Esterification of p-Coumaric Acid Improves the Control over Melanoma Cell Growth
Abstract
:1. Introduction
2. Materials and Methods
2.1. p-Coumaric acid and Ester Derivatives
2.2. Animals
2.3. Cell Lines and Cell Culture
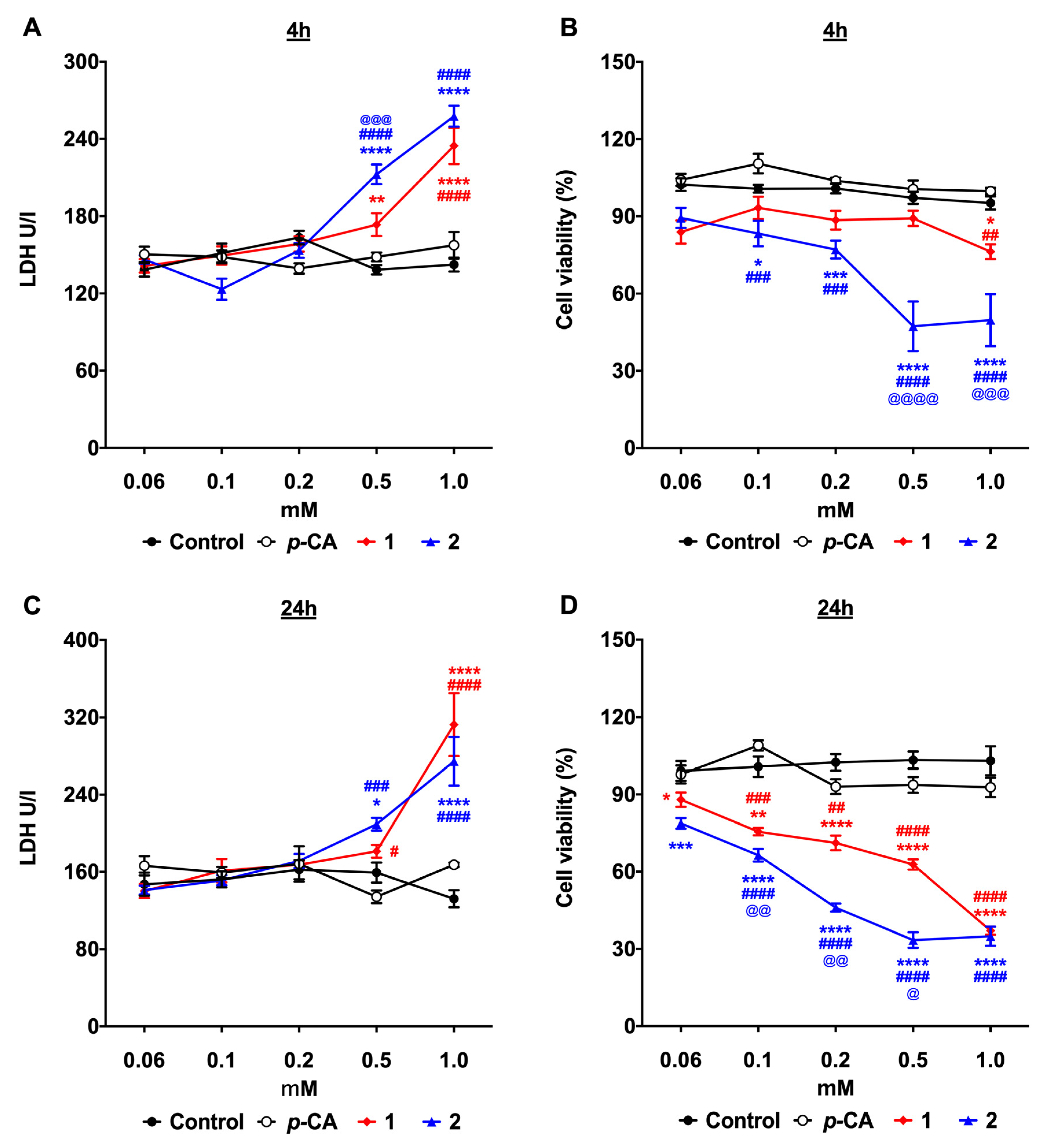
2.4. Cell Cytotoxicity and Viability Assay
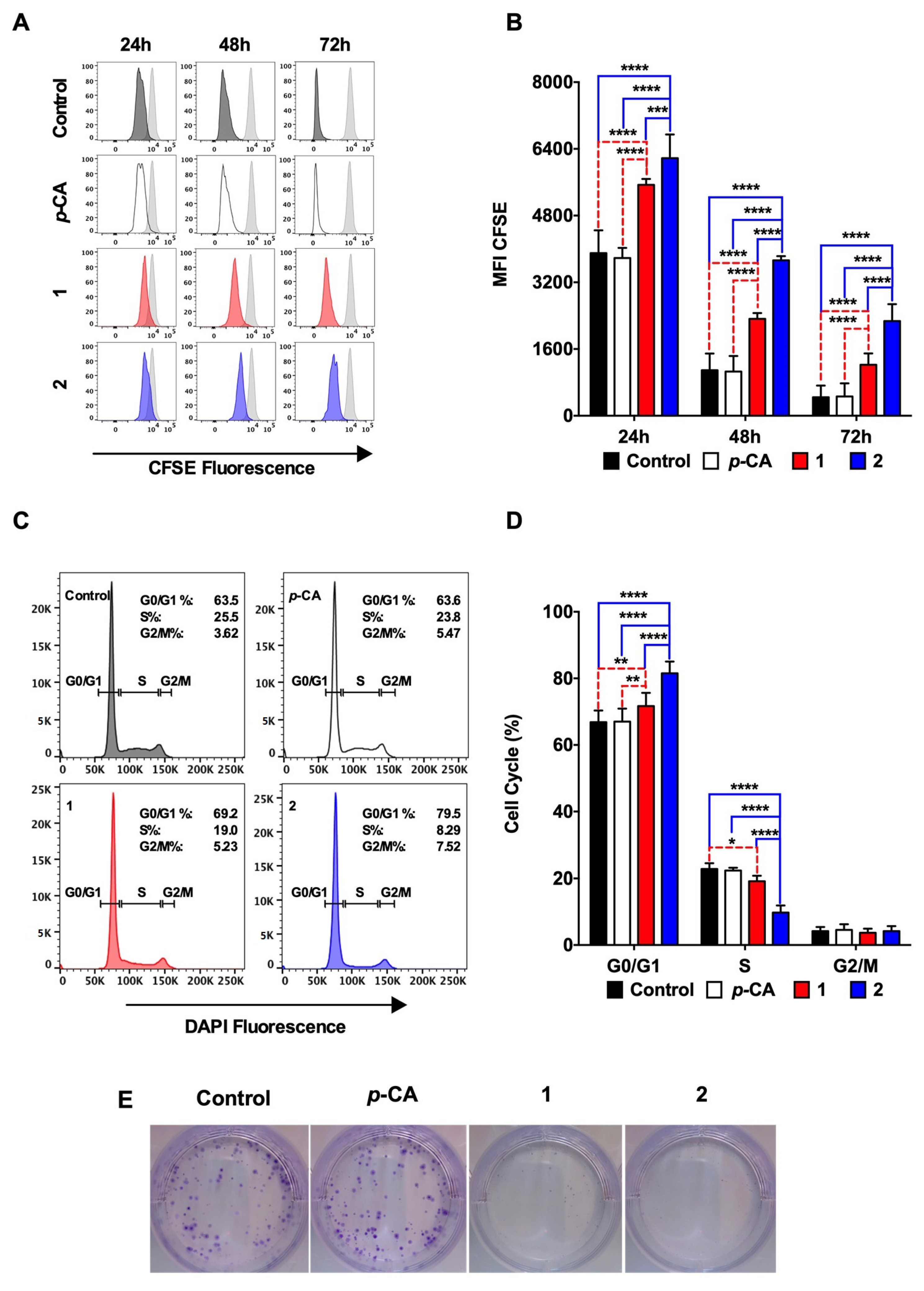
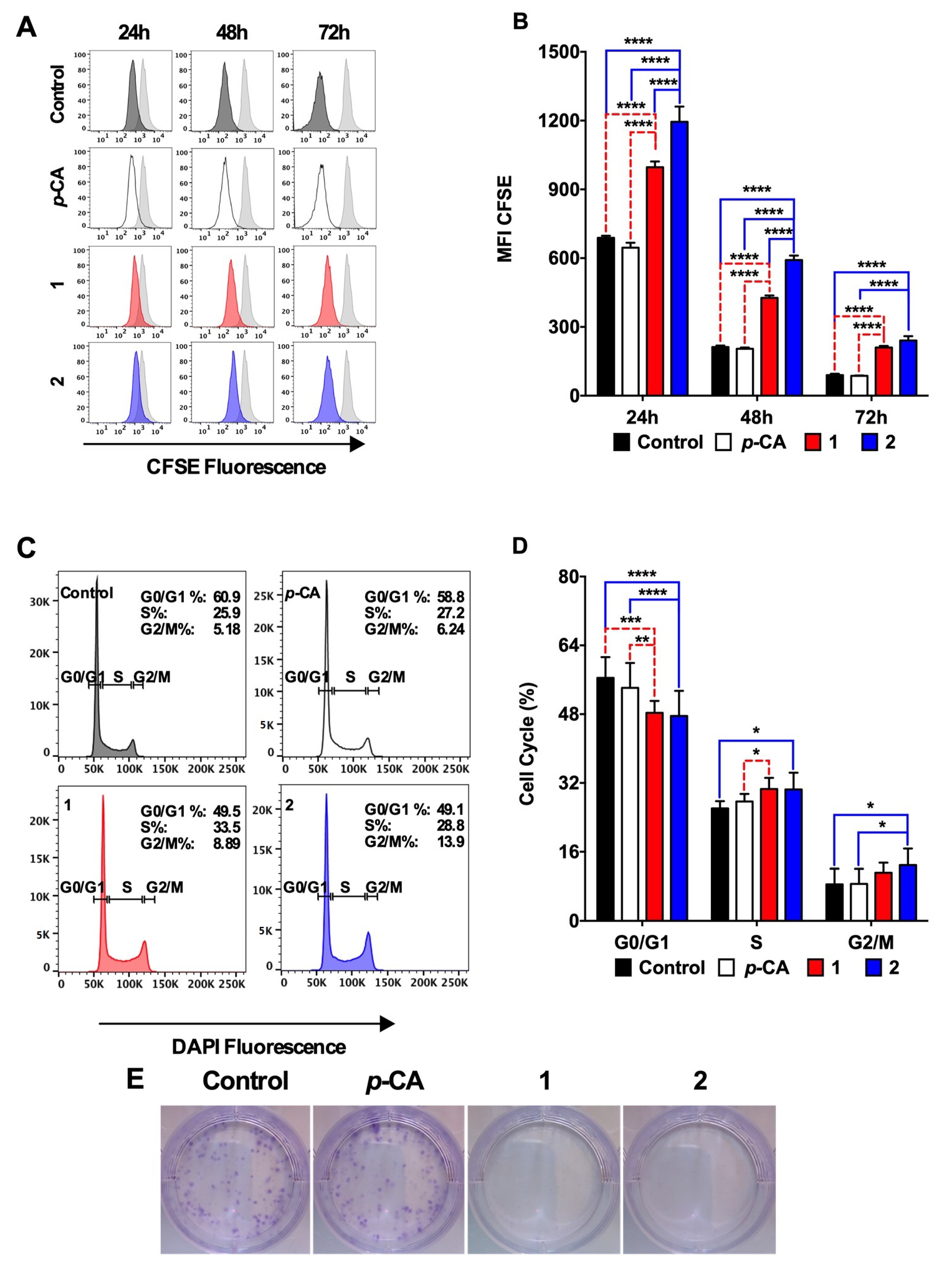
2.5. Cell Proliferation Assay
2.6. Cell Cycle Assay
2.7. Colony Formation Assay
2.8. In Vitro Splenocyte Stimulation
2.9. In Vivo Treatment and the Metastasis Model
2.10. Statistical Analysis
3. Results
3.1. p-Coumaric Acid Esterification Improves the Cytotoxicity against the B16-F10 Melanoma Cells
3.2. Ethyl p-Coumarate (1) and n-Butyl p-Coumarate (2) Inhibit the B16-F10 Cell Proliferation by Inducing the Cell Cycle Arrest at the G0/G1 Phase
3.3. Ethyl p-Coumarate (1) and n-Butyl p-Coumarate (2) Exert Cytotoxic and Antiproliferative Effects against the SK-MEL-25 Melanoma Cells
3.4. N-Butyl p-Coumarate (2) Controls the In Vivo Tumor Metastasis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stege, H.; Haist, M.; Nikfarjam, U.; Schultheis, M.; Heinz, J.; Pemler, S.; Loquai, C.; Grabbe, S. The Status of Adjuvant and Neoadjuvant Melanoma Therapy, New Developments and Upcoming Challenges. Target Oncol. 2021, 16, 537–552. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Yang, Z.; Liu, W.; Pan, Z.; Zhang, X.; Li, M.; Liu, X.; Zheng, Q.; Li, D. The Anti-tumor Effects of p-Coumaric Acid on Melanoma A375 and B16 Cells. Front Oncol. 2020, 10, 558414. [Google Scholar] [CrossRef]
- Arruda, C.; Pena Ribeiro, V.; Oliveira Almeida, M.; Aldana Mejia, J.A.; Casoti, R.; Kenupp Bastos, J. Effect of light, oxygen and temperature on the stability of artepillin C and p-coumaric acid from Brazilian green propolis. J. Pharm. Biomed. Anal. 2020, 178, 112922. [Google Scholar] [CrossRef] [PubMed]
- Varela, M.T.; Ferrarini, M.; Mercaldi, V.G.; Sufi, B.D.S.; Padovani, G.; Nazato, L.I.S.; Fernandes, J.P.S. Coumaric acid derivatives as tyrosinase inhibitors: Efficacy studies through in silico, in vitro and ex vivo approaches. Bioorg. Chem. 2020, 103, 104108. [Google Scholar] [CrossRef]
- Feoktistova, M.; Geserick, P.; Leverkus, M. Crystal Violet Assay for Determining Viability of Cultured Cells. Cold Spring Harb. Protoc. 2016, 2016, pdb.prot087379. [Google Scholar] [CrossRef]
- Ferreira, A.K.; Pasqualoto, K.F.; Kruyt, F.A.; Palace-Berl, F.; Azevedo, R.A.; Turra, K.M.; Rodrigues, C.P.; Ferreira, A.C.; Salomon, M.A.; de Sa Junior, P.L.; et al. BFD-22 a new potential inhibitor of BRAF inhibits the metastasis of B16F10 melanoma cells and simultaneously increased the tumor immunogenicity. Toxicol. Appl. Pharmacol. 2016, 295, 56–67. [Google Scholar] [CrossRef]
- Darzynkiewicz, Z.; Juan, G.; Bedner, E. Determining cell cycle stages by flow cytometry. Curr. Protoc. Cell Biol. 2001, 1, 8.4.1–8.4.18. [Google Scholar] [CrossRef]
- Massaro, R.R.; Faiao-Flores, F.; Rebecca, V.W.; Sandri, S.; Alves-Fernandes, D.K.; Pennacchi, P.C.; Smalley, K.S.M.; Maria-Engler, S.S. Inhibition of proliferation and invasion in 2D and 3D models by 2-methoxyestradiol in human melanoma cells. Pharmacol. Res. 2017, 119, 242–250. [Google Scholar] [CrossRef]
- Davis, L.E.; Coburn, N.G.; Hallet, J.; Earle, C.C.; Liu, Y.; Myrehaug, S.; Mahar, A.L. Material deprivation and access to cancer care in a universal health care system. Cancer 2020, 126, 4545–4552. [Google Scholar] [CrossRef]
- Nunes, B.P.; Thume, E.; Tomasi, E.; Duro, S.M.; Facchini, L.A. Socioeconomic inequalities in the access to and quality of health care services. Rev. Saude Publica 2014, 48, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, P.N., Jr.; Adashek, J.J.; Roitberg, F.; Noia Barreto, C.M.; Del Giglio, A.; Lopes, G.L., Jr. In the Era of Cost-Effectiveness Analysis, Affordability Is a Limiting Factor for Patients’ Access to Innovative Cancer Treatments. Value Health Reg. Issues 2019, 20, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Wani, M.C.; Taylor, H.L.; Wall, M.E.; Coggon, P.; McPhail, A.T. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J. Am. Chem. Soc. 1971, 93, 2325–2327. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kianmehr, Z.; Khorsandi, K.; Mohammadi, M.; Hosseinzadeh, R. Low-level laser irradiation potentiates anticancer activity of p-coumaric acid against human malignant melanoma cells. Melanoma Res. 2020, 30, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Gastaldello, G.H.; Cazeloto, A.C.V.; Ferreira, J.C.; Rodrigues, D.M.; Bastos, J.K.; Campo, V.L.; Zoccal, K.F.; Tefe-Silva, C. Green Propolis Compounds (Baccarin and p-Coumaric Acid) Show Beneficial Effects in Mice for Melanoma Induced by B16f10. Medicines 2021, 8, 20. [Google Scholar] [CrossRef]
- Sarna, M.; Krzykawska-Serda, M.; Jakubowska, M.; Zadlo, A.; Urbanska, K. Melanin presence inhibits melanoma cell spread in mice in a unique mechanical fashion. Sci. Rep. 2019, 9, 9280. [Google Scholar] [CrossRef] [Green Version]
- Sobecki, M.; Mrouj, K.; Colinge, J.; Gerbe, F.; Jay, P.; Krasinska, L.; Dulic, V.; Fisher, D. Cell-Cycle Regulation Accounts for Variability in Ki-67 Expression Levels. Cancer Res. 2017, 77, 2722–2734. [Google Scholar] [CrossRef] [Green Version]
- Remnant, L.; Kochanova, N.Y.; Reid, C.; Cisneros-Soberanis, F.; Earnshaw, W.C. The intrinsically disorderly story of Ki-67. Open Biol. 2021, 11, 210120. [Google Scholar] [CrossRef]
- Schwartz, G.K.; Shah, M.A. Targeting the cell cycle: A new approach to cancer therapy. J. Clin. Oncol. 2005, 23, 9408–9421. [Google Scholar] [CrossRef]
- Janicke, B.; Hegardt, C.; Krogh, M.; Onning, G.; Akesson, B.; Cirenajwis, H.M.; Oredsson, S.M. The antiproliferative effect of dietary fiber phenolic compounds ferulic acid and p-coumaric acid on the cell cycle of Caco-2 cells. Nutr. Cancer 2011, 63, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Pragasam, S.J.; Venkatesan, V.; Rasool, M. Immunomodulatory and anti-inflammatory effect of p-coumaric acid, a common dietary polyphenol on experimental inflammation in rats. Inflammation 2013, 36, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Kilani-Jaziri, S.; Mokdad-Bzeouich, I.; Krifa, M.; Nasr, N.; Ghedira, K.; Chekir-Ghedira, L. Immunomodulatory and cellular anti-oxidant activities of caffeic, ferulic, and p-coumaric phenolic acids: A structure-activity relationship study. Drug Chem. Toxicol. 2017, 40, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Cibrian, D.; Sanchez-Madrid, F. CD69: From activation marker to metabolic gatekeeper. Eur. J. Immunol. 2017, 47, 946–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borrego, F.; Robertson, M.J.; Ritz, J.; Pena, J.; Solana, R. CD69 is a stimulatory receptor for natural killer cell and its cytotoxic effect is blocked by CD94 inhibitory receptor. Immunology 1999, 97, 159–165. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carmo-Martins, J.I.; Gonzatti, M.B.; Varela, M.T.; Sousa, M.E.P.; Costa, L.V.S.; Rodrigues, E.G.; Fernandes, J.P.S.; Keller, A.C. Esterification of p-Coumaric Acid Improves the Control over Melanoma Cell Growth. Biomedicines 2023, 11, 196. https://doi.org/10.3390/biomedicines11010196
Carmo-Martins JI, Gonzatti MB, Varela MT, Sousa MEP, Costa LVS, Rodrigues EG, Fernandes JPS, Keller AC. Esterification of p-Coumaric Acid Improves the Control over Melanoma Cell Growth. Biomedicines. 2023; 11(1):196. https://doi.org/10.3390/biomedicines11010196
Chicago/Turabian StyleCarmo-Martins, Joana I., Michelangelo B. Gonzatti, Marina T. Varela, Maria Eduarda P. Sousa, Lucas V. S. Costa, Elaine Guadelupe Rodrigues, João Paulo S. Fernandes, and Alexandre C. Keller. 2023. "Esterification of p-Coumaric Acid Improves the Control over Melanoma Cell Growth" Biomedicines 11, no. 1: 196. https://doi.org/10.3390/biomedicines11010196
APA StyleCarmo-Martins, J. I., Gonzatti, M. B., Varela, M. T., Sousa, M. E. P., Costa, L. V. S., Rodrigues, E. G., Fernandes, J. P. S., & Keller, A. C. (2023). Esterification of p-Coumaric Acid Improves the Control over Melanoma Cell Growth. Biomedicines, 11(1), 196. https://doi.org/10.3390/biomedicines11010196





