Potential Protective Effect of Dengue NS1 Human Monoclonal Antibodies against Dengue and Zika Virus Infections
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. Virus Cultures
2.3. Preparation of MAbs
2.4. Neutralization Assay
2.5. DENV NS1 HuMAbs and Complement-Affected DENV Replication
2.6. Fluorescent Focus Assay
2.7. Antibody-Dependent Complement-Mediated Cytolytic Assay
2.8. Vascular Permeability Assay
2.9. Cytokine and Chemokine Analyses
2.10. Plasmid Construction and Transfection
2.11. Analysis of NS1 Sequences and Amino Acid Variation within Epitope Regions
2.12. Statistical Analysis
3. Results
3.1. DENV NS1 HuMAbs Neutralize Flaviviruses
3.2. DENV NS1 HuMAbs Stimulate Complement-Mediated Cell Cytolysis and Inhibit the Viral Replication of Infected Cells
3.3. Anti-DENV NS1 HuMAbs Protect against NS1-Induced Endothelial Leakage
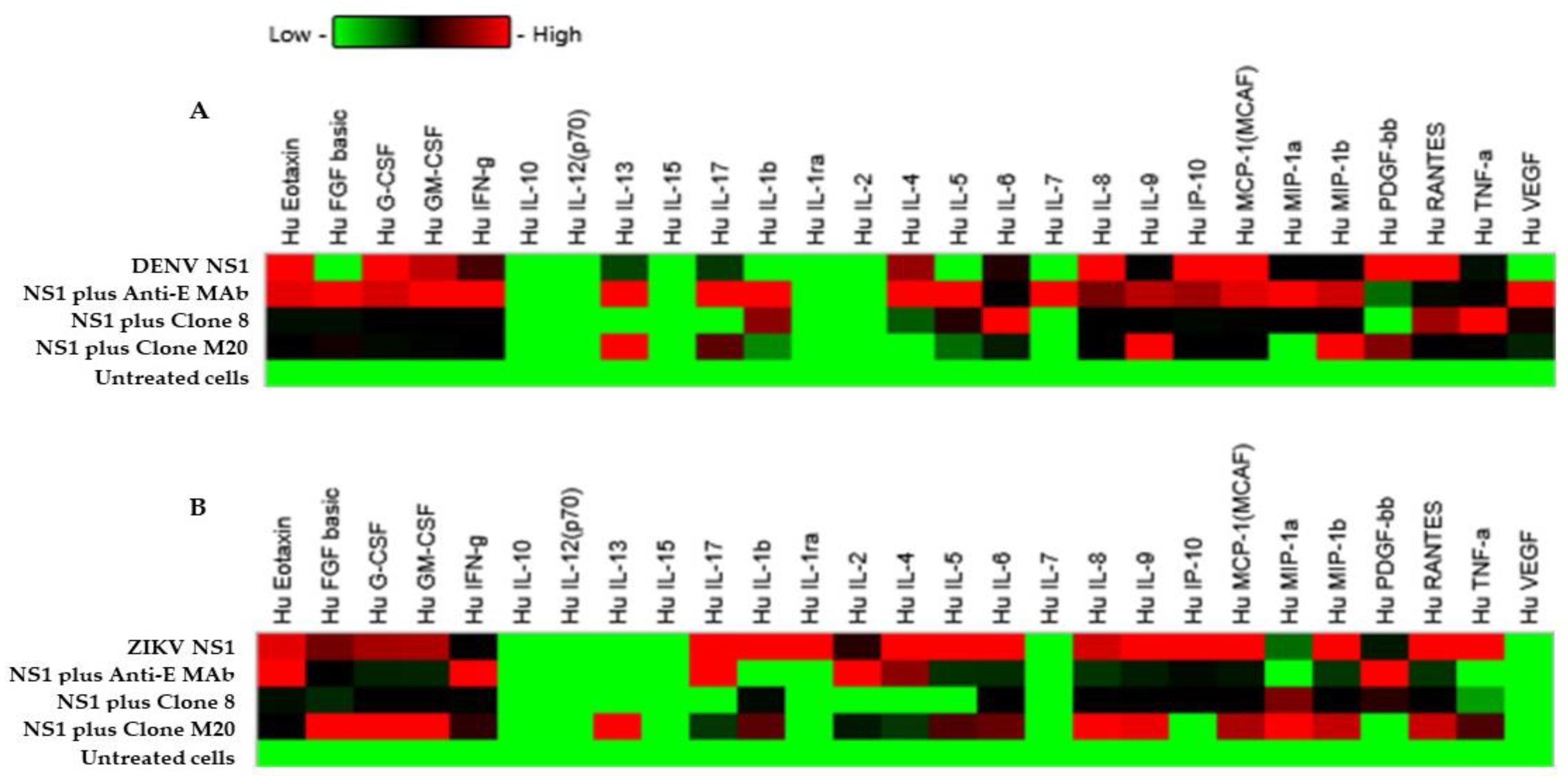
3.4. Effect of DENV NS1 HuMAbs on NS1-Mediated Inflammatory Cytokine Secretion In Vitro
3.5. Mapping of NS1-Specific HuMAbs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kyle, J.L.; Harris, E. Global Spread and Persistence of Dengue. Annu. Rev. Microbiol. 2008, 62, 71–92. [Google Scholar] [CrossRef] [PubMed]
- Gould, E.A.; Solomon, T. Pathogenic Flaviviruses. Lancet 2008, 371, 500–509. [Google Scholar] [CrossRef]
- Counotte, M.J.; Egli-Gany, D.; Riesen, M.; Abraha, M.; Porgo, T.V.; Wang, J.; Low, N. Zika Virus Infection as a Cause of Congenital Brain Abnormalities and Guillain-Barre Syndrome: From Systematic Review to Living Systematic Review. F1000Research 2018, 7, 196. [Google Scholar] [CrossRef]
- Rodriguez-Barraquer, I.; Costa, F.; Nascimento, E.J.M.; Nery, N.J.; Castanha, P.M.S.; Sacramento, G.A.; Cruz, J.; Carvalho, M.; De Olivera, D.; Hagan, J.E.; et al. Impact of Preexisting Dengue Immunity on Zika Virus Emergence in a Dengue Endemic Region. Science 2019, 363, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, R.J.; Zhang, W.; Rossmann, M.G.; Pletnev, S.V.; Corver, J.; Lenches, E.; Jones, C.T.; Mukhopadhyay, S.; Chipman, P.R.; Strauss, E.G.; et al. Structure of Dengue Virus: Implications for Flavivirus Organization, Maturation, and Fusion. Cell 2002, 108, 717–725. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Dengue Vaccine: WHO Position Paper, July 2016—Recommendations. Vaccine 2017, 35, 1200–1201. [Google Scholar] [CrossRef] [PubMed]
- Avirutnan, P.; Zhang, L.; Punyadee, N.; Manuyakorn, A.; Puttikhunt, C.; Kasinrerk, W.; Malasit, P.; Atkinson, J.P.; Diamond, M.S. Secreted NS1 of Dengue Virus Attaches to the Surface of Cells via Interactions with Heparan Sulfate and Chondroitin Sulfate E. PLoS Pathog. 2007, 3, e183. [Google Scholar] [CrossRef]
- Muller, D.A.; Young, P.R. The Flavivirus NS1 Protein: Molecular and Structural Biology, Immunology, Role in Pathogenesis and Application as a Diagnostic Biomarker. Antivir. Res. 2013, 98, 192–208. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Qi, J.; Haywood, J.; Shi, Y.; Gao, G.F. Zika Virus NS1 Structure Reveals Diversity of Electrostatic Surfaces Among Flaviviruses. Nat. Struct. Mol. Biol. 2016, 23, 456–458. [Google Scholar] [CrossRef]
- Mackenzie, J.M.; Jones, M.K.; Young, P.R. Immunolocalization of the Dengue Virus Nonstructural Glycoprotein NS1 Suggests a Role in Viral RNA Replication. Virology 1996, 220, 232–240. [Google Scholar] [CrossRef]
- Avirutnan, P.; Fuchs, A.; Hauhart, R.E.; Somnuke, P.; Youn, S.; Diamond, M.S.; Atkinson, J.P. Antagonism of the Complement Component C4 by Flavivirus Nonstructural Protein NS1. J. Exp. Med. 2010, 207, 793–806. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.C.; Wang, S.Y.; Lin, Y.S.; Chen, H.R.; Yeh, T.M. Re-Evaluation of the Pathogenic Roles of Nonstructural Protein 1 and Its Antibodies During Dengue Virus Infection. J. Biomed. Sci. 2013, 20, 42. [Google Scholar] [CrossRef] [PubMed]
- Modhiran, N.; Watterson, D.; Muller, D.A.; Panetta, A.K.; Sester, D.P.; Liu, L.; Hume, D.A.; Stacey, K.J.; Young, P.R. Dengue Virus NS1 Protein Activates Cells via Toll-Like Receptor 4 and Disrupts Endothelial Cell Monolayer Integrity. Sci. Transl. Med. 2015, 7, 304ra142. [Google Scholar] [CrossRef] [PubMed]
- Modhiran, N.; Watterson, D.; Blumenthal, A.; Baxter, A.G.; Young, P.R.; Stacey, K.J. Dengue Virus NS1 Protein Activates Immune Cells via TLR4 but Not TLR2 or TLR6. Immunol. Cell Biol. 2017, 95, 491–495. [Google Scholar] [CrossRef]
- Beatty, P.R.; Puerta-Guardo, H.; Killingbeck, S.S.; Glasner, D.R.; Hopkins, K.; Harris, E. Dengue Virus NS1 Triggers Endothelial Permeability and Vascular Leak That Is Prevented by NS1 Vaccination. Sci. Transl. Med. 2015, 7, 304ra141. [Google Scholar] [CrossRef]
- Chen, H.R.; Chuang, Y.C.; Lin, Y.S.; Liu, H.S.; Liu, C.C.; Perng, G.C.; Yeh, T.M. Dengue Virus Nonstructural Protein 1 Induces Vascular Leakage Through Macrophage Migration Inhibitory Factor and Autophagy. PLoS Negl. Trop. Dis. 2016, 10, e0004828. [Google Scholar] [CrossRef]
- Chen, H.R.; Lai, Y.C.; Yeh, T.M. Dengue Virus Non-structural Protein 1: A Pathogenic Factor, Therapeutic Target, and Vaccine Candidate. J. Biomed. Sci. 2018, 25, 58. [Google Scholar] [CrossRef]
- Wan, S.W.; Lu, Y.T.; Huang, C.H.; Lin, C.F.; Anderson, R.; Liu, H.S.; Yeh, T.M.; Yen, Y.T.; Wu-Hsieh, B.A.; Lin, Y.S. Protection Against Dengue Virus Infection in Mice by Administration of Antibodies Against Modified Nonstructural Protein 1. PLoS ONE 2014, 9, e92495. [Google Scholar] [CrossRef]
- Wan, S.W.; Chen, P.W.; Chen, C.Y.; Lai, Y.C.; Chu, Y.T.; Hung, C.Y.; Lee, H.; Wu, H.F.; Chuang, Y.C.; Lin, J.; et al. Therapeutic Effects of Monoclonal Antibody Against Dengue Virus NS1 in a STAT1 Knockout Mouse Model of Dengue Infection. J. Immunol. 2017, 199, 2834–2844. [Google Scholar] [CrossRef]
- Setthapramote, C.; Sasaki, T.; Puiprom, O.; Limkittikul, K.; Pitaksajjakul, P.; Pipattanaboon, C.; Sasayama, M.; Leuangwutiwong, P.; Phumratanaprapin, W.; Chamnachanan, S.; et al. Human Monoclonal Antibodies to Neutralize All Dengue Virus Serotypes Using Lymphocytes from Patients at Acute Phase of the Secondary Infection. Biochem. Biophys. Res. Commun. 2012, 423, 867–872. [Google Scholar] [CrossRef]
- Schlesinger, J.J.; Brandriss, M.W.; Walsh, E.E. Protection Against 17-D Yellow Fever Encephalitis in Mice by Passive Transfer of Monoclonal Antibodies to the Nonstructural Glycoprotein gp48 and by Active Immunization with gp48. J. Immunol. 1985, 135, 2805–2809. [Google Scholar] [CrossRef]
- Lin, Y.L.; Chen, L.K.; Liao, C.L.; Yeh, C.T.; Ma, S.H.; Chen, J.L.; Huang, Y.L.; Chen, S.S.; Chiang, H.Y. DNA Immunization with Japanese Encephalitis Virus Nonstructural Protein NS1 Elicits Protective Immunity in Mice. J. Virol. 1998, 72, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Kitai, Y.; Kondo, T.; Konishi, E. Complement-Dependent Cytotoxicity Assay for Differentiating West Nile Virus from Japanese Encephalitis Virus Infections in Horses. Clin. Vaccine Immunol. 2010, 17, 875–878. [Google Scholar] [CrossRef]
- Rothman, A.L. Immunity to Dengue Virus: A Tale of Original Antigenic sin and Tropical Cytokine Storms. Nat. Rev. Immunol. 2011, 11, 532–543. [Google Scholar] [CrossRef]
- Appanna, R.; Wang, S.M.; Ponnampalavanar, S.A.; Lum, L.C.; Sekaran, S.D. Cytokine Factors Present in Dengue Patient Sera Induces Alterations of Junctional Proteins in Human Endothelial Cells. Am. J. Trop. Med. Hyg. 2012, 87, 936–942. [Google Scholar] [CrossRef]
- Dewi, B.E.; Takasaki, T.; Kurane, I. In Vitro Assessment of Human Endothelial Cell Permeability: Effects of Inflammatory Cytokines and Dengue Virus Infection. J. Virol. Methods 2004, 121, 171–180. [Google Scholar] [CrossRef]
- Lee, Y.R.; Liu, M.T.; Lei, H.Y.; Liu, C.C.; Wu, J.M.; Tung, Y.C.; Lin, Y.S.; Yeh, T.M.; Chen, S.H.; Liu, H.S. MCP-1, a Highly Expressed Chemokine in Dengue Haemorrhagic Fever/Dengue Shock Syndrome Patients, May Cause Permeability Change, Possibly Through Reduced Tight Junctions of Vascular Endothelium Cells. J. Gen. Virol. 2006, 87, 3623–3630. [Google Scholar] [CrossRef] [PubMed]
- Puerta-Guardo, H.; Glasner, D.R.; Harris, E. Dengue Virus NS1 Disrupts the Endothelial Glycocalyx, Leading to Hyperpermeability. PLoS Pathog. 2016, 12, e1005738. [Google Scholar] [CrossRef] [PubMed]
- Alcon, S.; Talarmin, A.; Debruyne, M.; Falconar, A.; Deubel, V.; Flamand, M. Enzyme-Linked Immunosorbent Assay Specific to Dengue Virus Type 1 Nonstructural Protein NS1 Reveals Circulation of the Antigen in the Blood During the Acute Phase of Disease in Patients Experiencing Primary or Secondary Infections. J. Clin. Microbiol. 2002, 40, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.F.; Lei, H.Y.; Shiau, A.L.; Liu, H.S.; Yeh, T.M.; Chen, S.H.; Liu, C.C.; Chiu, S.C.; Lin, Y.S. Endothelial Cell Apoptosis Induced by Antibodies Against Dengue Virus Nonstructural Protein 1 via Production of Nitric Oxide. J. Immunol. 2002, 169, 657–664. [Google Scholar] [CrossRef]
- Lin, C.F.; Lei, H.Y.; Shiau, A.L.; Liu, C.C.; Liu, H.S.; Yeh, T.M.; Chen, S.H.; Lin, Y.S. Antibodies from Dengue Patient Sera Cross-React with Endothelial Cells and Induce Damage. J. Med. Virol. 2003, 69, 82–90. [Google Scholar] [CrossRef]
- Lin, Y.S.; Yeh, T.M.; Lin, C.F.; Wan, S.W.; Chuang, Y.C.; Hsu, T.K.; Liu, H.S.; Liu, C.C.; Anderson, R.; Lei, H.Y. Molecular Mimicry Between Virus and Host and Its Implications for Dengue Disease Pathogenesis. Exp. Biol. Med. (Maywood) 2011, 236, 515–523. [Google Scholar] [CrossRef]
- Cheng, H.J.; Lin, C.F.; Lei, H.Y.; Liu, H.S.; Yeh, T.M.; Luo, Y.H.; Lin, Y.S. Proteomic Analysis of Endothelial Cell Autoantigens Recognized by Anti-dengue Virus Nonstructural Protein 1 Antibodies. Exp. Biol. Med. (Maywood) 2009, 234, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.C.; Lin, C.F.; Lei, H.Y.; Lin, S.C.; Liu, H.S.; Yeh, T.M.; Anderson, R.; Lin, Y.S. Deletion of the C-Terminal Region of Dengue Virus Nonstructural Protein 1 (NS1) Abolishes Anti-NS1-Mediated Platelet Dysfunction and Bleeding Tendency. J. Immunol. 2009, 183, 1797–1803. [Google Scholar] [CrossRef]
- De Alwis, R.; Smith, S.A.; Olivarez, N.P.; Messer, W.B.; Huynh, J.P.; Wahala, W.M.; White, L.J.; Diamond, M.S.; Baric, R.S.; Crowe, J.E., Jr.; et al. Identification of Human Neutralizing Antibodies That Bind to Complex Epitopes on Dengue Virions. Proc. Natl. Acad. Sci. USA 2012, 109, 7439–7444. [Google Scholar] [CrossRef]
- Barba-Spaeth, G.; Dejnirattisai, W.; Rouvinski, A.; Vaney, M.C.; Medits, I.; Sharma, A.; Simon-Lorière, E.; Sakuntabhai, A.; Cao-Lormeau, V.M.; Haouz, A.; et al. Structural Basis of Potent Zika-Dengue Virus Antibody Cross-Neutralization. Nature 2016, 536, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Halstead, S.B. Neutralization and Antibody-Dependent Enhancement of Dengue Viruses. Adv. Virus Res. 2003, 60, 421–467. [Google Scholar] [CrossRef]
- Sridhar, S.; Luedtke, A.; Langevin, E.; Zhu, M.; Bonaparte, M.; Machabert, T.; Savarino, S.; Zambrano, B.; Moureau, A.; Khromava, A.; et al. Effect of Dengue Serostatus on Dengue Vaccine Safety and Efficacy. N. Engl. J. Med. 2018, 379, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, J.J.; Foltzer, M.; Chapman, S. The Fc Portion of Antibody to Yellow Fever Virus NS1 Is a Determinant of Protection Against YF Encephalitis in Mice. Virology 1993, 192, 132–141. [Google Scholar] [CrossRef]
- Chung, K.M.; Nybakken, G.E.; Thompson, B.S.; Engle, M.J.; Marri, A.; Fremont, D.H.; Diamond, M.S. Antibodies Against West Nile Virus Nonstructural Protein NS1 Prevent Lethal Infection Through Fc Gamma Receptor-Dependent and -Independent Mechanisms. J. Virol. 2006, 80, 1340–1351. [Google Scholar] [CrossRef]
- Henchal, E.A.; Henchal, L.S.; Schlesinger, J.J. Synergistic Interactions of Anti-NS1 Monoclonal Antibodies Protect Passively Immunized Mice from Lethal Challenge with Dengue 2 Virus. J. Gen. Virol. 1988, 69, 2101–2107. [Google Scholar] [CrossRef] [PubMed]
- Falgout, B.; Bray, M.; Schlesinger, J.J.; Lai, C.J. Immunization of Mice with Recombinant Vaccinia Virus Expressing Authentic Dengue Virus Nonstructural Protein NS1 Protects Against Lethal Dengue Virus Encephalitis. J. Virol. 1990, 64, 4356–4363. [Google Scholar] [CrossRef]
- Puerta-Guardo, H.; Glasner, D.R.; Espinosa, D.A.; Biering, S.B.; Patana, M.; Ratnasiri, K.; Wang, C.; Beatty, P.R.; Harris, E. Flavivirus NS1 Triggers Tissue-Specific Vascular Endothelial Dysfunction Reflecting Disease Tropism. Cell Rep. 2019, 26, 1598–1613. [Google Scholar] [CrossRef] [PubMed]
- Puerta-Guardo, H.; Tabata, T.; Petitt, M.; Dimitrova, M.; Glasner, D.R.; Pereira, L.; Harris, E.; Virus, Z. Zika Virus Nonstructural Protein 1 Disrupts Glycosaminoglycans and Causes Permeability in Developing Human Placentas. J. Infect. Dis. 2020, 221, 313–324. [Google Scholar] [CrossRef]
- Falconar, A.K. The Dengue Virus nonstructural-1 Protein (NS1) Generates Antibodies to Common Epitopes on Human Blood Clotting, Integrin/Adhesin Proteins and Binds to Human Endothelial Cells: Potential Implications in Haemorrhagic Fever Pathogenesis. Arch. Virol. 1997, 142, 897–916. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.J.; Li, S.J.; Chen, S.C.; Liu, H.S.; Lin, Y.S.; Yeh, T.M.; Liu, C.C.; Lei, H.Y. Manifestation of Thrombocytopenia in dengue-2-virus-infected Mice. J. Gen. Virol. 2000, 81, 2177–2182. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.J.; Lei, H.Y.; Lin, C.F.; Luo, Y.H.; Wan, S.W.; Liu, H.S.; Yeh, T.M.; Lin, Y.S. Anti-dengue Virus Nonstructural Protein 1 Antibodies Recognize Protein Disulfide Isomerase on Platelets and Inhibit Platelet Aggregation. Mol. Immunol. 2009, 47, 398–406. [Google Scholar] [CrossRef]
- Chuang, Y.C.; Lei, H.Y.; Lin, Y.S.; Liu, H.S.; Wu, H.L.; Yeh, T.M. Dengue Virus-Induced Autoantibodies Bind to Plasminogen and Enhance Its Activation. J. Immunol. 2011, 187, 6483–6490. [Google Scholar] [CrossRef]
- Chuang, Y.C.; Lin, Y.S.; Liu, H.S.; Wang, J.R.; Yeh, T.M. Antibodies Against Thrombin in Dengue Patients Contain Both Anti-thrombotic and Pro-fibrinolytic Activities. Thromb. Haemost. 2013, 110, 358–365. [Google Scholar] [CrossRef]
- Smith, J.L.; Flavivirus, N.S. Flavivirus NS1: Structure and Function of an Enigmatic Virulence Factor. FASEB J. 2022, 36 (Suppl. S1). [Google Scholar] [CrossRef]
- Poungpair, O.; Bangphoomi, K.; Chaowalit, P.; Sawasdee, N.; Saokaew, N.; Choowongkomon, K.; Chaicumpa, W.; Yenchitsomanus, P.T. Generation of Human Single-Chain Variable Fragment Antibodies Specific to Dengue Virus Non-structural Protein 1 That Interfere with the Virus Infectious Cycle. Mabs 2014, 6, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.; Li, G.; Shen, M.; Yu, Z.; Ge, W.; Lao, Z.; Fan, Y.; Chen, K.; Ding, Z.; Wang, W.; et al. DENV NS1 and MMP-9 Cooperate to Induce Vascular Leakage by Altering Endothelial Cell Adhesion and Tight Junction. PLoS Pathog. 2021, 17, e1008603. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ng, M.M.; Chu, J.J. Activation of TLR2 and TLR6 by Dengue NS1 Protein and Its Implications in the Immunopathogenesis of Dengue Virus Infection. PLoS Pathog. 2015, 11, e1005053. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.T.; Wan, S.W.; Chang, Y.C.; Lee, C.K.; Wu-Hsieh, B.A.; Anderson, R.; Lin, Y.S. Antibodies Against Nonstructural Protein 1 Protect Mice from Dengue Virus-Induced Mast Cell Activation. Lab. Investig. 2017, 97, 602–614. [Google Scholar] [CrossRef]
- Patro, A.R.K.; Mohanty, S.; Prusty, B.K.; Singh, D.K.; Gaikwad, S.; Saswat, T.; Chattopadhyay, S.; Das, B.K.; Tripathy, R.; Ravindran, B. Cytokine Signature Associated with Disease Severity in Dengue. Viruses 2019, 11, 34. [Google Scholar] [CrossRef] [PubMed]
- Rathakrishnan, A.; Wang, S.M.; Hu, Y.; Khan, A.M.; Ponnampalavanar, S.; Lum, L.C.; Manikam, R.; Sekaran, S.D. Cytokine Expression Profile of Dengue Patients at Different Phases of Illness. PLoS ONE 2012, 7, e52215. [Google Scholar] [CrossRef] [PubMed]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte Chemoattractant Protein-1 (MCP-1): An Overview. J. Interferon Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef]
- Sierra, B.; Perez, A.B.; Vogt, K.; Garcia, G.; Schmolke, K.; Aguirre, E.; Alvarez, M.; Volk, H.D.; Guzman, M.G. MCP-1 and MIP-1Alpha Expression in a Model Resembling Early Immune Response to Dengue. Cytokine 2010, 52, 175–183. [Google Scholar] [CrossRef]
- Kelley, J.F.; Kaufusi, P.H.; Nerurkar, V.R. Dengue Hemorrhagic Fever-Associated Immunomediators Induced via Maturation of Dengue Virus Nonstructural 4B Protein in Monocytes Modulate Endothelial Cell Adhesion Molecules and Human Microvascular Endothelial Cells Permeability. Virology 2012, 422, 326–337. [Google Scholar] [CrossRef]
- Soe, H.J.; Khan, A.M.; Manikam, R.; Samudi Raju, C.; Vanhoutte, P.; Sekaran, S.D. High Dengue Virus Load Differentially Modulates Human Microvascular Endothelial Barrier Function During Early Infection. J. Gen. Virol. 2017, 98, 2993–3007. [Google Scholar] [CrossRef]
- Talavera, D.; Castillo, A.M.; Dominguez, M.C.; Gutierrez, A.E.; Meza, I. IL8 Release, Tight Junction and Cytoskeleton Dynamic Reorganization Conducive to Permeability Increase Are Induced by Dengue Virus Infection of Microvascular Endothelial Monolayers. J. Gen. Virol. 2004, 85, 1801–1813. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.C.; Lei, H.Y.; Liu, H.S.; Lin, Y.S.; Fu, T.F.; Yeh, T.M. Macrophage Migration Inhibitory Factor Induced by Dengue Virus Infection Increases Vascular Permeability. Cytokine 2011, 54, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Glasner, D.R.; Ratnasiri, K.; Puerta-Guardo, H.; Espinosa, D.A.; Beatty, P.R.; Harris, E. Dengue Virus NS1 Cytokine-Independent Vascular Leak Is Dependent on Endothelial Glycocalyx Components. PLoS Pathog. 2017, 13, e1006673. [Google Scholar] [CrossRef] [PubMed]
- Pries, A.R.; Kuebler, W.M. Normal Endothelium. Handb. Exp. Pharmacol. 2006, 176 Pt 1, 1–40. [Google Scholar] [CrossRef]
- Page, A.V.; Liles, W.C. Biomarkers of Endothelial Activation/Dysfunction in Infectious Diseases. Virulence 2013, 4, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, J.J.; Brandriss, M.W.; Walsh, E.E. Protection of Mice Against Dengue 2 Virus Encephalitis by Immunization with the Dengue 2 Virus Non-structural Glycoprotein NS1. J. Gen. Virol. 1987, 68, 853–857. [Google Scholar] [CrossRef]
- Modhiran, N.; Song, H.; Liu, L.; Bletchly, C.; Brillault, L.; Amarilla, A.A.; Xu, X.; Qi, J.; Chai, Y.; Cheung, S.T.M.; et al. A Broadly Protective Antibody That Targets the Flavivirus NS1 Protein. Science 2021, 371, 190–194. [Google Scholar] [CrossRef]
- Biering, S.B.; Akey, D.L.; Wong, M.P.; Brown, W.C.; Lo, N.T.N.; Puerta-Guardo, H.; Tramontini Gomes de Sousa, F.; Wang, C.; Konwerski, J.R.; Espinosa, D.A.; et al. Structural Basis for Antibody Inhibition of Flavivirus NS1-Triggered Endothelial Dysfunction. Science 2021, 371, 194–200. [Google Scholar] [CrossRef]







| Patients | Gender | Age | Diagnosis | Blood Collection | Rapid Test | PCR Serotyping | HuMAbs | ||
|---|---|---|---|---|---|---|---|---|---|
| Days | Phase | IgG | IgM | ||||||
| D25 | Male | 27 | DF | 14 | Convalescent | + | + | DENV2 | 8, M1, M20 |
| D26 | Female | 33 | DF | 19 | Convalescent | + | + | DENV2 | 238 |
| Primer | Sequence |
|---|---|
| DVNS1 60-Fw | 5-GGC GGA TCC ATG GTA ACA AGA CTG GAA AAT CTG-3 |
| DVNS1 120-Fw | 5-GGC GGA TCC ATG AAA GCG AAA ATG CTC TCT ACA GAG-3 |
| DVNS1 221-Fw | 5-GGC GGA TCC ATG AAA AGC TGC CAC TGG CCA AAG-3 |
| DVNS1 300-Fw | 5-GGC GGA TCC ATG ACA ACT ACT GCC TCT GGA AAA CTC-3 |
| DVNS1 352-Rv | 5-CCG CTC GAG TTA GGC TGT GAC CAA GGA GTT GAC-3 |
| Cytokines | NS1 | NS1 Plus Anti-E mAb | NS1 Plus DENV NS1 HuMAb-Clone M20 | NS1 Plus DENV NS1 HuMAb-Clone 8 | ||||
|---|---|---|---|---|---|---|---|---|
| DENV | ZIKV | DENV | ZIKV | DENV | ZIKV | DENV | ZIKV | |
| IL-1β | NS | NS | NS | NS | NS | NS | NS | NS |
| IL-1ra | NS | NS | NS | NS | NS | NS | NS | NS |
| IL-2 | NS | NS | NS | NS | NS | NS | NS | NS |
| IL-4 | NS | NS | NS | NS | NS | NS | NS | NS |
| IL-5 | NS | NS | NS | NS | NS | NS | NS | NS |
| IL-6 | NS | NS | NS | NS | NS | NS | NS | NS |
| IL-7 | NS | NS | NS | NS | NS | NS | NS | NS |
| IL-8 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| IL-9 | NS | NS | NS | NS | NS | NS | NS | NS |
| IL-10 | NS | NS | NS | NS | NS | NS | NS | NS |
| IL-12 | NS | NS | NS | NS | NS | NS | NS | NS |
| IL-13 | NS | NS | NS | NS | NS | NS | NS | NS |
| IL-15 | NS | NS | NS | NS | NS | NS | NS | NS |
| IL-17 | NS | NS | NS | NS | NS | NS | NS | NS |
| Eotaxin | NS | NS | NS | NS | NS | NS | NS | NS |
| FGF-β | NS | NS | NS | NS | NS | NS | NS | NS |
| G-CSF | NS | NS | NS | NS | NS | NS | NS | NS |
| GM-CSF | NS | NS | NS | NS | NS | NS | NS | NS |
| IFN-γ | NS | NS | NS | NS | NS | NS | NS | NS |
| IP-10 | <0.01 | <0.01 | <0.01 | NS | <0.05 | <0.05 | <0.05 | <0.05 |
| MCP-1 | <0.01 | <0.05 | <0.01 | NS | <0.01 | <0.05 | <0.01 | NS |
| MIP-1α | NS | NS | NS | NS | NS | NS | NS | NS |
| PDGF | NS | NS | NS | NS | NS | NS | NS | NS |
| MIP-1β | NS | NS | NS | NS | NS | NS | NS | NS |
| RANTES | NS | NS | NS | NS | NS | NS | NS | NS |
| TNF-α | NS | NS | NS | NS | NS | NS | NS | NS |
| VEGF | NS | NS | NS | NS | NS | NS | NS | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sootichote, R.; Puangmanee, W.; Benjathummarak, S.; Kowaboot, S.; Yamanaka, A.; Boonnak, K.; Ampawong, S.; Chatchen, S.; Ramasoota, P.; Pitaksajjakul, P. Potential Protective Effect of Dengue NS1 Human Monoclonal Antibodies against Dengue and Zika Virus Infections. Biomedicines 2023, 11, 227. https://doi.org/10.3390/biomedicines11010227
Sootichote R, Puangmanee W, Benjathummarak S, Kowaboot S, Yamanaka A, Boonnak K, Ampawong S, Chatchen S, Ramasoota P, Pitaksajjakul P. Potential Protective Effect of Dengue NS1 Human Monoclonal Antibodies against Dengue and Zika Virus Infections. Biomedicines. 2023; 11(1):227. https://doi.org/10.3390/biomedicines11010227
Chicago/Turabian StyleSootichote, Rochanawan, Wilarat Puangmanee, Surachet Benjathummarak, Siriporn Kowaboot, Atsushi Yamanaka, Korbporn Boonnak, Sumate Ampawong, Supawat Chatchen, Pongrama Ramasoota, and Pannamthip Pitaksajjakul. 2023. "Potential Protective Effect of Dengue NS1 Human Monoclonal Antibodies against Dengue and Zika Virus Infections" Biomedicines 11, no. 1: 227. https://doi.org/10.3390/biomedicines11010227
APA StyleSootichote, R., Puangmanee, W., Benjathummarak, S., Kowaboot, S., Yamanaka, A., Boonnak, K., Ampawong, S., Chatchen, S., Ramasoota, P., & Pitaksajjakul, P. (2023). Potential Protective Effect of Dengue NS1 Human Monoclonal Antibodies against Dengue and Zika Virus Infections. Biomedicines, 11(1), 227. https://doi.org/10.3390/biomedicines11010227





