Subcortical Structures in Demented Schizophrenia Patients: A Comparative Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Cognitive Evaluation
2.3. Structural Reconstruction
2.4. Cortical Surface-Based Analysis
2.4.1. FreeSurfer 6
2.4.2. Cortical and Volumetric Segmentation
2.4.3. Statistical Analysis
3. Results
3.1. Structural Analysis
3.1.1. Hippocampus
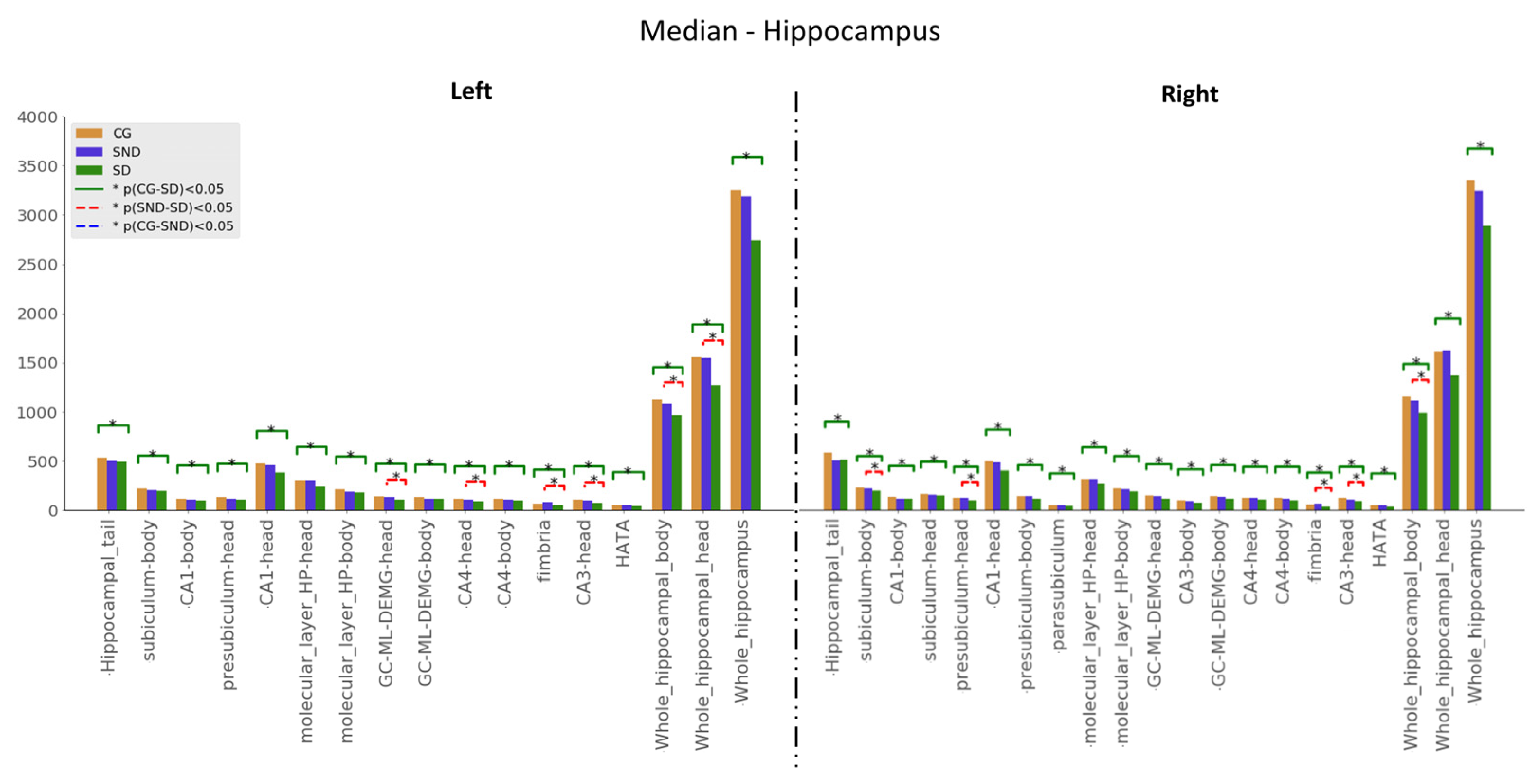
3.1.2. Thalamus
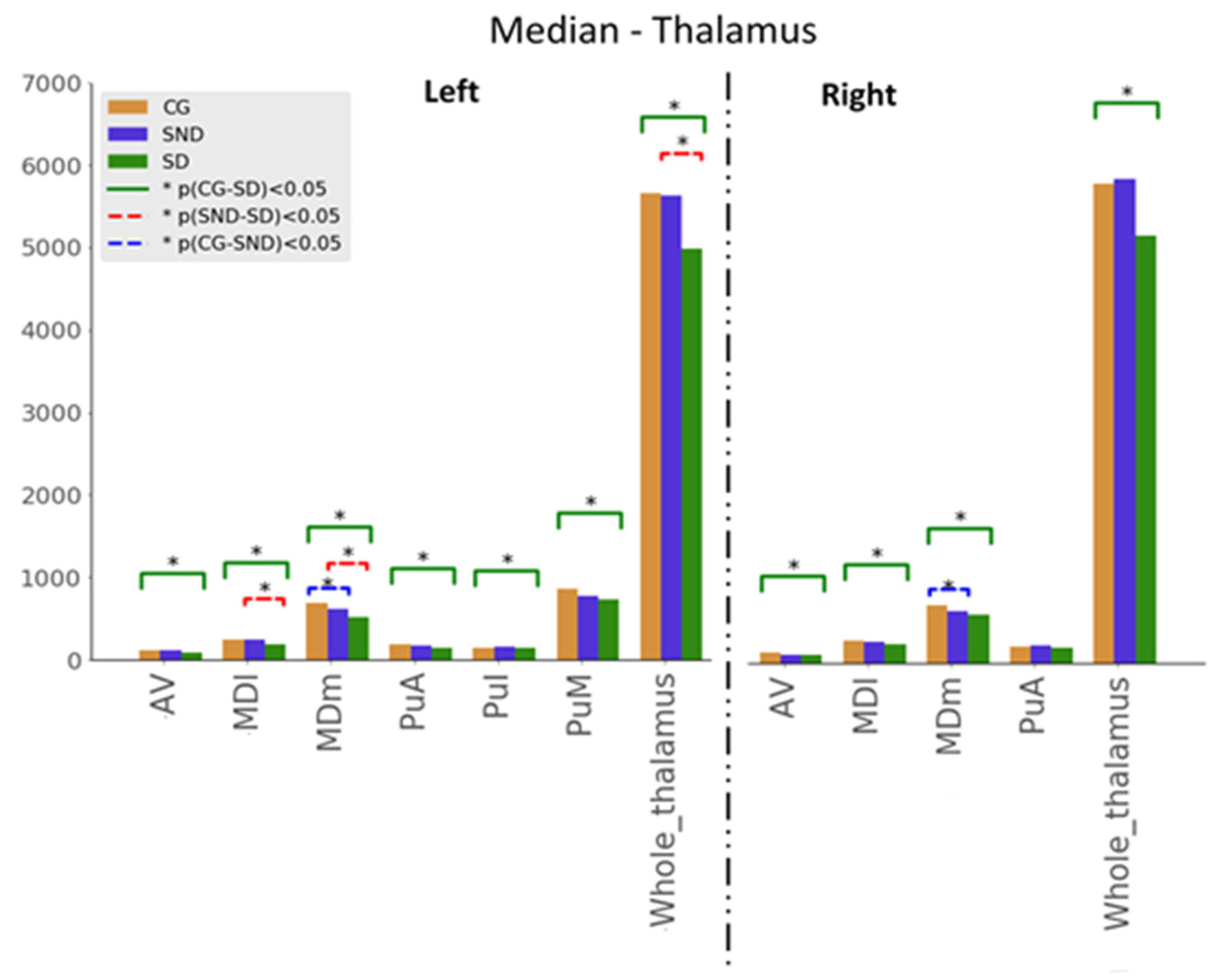
3.1.3. Amygdala
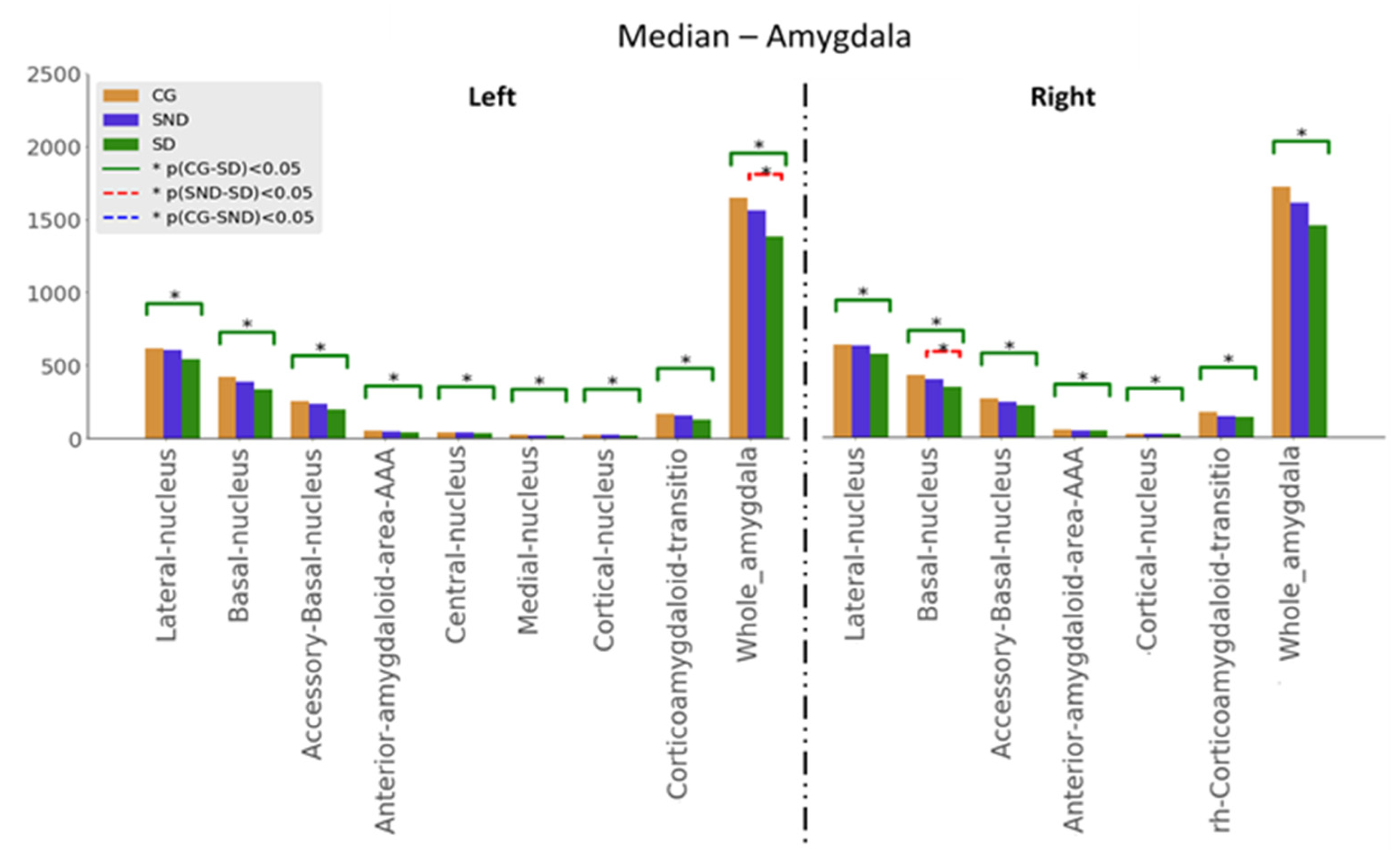
3.2. Structure vs. Function Analysis
3.2.1. Hippocampus

3.2.2. Thalamus
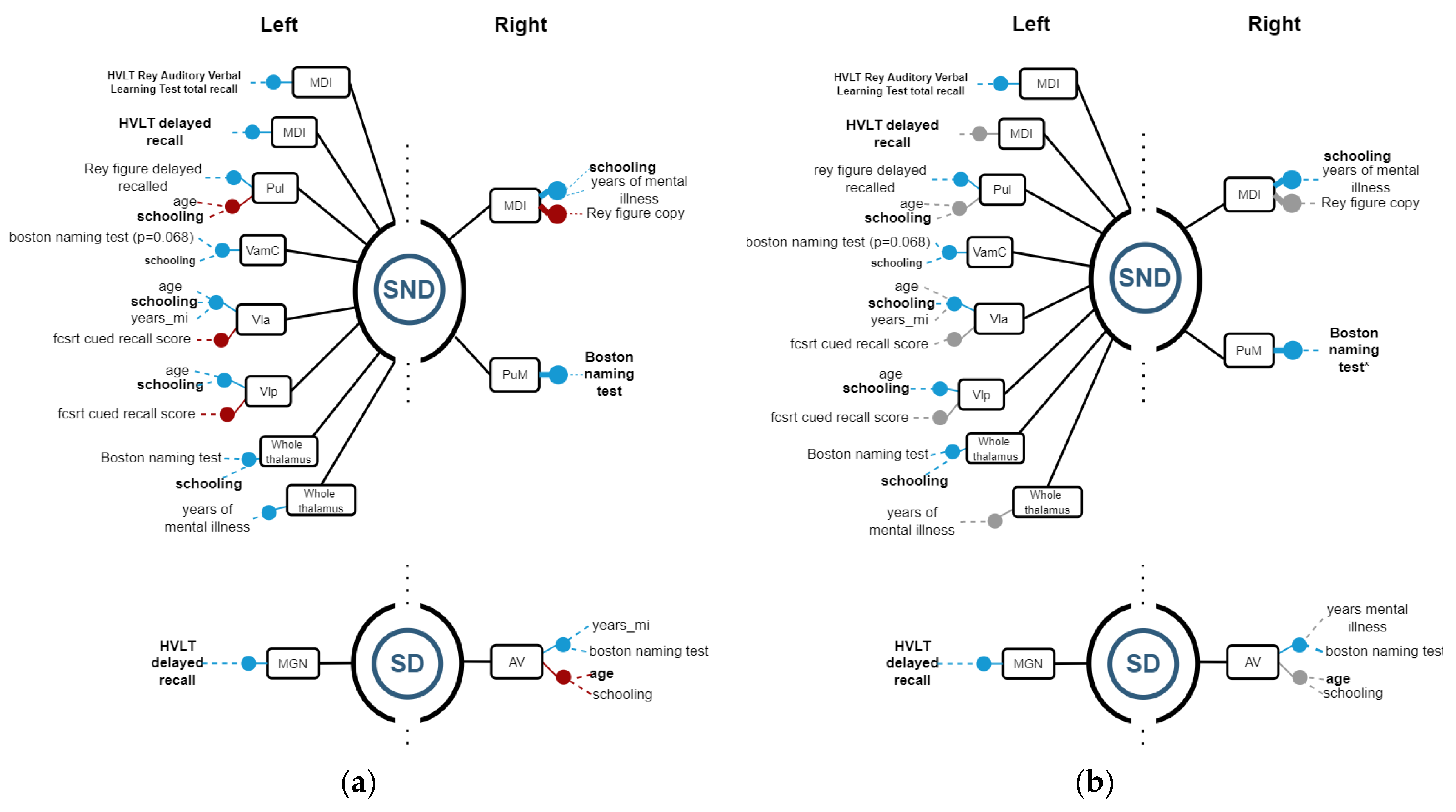
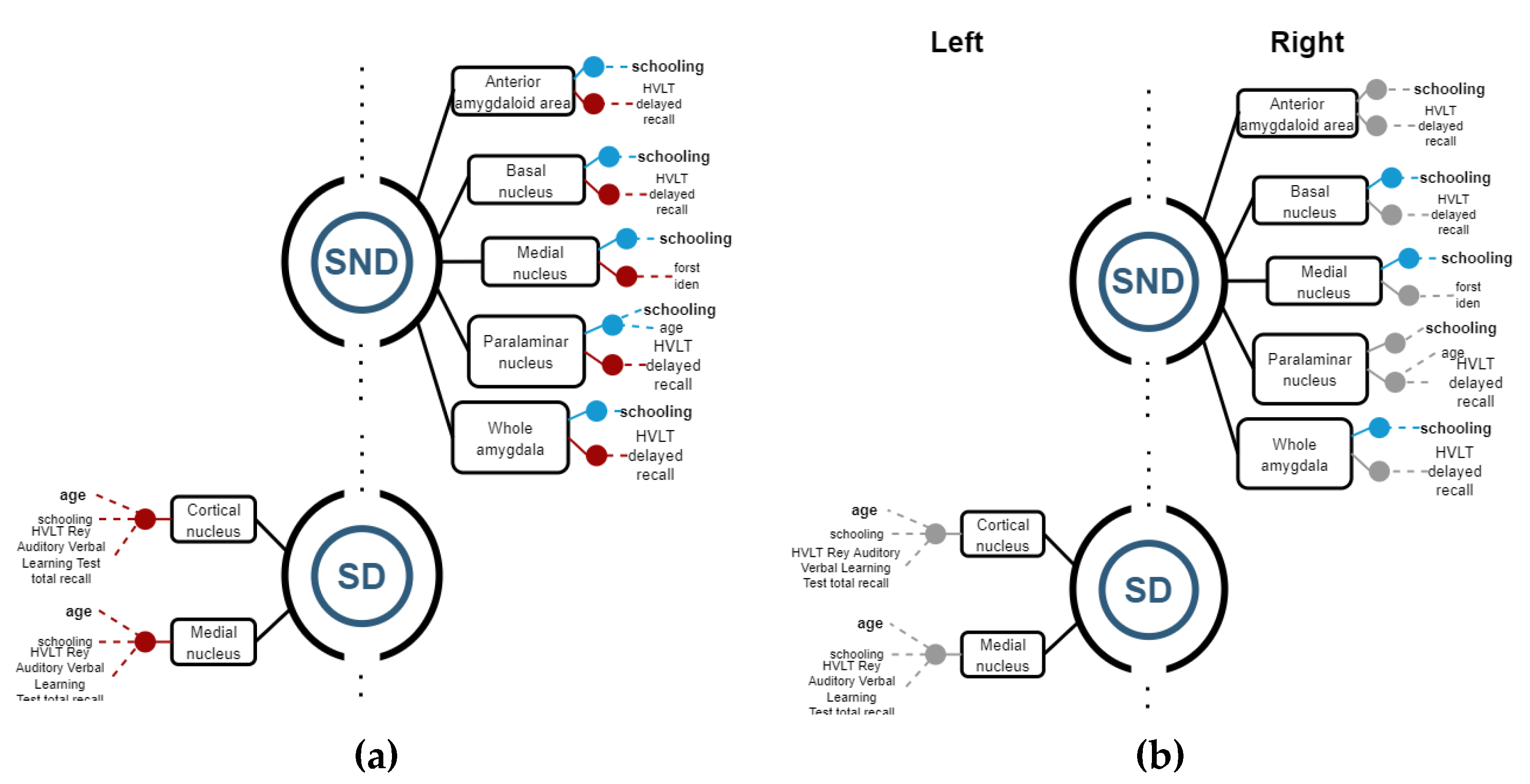
3.2.3. Amygdala
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Variable | SND Q2 (Q1–Q3) | SD Q2 (Q1–Q3) | p-Value |
|---|---|---|---|
| P1 Delusion | 4.5 (2.75–5) | 3.5 (3–5.25) | 1.000 |
| P2 Conceptual disorganisation | 3.5 (2.75–4.25) | 4.5 (3.75–5) | 0.078 |
| P3 Hallucinatory behaviour | 2.5 (2–4) | 2.5 (2–4.25) | 0.522 |
| P4 Excitement | 2 (2–2.25) | 3 (2–3.25) | 0.072 |
| P5 Grandiosity | 2.5 (2–3.25) | 3 (2–3.25) | 0.684 |
| P6 Suspiciousness/persecution | 4 (3–4) | 4 (2–5) | 0.905 |
| P7 Hostility | 2 (1.75–3) | 2 (2–4) | 0.245 |
| N1 Blunted affect | 5 (3.75–6) | 4 (3.75–6) | 0.580 |
| N2 Emotional withdrawal | 5 (3.75–6) | 5 (3.75–6) | 0.937 |
| N3 Poor rapport | 5 (3.5–6) | 5 (3.75–6) | 0.724 |
| N4 Passive/apathetic socialwithdrawal | 5 (3.5–5.25) | 5 (4–5.25) | 0.691 |
| N5 Difficulty in abstract thinking | 5 (4.5–5) | 6 (5.5–6) | 0.015 |
| N6 Lack of spontaneity andflow of conversation | 5 (3.75–6) | 6 (4.75–6) | 0.094 |
| N7 Stereotyped thinking | 5 (3.75–5) | 6 (4.75–6) | 0.039 |
| G1 Somatic concern | 4 (3–4.25) | 5 (4–6) | 0.056 |
| G2 Anxiety | 3 (2–4) | 4 (3.75–5) | 0.034 |
| G3 Guilt feelings | 2 (1.75–2) | 2 (2–3) | 0.061 |
| G4 Tension | 3 (2–4.25) | 3 (2–4) | 1.000 |
| G5 Mannerisms and posturing | 3 (2–3) | 2 (2–3) | 0.622 |
| G6 Depression | 2 (2–3) | 2.5 (2–4) | 0.270 |
| G7 Motor retardation | 3.5 (2–4) | 3 (2–4) | 0.872 |
| G8 Uncooperativeness | 3 (2–4) | 5 (4–6) | 0.009 |
| G9 Unusual thought content | 5 (2.75–5) | 5.5 (3.75–6) | 0.138 |
| G10 Disorientation | 2 (2–2) | 6 (5.75–6) | 0.001 |
| G 11 Poor attention | 2 (2–3) | 6 (5–6) | 0.001 |
| G12 Lack of judgement and insight | 4 (3.75–5) | 6 (5–6) | 0.001 |
| G13 Disturbance of volition | 4.5 (3.75–5.25) | 6 (5.75–6) | 0.008 |
| G14 Poor impulse control | 2.5 (2–3.25) | 5 (3.5–5.25) | 0.016 |
| G15 Preoccupation | 2.5 (2–4.25) | 4 (3.5–5.25) | 0.072 |
| G16 Active social avoidance | 5 (3–5.25) | 6 (4.75–6) | 0.068 |
| Total P—PANSS | 21 (16.75–24.25) | 22.5 (17.75–29.25) | 0.343 |
| Total N—PANSS | 35 (27.25–38.5) | 36.5 (32.5–39.5) | 0.472 |
| Total G—PANSS | 53 (42–55.75) | 67 (64.75–77.5) | 0.001 |
Appendix B
| Variable | CG | SND | SD |
|---|---|---|---|
| lh-Hippocampal_tail | 533.1768 | 502.179 | 491.2918 |
| lh-subiculum-body | 221.7534 | 209.4655 | 198.4704 |
| lh-CA1-body | 118.8101 | 108.485 | 102.6303 |
| lh-subiculum-head | 184.9953 | 178.9422 | 165.2528 |
| lh-hippocampal-fissure | 138.3868 | 137.3062 | 155.3961 |
| lh-presubiculum-head | 134.1008 | 120.8248 | 109.1787 |
| lh-CA1-head | 474.0209 | 460.1142 | 384.2375 |
| lh-presubiculum-body | 153.4684 | 147.7923 | 125.0648 |
| lh-parasubiculum | 56.16448 | 50.60878 | 51.01351 |
| lh-molecular_layer_HP-head | 301.2178 | 305.4716 | 247.7981 |
| lh-molecular_layer_HP-body | 213.2131 | 192.8378 | 181.2546 |
| lh-GC-ML-DEMG-head | 138.8135 | 131.2461 | 111.1553 |
| lh-CA3-body | 83.18301 | 75.73118 | 77.4677 |
| lh-GC-ML-DEMG-body | 129.4957 | 116.4267 | 113.3807 |
| lh-CA4-head | 116.6823 | 110.3765 | 92.76147 |
| lh-CA4-body | 116.1934 | 106.2959 | 103.3087 |
| lh-fimbria | 66.43488 | 81.87439 | 50.07478 |
| lh-CA3-head | 109.5836 | 99.03824 | 75.07533 |
| lh-HATA | 52.55361 | 53.39614 | 41.60395 |
| lh-Whole_hippocampal_body | 1122.199 | 1083.858 | 966.5157 |
| lh-Whole_hippocampal_head | 1553.4 | 1545.423 | 1272.626 |
| lh-Whole_hippocampus | 3250.59 | 3191.603 | 2744.368 |
| rh-Hippocampal_tail | 580.5496 | 505.6549 | 510.067 |
| rh-subiculum-body | 231.9206 | 227.1127 | 199.9714 |
| rh-CA1-body | 134.9102 | 115.9702 | 116.1926 |
| rh-subiculum-head | 168.9085 | 162.5255 | 153.7425 |
| rh-hippocampal-fissure | 156.8318 | 145.9764 | 174.7104 |
| rh-presubiculum-head | 124.5519 | 122.7295 | 103.8624 |
| rh-CA1-head | 497.8928 | 489.8866 | 406.8071 |
| rh-presubiculum-body | 142.5037 | 138.6225 | 119.0961 |
| rh-parasubiculum | 55.66079 | 54.70945 | 43.47544 |
| rh-molecular_layer_HP-head | 317.6802 | 313.378 | 270.7611 |
| rh-molecular_layer_HP-body | 226.9824 | 213.1586 | 187.8682 |
| rh-GC-ML-DEMG-head | 150.4251 | 145.2717 | 119.7443 |
| rh-CA3-body | 98.27856 | 96.03128 | 78.87846 |
| rh-GC-ML-DEMG-body | 138.4234 | 133.6741 | 113.8646 |
| rh-CA4-head | 124.3385 | 122.6584 | 106.5683 |
| rh-CA4-body | 122.7553 | 117.8695 | 103.7635 |
| rh-fimbria | 64.06755 | 65.34351 | 38.52792 |
| rh-CA3-head | 122.4057 | 107.8549 | 94.62282 |
| rh-HATA | 54.12791 | 52.05819 | 37.70217 |
| rh-Whole_hippocampal_body | 1157.326 | 1109.135 | 985.3122 |
| rh-Whole_hippocampal_head | 1600.391 | 1616.814 | 1371.619 |
| rh-Whole_hippocampus | 3334.209 | 3221.467 | 2877.293 |
| Variable | CG Q2 | SND Q2 | SD Q2 |
|---|---|---|---|
| lf-AV | 123.2183 | 113.6766 | 95.21425 |
| lf-CeM | 58.49963 | 54.05158 | 44.02321 |
| lf-CL | 32.81186 | 30.63348 | 27.25588 |
| lf-CM | 224.751 | 256.3122 | 223.2634 |
| lf-LD | 25.71389 | 25.69633 | 16.54721 |
| lf-LGN | 155.1749 | 142.4712 | 114.0722 |
| lf-LP | 113.6153 | 110.5227 | 99.43813 |
| lf-L-Sg | 17.44105 | 22.1667 | 24.09774 |
| lf-MDl | 252.15 | 241.727 | 193.1979 |
| lf-MDm | 685.4231 | 613.685 | 511.9704 |
| lf-MGN | 110.7312 | 96.8326 | 92.98687 |
| lf-MV(Re) | 11.19719 | 9.730469 | 8.056666 |
| lf-Pc | 3.213006 | 3.061099 | 2.771158 |
| lf-Pf | 50.18333 | 58.4608 | 50.36604 |
| lf-Pt | 6.539254 | 6.629584 | 6.239184 |
| lf-PuA | 182.463 | 175.5583 | 150.0718 |
| lf-PuI | 151.2756 | 154.531 | 138.726 |
| lf-PuL | 123.1327 | 134.7406 | 125.0957 |
| lf-PuM | 854.6671 | 779.0034 | 736.5568 |
| lf-VA | 364.8248 | 397.8157 | 340.5276 |
| lf-VAmc | 28.55659 | 31.07307 | 27.04921 |
| lf-VLa | 559.377 | 585.9731 | 534.2324 |
| lf-VLp | 699.2602 | 759.2448 | 672.0896 |
| lf-VM | 18.55997 | 19.64559 | 17.59458 |
| lf-VPL | 783.8474 | 844.3912 | 734.5783 |
| lf-Whole_thalamus | 5652.642 | 5622.447 | 4979.5 |
| rh-AV | 130.8434 | 107.5276 | 104.285 |
| rh-CeM | 62.1244 | 55.45355 | 48.06551 |
| rh-CL | 33.63634 | 28.65087 | 26.14143 |
| rh-CM | 213.33 | 221.675 | 208.5581 |
| rh-LD | 25.83505 | 18.26258 | 12.13563 |
| rh-LGN | 183.3781 | 164.859 | 134.9011 |
| rh-LP | 105.6465 | 95.88531 | 83.27562 |
| rh-L-Sg | 16.47282 | 18.80542 | 18.71431 |
| rh-MDl | 269.9493 | 253.1327 | 230.6535 |
| rh-MDm | 708.8589 | 633.1818 | 593.9285 |
| rh-MGN | 114.918 | 117.1189 | 102.668 |
| rh-MV(Re) | 11.02887 | 9.390319 | 6.728964 |
| rh-Pc | 3.374931 | 2.94308 | 2.593708 |
| rh-Pf | 48.73434 | 54.78222 | 47.45407 |
| rh-Pt | 5.996214 | 6.098988 | 5.437686 |
| rh-PuA | 208.8913 | 223.5418 | 193.5258 |
| rh-PuI | 182.626 | 203.4928 | 177.7739 |
| rh-PuL | 161.889 | 188.4466 | 163.0043 |
| rh-PuM | 956.5881 | 1002.704 | 898.1172 |
| rh-VA | 340.8498 | 370.3286 | 339.9549 |
| rh-VAmc | 29.64441 | 30.86262 | 28.17401 |
| rh-VLa | 541.2549 | 579.2112 | 519.2811 |
| rh-VLp | 700.2594 | 737.5595 | 657.7377 |
| rh-VM | 17.45699 | 19.59703 | 17.50687 |
| rh-VPL | 742.177 | 810.5024 | 704.2473 |
| rh-Whole_thalamus | 5813.964 | 5865.795 | 5178.104 |
| Variable | CG Q2 | SND Q2 | SD Q2 |
|---|---|---|---|
| lh-Lateral-nucleus | 616.3684 | 608.9339 | 542.3933 |
| lh-Basal-nucleus | 419.4339 | 390.2282 | 337.067 |
| lh-Accessory-Basal-nucleus | 257.6557 | 236.9855 | 197.4864 |
| lh-Anterior-amygdaloid-area-AAA | 51.55009 | 50.54429 | 41.65633 |
| lh-Central-nucleus | 45.46808 | 40.86922 | 39.81099 |
| lh-Medial-nucleus | 25.89562 | 21.13942 | 17.60892 |
| lh-Cortical-nucleus | 25.32682 | 25.28271 | 21.66816 |
| lh-Corticoamygdaloid-transitio | 170.3703 | 155.4683 | 127.7356 |
| lh-Paralaminar-nucleus | 45.41394 | 44.19915 | 41.82177 |
| lh-Whole_amygdala | 1644.198 | 1562.221 | 1383.873 |
| rh-Lateral-nucleus | 633.5202 | 631.8963 | 572.0321 |
| rh-Basal-nucleus | 425.5786 | 399.0396 | 346.8798 |
| rh-Accessory-Basal-nucleus | 266.5776 | 246.6755 | 220.4747 |
| rh-Anterior-amygdaloid-area-AAA | 53.56824 | 50.89934 | 46.4302 |
| rh-Central-nucleus | 46.06809 | 43.14087 | 43.48341 |
| rh-Medial-nucleus | 28.1996 | 22.82126 | 25.00227 |
| rh-Cortical-nucleus | 27.63528 | 27.46292 | 24.37196 |
| rh-Corticoamygdaloid-transitio | 172.166 | 147.3925 | 141.5533 |
| rh-Paralaminar-nucleus | 46.9591 | 42.19772 | 41.0332 |
| rh-Whole_amygdala | 1717.07 | 1604.96 | 1452.154 |
References
- Owen, M.J.; Sawa, A.; Mortensen, P.B. Schizophrenia. Lancet 2016, 388, 86–97. [Google Scholar] [CrossRef] [Green Version]
- Green, M.F.; Hellemann, G.; Horan, W.P.; Lee, J.; Wynn, J.K. From Perception to Functional Outcome in Schizophrenia: Modeling the Role of Ability and Motivation. Arch. Gen. Psychiatry 2012, 69, 1216–1224. [Google Scholar] [CrossRef] [Green Version]
- Urfer-Parnas, A.; Mortensen, E.L.; Parnas, J. Core of Schizophrenia: Estrangement, Dementia or Neurocognitive Disorder? Psychopathology 2010, 43, 300–311. [Google Scholar] [CrossRef]
- Stroup, T.S.; Olfson, M.; Huang, C.; Wall, M.M.; Goldberg, T.; Devanand, D.P.; Gerhard, T. Age-Specific Prevalence and Incidence of Dementia Diagnoses Among Older US Adults with Schizophrenia. JAMA Psychiatry 2021, 78, 632–641. [Google Scholar] [CrossRef]
- Lin, C.-E.; Chung, C.-H.; Chen, L.-F.; Chi, M.-J. Increased Risk of Dementia in Patients with Schizophrenia: A Population-Based Cohort Study in Taiwan. Eur. Psychiatry 2018, 53, 7–16. [Google Scholar] [CrossRef]
- Cai, L.; Huang, J. Schizophrenia and Risk of Dementia: A Meta-Analysis Study. Neuropsychiatr. Dis. Treat. 2018, 14, 2047–2055. [Google Scholar] [CrossRef] [Green Version]
- Herold, C.J.; Schmid, L.A.; Lässer, M.M.; Seidl, U.; Schröder, J. Cognitive Performance in Patients with Chronic Schizophrenia Across the Lifespan. GeroPsych 2017, 30, 35–44. [Google Scholar] [CrossRef]
- Palmer, B.W.; Moore, R.C.; Eyler, L.T.; Pinto, L.L.; Saks, E.R.; Jeste, D.V. Avoidance of Accelerated Aging in Schizophrenia?: Clinical and Biological Characterization of an Exceptionally High Functioning Individual. Schizophr. Res. 2018, 196, 45–52. [Google Scholar] [CrossRef]
- Sheffield, J.M.; Karcher, N.R.; Barch, D.M. Cognitive Deficits in Psychotic Disorders: A Lifespan Perspective. Neuropsychol. Rev. 2018, 28, 509–533. [Google Scholar] [CrossRef]
- Thuaire, F.; Rondepierre, F.; Bacon, E.; Vallet, G.T.; Jalenques, I.; Izaute, M. Executive Functions in Schizophrenia Aging: Differential Effects of Age within Specific Executive Functions. Cortex 2020, 125, 109–121. [Google Scholar] [CrossRef]
- Gauthier, S.; Loft, H.; Cummings, J. Improvement in Behavioural Symptoms in Patients with Moderate to Severe Alzheimer’s Disease by Memantine: A Pooled Data Analysis. Int. J. Geriatr. Psychiatry 2008, 23, 537–545. [Google Scholar] [CrossRef]
- Lyketsos, C.G.; Peters, M.E. Dementia in Patients with Schizophrenia: Evidence for Heterogeneity. JAMA Psychiatry 2015, 72, 1075–1076. [Google Scholar] [CrossRef]
- Harvey, P.D. Cognitive Impairment in Elderly Patients with Schizophrenia: Age Related Changes. Int. J. Geriatr. Psychiatry 2001, 16, S78–S85. [Google Scholar] [CrossRef]
- Davidson, M.; Harvey, P.; Welsh, K.A.; Powchik, P.; Putnam, K.M.; Mohs, R.C. Cognitive Functioning in Late-Life Schizophrenia: A Comparison of Elderly Schizophrenic Patients and Patients with Alzheimer’s Disease. Am. J. Psychiatry 1996, 153, 1274–1279. [Google Scholar] [CrossRef] [Green Version]
- Rohde, C.; Agerbo, E.; Nielsen, P.R. Does Schizophrenia in Offspring Increase the Risk of Developing Alzheimer’s Dementia. Dement. Geriatr. Cogn. Disord. Extra 2016, 6, 361–373. [Google Scholar] [CrossRef]
- Shah, J.N.; Qureshi, S.U.; Jawaid, A.; Schulz, P.E. Is There Evidence for Late Cognitive Decline in Chronic Schizophrenia? Psychiatr. Q. 2012, 83, 127–144. [Google Scholar] [CrossRef]
- Okada, N.; Fukunaga, M.; Yamashita, F.; Koshiyama, D.; Yamamori, H.; Ohi, K.; Yasuda, Y.; Fujimoto, M.; Watanabe, Y.; Yahata, N.; et al. Abnormal Asymmetries in Subcortical Brain Volume in Schizophrenia. Mol. Psychiatry 2016, 21, 1460–1466. [Google Scholar] [CrossRef] [Green Version]
- Shahab, S.; Mulsant, B.H.; Levesque, M.L.; Calarco, N.; Nazeri, A.; Wheeler, A.L.; Foussias, G.; Rajji, T.K.; Voineskos, A.N. Brain Structure, Cognition, and Brain Age in Schizophrenia, Bipolar Disorder, and Healthy Controls. Neuropsychopharmacology 2019, 44, 898–906. [Google Scholar] [CrossRef] [Green Version]
- van Erp, T.G.M.; Hibar, D.P.; Rasmussen, J.M.; Glahn, D.C.; Pearlson, G.D.; Andreassen, O.A.; Agartz, I.; Westlye, L.T.; Haukvik, U.K.; Dale, A.M.; et al. Subcortical Brain Volume Abnormalities in 2028 Individuals with Schizophrenia and 2540 Healthy Controls via the ENIGMA Consortium. Mol. Psychiatry 2016, 21, 547–553. [Google Scholar] [CrossRef]
- Narr, K.L.; Thompson, P.M.; Szeszko, P.; Robinson, D.; Jang, S.; Woods, R.P.; Kim, S.; Hayashi, K.M.; Asunction, D.; Toga, A.W.; et al. Regional Specificity of Hippocampal Volume Reductions in First-Episode Schizophrenia. NeuroImage 2004, 21, 1563–1575. [Google Scholar] [CrossRef]
- Heckers, S.; Konradi, C. Hippocampal Pathology in Schizophrenia. In Behavioral Neurobiology of Schizophrenia and Its Treatment; Swerdlow, N.R., Ed.; Current Topics in Behavioral Neurosciences; Springer: Berlin/Heidelberg, Germany, 2010; Volume 4, pp. 529–553. ISBN 978-3-642-13716-7. [Google Scholar]
- Zheng, F.; Li, C.; Zhang, D.; Cui, D.; Wang, Z.; Qiu, J. Study on the Sub-Regions Volume of Hippocampus and Amygdala in Schizophrenia. Quant. Imaging Med. Surg. 2019, 9, 1025–1036. [Google Scholar] [CrossRef]
- Dorph-Petersen, K.-A.; Lewis, D.A. Postmortem Structural Studies of the Thalamus in Schizophrenia. Schizophr. Res. 2017, 180, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Adriano, F.; Spoletini, I.; Caltagirone, C.; Spalletta, G. Updated Meta-Analyses Reveal Thalamus Volume Reduction in Patients with First-Episode and Chronic Schizophrenia. Schizophr. Res. 2010, 123, 1–14. [Google Scholar] [CrossRef]
- Dietsche, B.; Kircher, T.; Falkenberg, I. Structural Brain Changes in Schizophrenia at Different Stages of the Illness: A Selective Review of Longitudinal Magnetic Resonance Imaging Studies. Aust. N. Z. J. Psychiatry 2017, 51, 500–508. [Google Scholar] [CrossRef]
- Harvey, P.D.; Rosenthal, J.B. Cognitive and Functional Deficits in People with Schizophrenia: Evidence for Accelerated or Exaggerated Aging? Schizophr. Res. 2018, 196, 14–21. [Google Scholar] [CrossRef]
- Uwatoko, T.; Yoshizumi, M.; Miyata, J.; Ubukata, S.; Fujiwara, H.; Kawada, R.; Kubota, M.; Sasamoto, A.; Sugihara, G.; Aso, T.; et al. Insular Gray Matter Volume and Objective Quality of Life in Schizophrenia. PLoS ONE 2015, 10, e0142018. [Google Scholar] [CrossRef] [Green Version]
- Yue, Y.; Kong, L.; Wang, J.; Li, C.; Tan, L.; Su, H.; Xu, Y. Regional Abnormality of Grey Matter in Schizophrenia: Effect from the Illness or Treatment? PLoS ONE 2016, 11, e0147204. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Ma, X.; Wang, D.; Qin, W.; Zhu, J.; Zhuo, C.; Yu, C. The Selective Impairment of Resting-State Functional Connectivity of the Lateral Subregion of the Frontal Pole in Schizophrenia. PLoS ONE 2015, 10, e0119176. [Google Scholar] [CrossRef]
- Zhuo, C.; Ma, X.; Qu, H.; Wang, L.; Jia, F.; Wang, C. Schizophrenia Patients Demonstrate Both Inter-Voxel Level and Intra-Voxel Level White Matter Alterations. PLoS ONE 2016, 11, e0162656. [Google Scholar] [CrossRef]
- Russell, A.J.; Munro, J.C.; Jones, P.B.; Hemsley, D.R.; Murray, R.M. Schizophrenia and the Myth of Intellectual Decline. Am. J. Psychiatry 1997, 154, 635–639. [Google Scholar] [CrossRef]
- Laks, J.; Fontenelle, L.F.; Chalita, A.; Mendlowicz, M.V. Absence of Dementia in Late-Onset Schizophrenia: A One Year Follow-up of a Brazilian Case Series. Arq. Neuropsiquiatr. 2006, 64, 946–949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivas, J.; Libreros, J.; Trujillo, M.; Hurtado, A.; Camprodon, J. Experimental Data on Demographic, Functional and Structures of Patients with Schizophrenia and Schizophrenia-Dementia. Data Brief 2020, 32, 106286. [Google Scholar] [CrossRef] [PubMed]
- Hachinski, V.C.; Iliff, L.D.; Zilhka, E.; Du Boulay, G.H.; McAllister, V.L.; Marshall, J.; Russell, R.W.R.; Symon, L. Cerebral Blood Flow in Dementia. Arch. Neurol. 1975, 32, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Yesavage, J.A.; Brink, T.L.; Rose, T.L.; Lum, O.; Huang, V.; Adey, M.; Leirer, V.O. Development and Validation of a Geriatric Depression Screening Scale: A Preliminary Report. J. Psychiatr. Res. 1982, 17, 37–49. [Google Scholar] [CrossRef]
- Brandt, J. The Hopkins Verbal Learning Test: Development of a New Memory Test with Six Equivalent Forms. Clin. Neuropsychol. 1991, 5, 125–142. [Google Scholar] [CrossRef]
- Osterrieth, P.A. Le Test de Copie d’une Figure Complexe; Contribution à l’étude de La Perception et de La Mémoire. [Test of Copying a Complex Figure; Contribution to the Study of Perception and Memory]. Arch. Psychol. 1944, 30, 206–356. [Google Scholar]
- Buschke, H.; Fuld, P.A. Evaluating Storage, Retention, and Retrieval in Disordered Memory and Learning. Neurology 1974, 24, 1019. [Google Scholar] [CrossRef] [Green Version]
- Nicholas, L.E.; Brookshire, R.H.; Maclennan, D.L.; Schumacher, J.G.; Porrazzo, S.A. Revised Administration and Scoring Procedures for the Boston Naming Test and Norms for Non-Brain-Damaged Adults. Aphasiology 1989, 3, 569–580. [Google Scholar] [CrossRef]
- Belleville, S.; Fouquet, C.; Hudon, C.; Zomahoun, H.T.V.; Croteau, J. Consortium for the Early Identification of Alzheimer’s disease-Quebec Neuropsychological Measures That Predict Progression from Mild Cognitive Impairment to Alzheimer’s Type Dementia in Older Adults: A Systematic Review and Meta-Analysis. Neuropsychol. Rev. 2017, 27, 328–353. [Google Scholar] [CrossRef]
- Dale, A.M.; Sereno, M.I. Improved Localizadon of Cortical Activity by Combining EEG and MEG with MRI Cortical Surface Reconstruction: A Linear Approach. J. Cogn. Neurosci. 1993, 5, 162–176. [Google Scholar] [CrossRef]
- Fischl, B.; Salat, D.H.; van der Kouwe, A.J.W.; Makris, N.; Ségonne, F.; Quinn, B.T.; Dale, A.M. Sequence-Independent Segmentation of Magnetic Resonance Images. NeuroImage 2004, 23, S69–S84. [Google Scholar] [CrossRef] [PubMed]
- Fischl, B.; Sereno, M.I.; Dale, A.M. Cortical Surface-Based Analysis. NeuroImage 1999, 9, 195–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischl, B.; Sereno, M.I.; Tootell, R.B.H.; Dale, A.M. High-Resolution Intersubject Averaging and a Coordinate System for the Cortical Surface. Hum. Brain Mapp. 1999, 8, 272–284. [Google Scholar] [CrossRef]
- Han, X.; Jovicich, J.; Salat, D.; van der Kouwe, A.; Quinn, B.; Czanner, S.; Busa, E.; Pacheco, J.; Albert, M.; Killiany, R.; et al. Reliability of MRI-Derived Measurements of Human Cerebral Cortical Thickness: The Effects of Field Strength, Scanner Upgrade and Manufacturer. NeuroImage 2006, 32, 180–194. [Google Scholar] [CrossRef] [PubMed]
- Reuter, M.; Rosas, H.D.; Fischl, B. Highly Accurate Inverse Consistent Registration: A Robust Approach. NeuroImage 2010, 53, 1181–1196. [Google Scholar] [CrossRef] [Green Version]
- Sled, J.G.; Zijdenbos, A.P.; Evans, A.C. A Nonparametric Method for Automatic Correction of Intensity Nonuniformity in MRI Data. IEEE Trans. Med. Imaging 1998, 17, 87–97. [Google Scholar] [CrossRef] [Green Version]
- Fischl, B.; Liu, A.; Dale, A.M. Automated Manifold Surgery: Constructing Geometrically Accurate and Topologically Correct Models of the Human Cerebral Cortex. IEEE Trans. Med. Imaging 2001, 20, 70–80. [Google Scholar] [CrossRef] [Green Version]
- Fischl, B.; Dale, A.M. Measuring the Thickness of the Human Cerebral Cortex from Magnetic Resonance Images. Proc. Natl. Acad. Sci. USA 2000, 97, 11050–11055. [Google Scholar] [CrossRef] [Green Version]
- Reuter, M.; Schmansky, N.J.; Rosas, H.D.; Fischl, B. Within-Subject Template Estimation for Unbiased Longitudinal Image Analysis. NeuroImage 2012, 61, 1402–1418. [Google Scholar] [CrossRef] [Green Version]
- Iglesias, J.E.; Augustinack, J.C.; Nguyen, K.; Player, C.M.; Player, A.; Wright, M.; Roy, N.; Frosch, M.P.; McKee, A.C.; Wald, L.L.; et al. A Computational Atlas of the Hippocampal Formation Using Ex Vivo, Ultra-High Resolution MRI: Application to Adaptive Segmentation of in Vivo MRI. NeuroImage 2015, 115, 117–137. [Google Scholar] [CrossRef]
- Iglesias, J.E.; Insausti, R.; Lerma-Usabiaga, G.; Bocchetta, M.; Van Leemput, K.; Greve, D.N.; van der Kouwe, A.; Fischl, B.; Caballero-Gaudes, C.; Paz-Alonso, P.M. A Probabilistic Atlas of the Human Thalamic Nuclei Combining Ex Vivo MRI and Histology. NeuroImage 2018, 183, 314–326. [Google Scholar] [CrossRef] [PubMed]
- Aylward, E.; Walker, E.; Bettes, B. Intelligence in Schizophrenia: Meta-Analysis of the Research. Schizophr. Bull. 1984, 10, 430–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bilder, R.M.; Lipschutz-Broch, L.; Reiter, G.; Geisler, S.H.; Mayerhoff, D.I.; Lieberman, J.A. Intellectual Deficits in First-Episode Schizophrenia: Evidence for Progressive Deterioration. Schizophr. Bull. 1992, 18, 437–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caspi, A.; Reichenberg, A.; Weiser, M.; Rabinowitz, J.; Kaplan, Z.; Knobler, H.; Davidson-Sagi, N.; Davidson, M. Cognitive Performance in Schizophrenia Patients Assessed before and Following the First Psychotic Episode. Schizophr. Res. 2003, 65, 87–94. [Google Scholar] [CrossRef]
- Duan, X.; He, C.; Ou, J.; Wang, R.; Xiao, J.; Li, L.; Wu, R.; Zhang, Y.; Zhao, J.; Chen, H. Reduced Hippocampal Volume and Its Relationship with Verbal Memory and Negative Symptoms in Treatment-Naive First-Episode Adolescent-Onset Schizophrenia. Schizophr. Bull. 2021, 47, 64–74. [Google Scholar] [CrossRef]
- Heaton, R.K.; Gladsjo, J.A.; Palmer, B.W.; Kuck, J.; Marcotte, T.D.; Jeste, D.V. Stability and Course of Neuropsychological Deficits in Schizophrenia. Arch. Gen. Psychiatry 2001, 58, 24–32. [Google Scholar] [CrossRef]
- Heinrichs, R.W.; Zakzanis, K.K. Neurocognitive Deficit in Schizophrenia: A Quantitative Review of the Evidence. Neuropsychology 1998, 12, 426–445. [Google Scholar] [CrossRef]
- Hoff, A.L.; Svetina, C.; Shields, G.; Stewart, J.; DeLisi, L.E. Ten Year Longitudinal Study of Neuropsychological Functioning Subsequent to a First Episode of Schizophrenia. Schizophr. Res. 2005, 78, 27–34. [Google Scholar] [CrossRef]
- Hyde, T.M.; Nawroz, S.; Goldberg, T.E.; Bigelow, L.B.; Strong, D.; Ostrem, J.L.; Weinberger, D.R.; Kleinman, J.E. Is There Cognitive Decline in Schizophrenia?: A Cross-Sectional Study. Br. J. Psychiatry 1994, 164, 494–500. [Google Scholar] [CrossRef] [Green Version]
- Kremen, W.S.; Buka, S.L.; Seidman, L.J.; Goldstein, J.M.; Koren, D.; Tsuang, M.T. IQ Decline During Childhood and Adult Psychotic Symptoms in a Community Sample: A 19-Year Longitudinal Study. Am. J. Psychiatry 1998, 155, 672–677. [Google Scholar] [CrossRef]
- Kurtz, M. Neurocognitive Impairment across the Lifespan in Schizophrenia: An Update. Schizophr. Res. 2005, 74, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Mockler, D.; Riordan, J.; Sharma, T. Memory and Intellectual Deficits Do Not Decline with Age in Schizophrenia. Schizophr. Res. 1997, 26, 1–7. [Google Scholar] [CrossRef]
- Palmer, B.W.; Dawes, S.E.; Heaton, R.K. What Do We Know About Neuropsychological Aspects of Schizophrenia? Neuropsychol. Rev. 2009, 19, 365–384. [Google Scholar] [CrossRef] [Green Version]
- Reichenberg, A.; Weiser, M.; Rapp, M.A.; Rabinowitz, J.; Caspi, A.; Schmeidler, J.; Knobler, H.Y.; Lubin, G.; Nahon, D.; Harvey, P.D.; et al. Elaboration on Premorbid Intellectual Performance in Schizophrenia: Premorbid Intellectual Decline and Risk for Schizophrenia. Arch. Gen. Psychiatry 2005, 62, 1297–1304. [Google Scholar] [CrossRef] [PubMed]
- Rund, B.R.; Melle, I.; Friis, S.; Larsen, T.K.; Midbøe, L.J.; Opjordsmoen, S.; Simonsen, E.; Vaglum, P.; McGlashan, T. Neurocognitive Dysfunction in First-Episode Psychosis: Correlates with Symptoms, Premorbid Adjustment, and Duration of Untreated Psychosis. Am. J. Psychiatry 2004, 161, 466–472. [Google Scholar] [CrossRef]
- Seidman, L.J.; Buka, S.L.; Goldstein, J.M.; Tsuang, M.T. Intellectual Decline in Schizophrenia: Evidence from a Prospective Birth Cohort 28 Year Follow-up Study. J. Clin. Exp. Neuropsychol. 2006, 28, 225–242. [Google Scholar] [CrossRef] [PubMed]
- Sheitman, B.B.; Murray, M.G.; Snyder, J.A.; Silva, S.; Goldman, R.; Chakos, M.; Volavka, J.; Lieberman, J.A. IQ Scores of Treatment-Resistant Schizophrenia Patients before and after the Onset of the Illness. Schizophr. Res. 2000, 46, 203–207. [Google Scholar] [CrossRef]
- van Winkel, R.; Myin-Germeys, I.; Delespaul, P.; Peuskens, J.; De Hert, M.; van Os, J. Premorbid IQ as a Predictor for the Course of IQ in First Onset Patients with Schizophrenia: A 10-Year Follow-up Study. Schizophr. Res. 2006, 88, 47–54. [Google Scholar] [CrossRef]
- Weickert, T.W.; Goldberg, T.E.; Gold, J.M.; Bigelow, L.B.; Egan, M.F.; Weinberger, D.R. Cognitive Impairments in Patients with Schizophrenia Displaying Preserved and Compromised Intellect. Arch. Gen. Psychiatry 2000, 57, 907–913. [Google Scholar] [CrossRef] [Green Version]
- Woodberry, K.A.; Giuliano, A.J.; Seidman, L.J. Premorbid IQ in Schizophrenia: A Meta-Analytic Review. Am. J. Psychiatry 2008, 165, 579–587. [Google Scholar] [CrossRef]
- Casanova, M.F.; Rothberg, B. Shape Distortion of the Hippocampus: A Possible Explanation of the Pyramidal Cell Disarray Reported in Schizophrenia. Schizophr. Res. 2002, 55, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Small, S.A.; Schobel, S.A.; Buxton, R.B.; Witter, M.P.; Barnes, C.A. A Pathophysiological Framework of Hippocampal Dysfunction in Ageing and Disease. Nat. Rev. Neurosci. 2011, 12, 585–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byne, W.; Buchsbaum, M.S.; Kemether, E.; Hazlett, E.A.; Shinwari, A.; Mitropoulou, V.; Siever, L.J. Magnetic Resonance Imaging of the Thalamic Mediodorsal Nucleus and Pulvinar in Schizophrenia and Schizotypal Personality Disorder. Arch. Gen. Psychiatry 2001, 58, 133–140. [Google Scholar] [CrossRef] [Green Version]
- Kemether, E.M.; Buchsbaum, M.S.; Byne, W.; Hazlett, E.A.; Haznedar, M.; Brickman, A.M.; Platholi, J.; Bloom, R. Magnetic Resonance Imaging of Mediodorsal, Pulvinar, and Centromedian Nuclei of the Thalamus in Patients with Schizophrenia. Arch. Gen. Psychiatry 2003, 60, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Sim, K.; Cullen, T.; Ongur, D.; Heckers, S. Testing Models of Thalamic Dysfunction in Schizophrenia Using Neuroimaging. J. Neural Transm. 2006, 113, 907–928. [Google Scholar] [CrossRef]
- Andreasen, N.C.; Paradiso, S.; O’Leary, D.S. “Cognitive Dysmetria” as an Integrative Theory of Schizophrenia: A Dysfunction in Cortical-Subcortical-Cerebellar Circuitry? Schizophr. Bull. 1998, 24, 203–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coscia, D.M.; Narr, K.L.; Robinson, D.G.; Hamilton, L.S.; Sevy, S.; Burdick, K.E.; Gunduz-Bruce, H.; McCormack, J.; Bilder, R.M.; Szeszko, P.R. Volumetric and Shape Analysis of the Thalamus in First-Episode Schizophrenia. Hum. Brain Mapp. 2009, 30, 1236–1245. [Google Scholar] [CrossRef]
- Phelps, E.A. Human Emotion and Memory: Interactions of the Amygdala and Hippocampal Complex. Curr. Opin. Neurobiol. 2004, 14, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Killgore, W.D.S.; Rosso, I.M.; Gruber, S.A.; Yurgelun-Todd, D.A. Amygdala Volume and Verbal Memory Performance in Schizophrenia and Bipolar Disorder. Cogn. Behav. Neurol. 2009, 22, 28–37. [Google Scholar] [CrossRef]
- Ho, N.F.; Chong, P.L.H.; Lee, D.R.; Chew, Q.H.; Chen, G.; Sim, K. The Amygdala in Schizophrenia and Bipolar Disorder: A Synthesis of Structural MRI, Diffusion Tensor Imaging, and Resting-State Functional Connectivity Findings. Harv. Rev. Psychiatry 2019, 27, 150–164. [Google Scholar] [CrossRef]
- Rivas, J.C.; Libreros, J.; Trujillo, M. Control—Schizophrenia—Schizophrenia-Dementia. Zenodo 2020. [Google Scholar] [CrossRef]
| CG Q2 (Q1–Q3) | SND Q2 (Q1–Q3) | SD Q2 (Q1–Q3) | Mann–Whitney p-Value | |||
|---|---|---|---|---|---|---|
| CG vs. SND | CG vs. SD | SD vs. SND | ||||
| Age (years) | 60.0 (56.0–64.0) | 58.0 (52.0–59.8) | 69.5 (66.5–72.5) | 0.1810 | 0.0060 | 0.0030 |
| Schooling: completed years | 15.6 (11.0–22.0) | 8.9 (5.0–14.0) | 6.8 (0–15.0) | 0.0001 | 0.0001 | 0.2122 |
| Years of schizophrenia | - | 26.9 (15.0–45.0) | 41.3 (27.0–55.0) | - | - | 0.0042 |
| CG Q2 (Q1–Q3) | SND Q2 (Q1–Q3) | SD Q2 (Q1–Q3) | Mann–Whitney p-Value | |||
|---|---|---|---|---|---|---|
| CG vs. SND | CG vs. SD | SD vs. SND | ||||
| LetterF | 6 (4–7) | 4 (3.8–5.3) | 0 (0–2.3) | 0.039 | 0.001 | 0.001 |
| Animal Fluency | 7 (6–7) | 3 (1.8–4.3) | 0 (0–2) | 0.001 | 0.001 | 0.005 |
| LetterS | 6 (4–7) | 3 (0–4) | 0 (0–0.3) | 0.002 | 0.001 | 0.034 |
| HVLT Rey words-Total Recall | 105 (87–116) | 56.5 (41–75) | 25.5 (14.3–42.3) | 0.001 | 0.001 | 0.009 |
| HVLT-Delayed Recall | 11 (9–14) | 4.5 (3.8–7.3) | 0 (0–3) | 0.001 | 0.001 | 0.007 |
| Rey Figure-Copy | 35 (32–36) | 25.5 (18.4–32) | 7 (0–14) | 0.003 | 0.001 | 0.025 |
| Rey Figure-Immediate Recall | 18 (14.5–28) | 7.5 (0–11) | 0 (0–3.8) | 0.001 | 0.001 | 0.122 |
| Rey Figure-Delayed Recalled | 18 (14–22.5) | 7.5 (0.4–13.5) | 0 (0–2) | 0.001 | 0.001 | 0.024 |
| Digit Span | 5 (4–7) | 4 (2.8–4) | 2.5 (1.5–3.0) | 0.005 | 0.001 | 0.012 |
| Boston Naming Test | 19 (19–20) | 8 (0–19.3) | 11.5 (0–15) | 0.018 | 0.001 | 0.617 |
| FCSRT-IDEN | 16 (16–16) | 15 (13.8–16) | 12.5 (7.5–15.3) | 0.001 | 0.001 | 0.077 |
| FCSRT-Free Recall score | 35 (31–40) | 18 (12.8–26.5) | 8.5 (0–13.5) | 0.004 | 0.001 | 0.022 |
| FCSRT-Cued Recall score | 43 (40–46) | 24 (17.3–30.8) | 7 (0–21.3) | 0.001 | 0.001 | 0.033 |
| FCSRT-Total recall score | 73 (55–84) | 46.5 (37.5–55.5) | 18 (0–4) | 0.017 | 0.001 | 0.015 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivas, J.; Gutierrez-Gomez, S.; Villanueva-Congote, J.; Libreros, J.; Camprodon, J.A.; Trujillo, M. Subcortical Structures in Demented Schizophrenia Patients: A Comparative Study. Biomedicines 2023, 11, 233. https://doi.org/10.3390/biomedicines11010233
Rivas J, Gutierrez-Gomez S, Villanueva-Congote J, Libreros J, Camprodon JA, Trujillo M. Subcortical Structures in Demented Schizophrenia Patients: A Comparative Study. Biomedicines. 2023; 11(1):233. https://doi.org/10.3390/biomedicines11010233
Chicago/Turabian StyleRivas, Juan, Santiago Gutierrez-Gomez, Juliana Villanueva-Congote, Jose Libreros, Joan Albert Camprodon, and María Trujillo. 2023. "Subcortical Structures in Demented Schizophrenia Patients: A Comparative Study" Biomedicines 11, no. 1: 233. https://doi.org/10.3390/biomedicines11010233
APA StyleRivas, J., Gutierrez-Gomez, S., Villanueva-Congote, J., Libreros, J., Camprodon, J. A., & Trujillo, M. (2023). Subcortical Structures in Demented Schizophrenia Patients: A Comparative Study. Biomedicines, 11(1), 233. https://doi.org/10.3390/biomedicines11010233





