriksen, S.J. Pharmacology of ethanol and glutamate antagonists on rodent sleep: A comparative study. Pharmacol. Biochem. Behav. 1994, 49, 413–416. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moulin, T.C.; Stojanovic, T.; Rajesh, R.P.; Pareek, T.; Donzelli, L.; Williams, M.J.; Schiöth, H.B. Effects of Transient Administration of the NMDA Receptor Antagonist MK-801 in Drosophila melanogaster Activity, Sleep, and Negative Geotaxis. Biomedicines 2023, 11, 192. https://doi.org/10.3390/biomedicines11010192
Moulin TC, Stojanovic T, Rajesh RP, Pareek T, Donzelli L, Williams MJ, Schiöth HB. Effects of Transient Administration of the NMDA Receptor Antagonist MK-801 in Drosophila melanogaster Activity, Sleep, and Negative Geotaxis. Biomedicines. 2023; 11(1):192. https://doi.org/10.3390/biomedicines11010192
Chicago/Turabian StyleMoulin, Thiago C., Tijana Stojanovic, Rasika P. Rajesh, Tirusha Pareek, Laura Donzelli, Michael J. Williams, and Helgi B. Schiöth. 2023. "Effects of Transient Administration of the NMDA Receptor Antagonist MK-801 in Drosophila melanogaster Activity, Sleep, and Negative Geotaxis" Biomedicines 11, no. 1: 192. https://doi.org/10.3390/biomedicines11010192
APA StyleMoulin, T. C., Stojanovic, T., Rajesh, R. P., Pareek, T., Donzelli, L., Williams, M. J., & Schiöth, H. B. (2023). Effects of Transient Administration of the NMDA Receptor Antagonist MK-801 in Drosophila melanogaster Activity, Sleep, and Negative Geotaxis. Biomedicines, 11(1), 192. https://doi.org/10.3390/biomedicines11010192




